Introduction
There has been a rapid development in invasive strategies for coronary artery disease from balloon angioplasty alone (no scaffolds) to bare metal stents and drug eluting stents (permanent scaffolds) to bioabsorbable stents (temporary scaffolds). While the introduction of durable stents (permanent scaffolds) resolved issues relating to arterial recoil, restenosis was overcome (to a greater extent) by including drugs to suppress neointimal hyperplasia. Bioabsorbable stents are temporary scaffolds which disappear over a period of time and may also incorporate drug-elution characteristics. We review how the interaction of the mechanical and biological forces in bioabsorbable stents differs from durable stents.
Coronary restenosis
Neointimal hyperplasia after stent placement and the resulting reduction in coronary lumen remains a problem in patients undergoing percutaneous coronary intervention, with bare metal stents in particular. While drug-eluting stents have focused on suppressing smooth muscle cell proliferation, they have required durable scaffolds and polymers. It is important, however, to recall that the substantial impact of stents have been to optimise lumen gain by preventing vessel recoil (“the bigger the better” hypothesis). Modern durable stents consistently yield acute residual stenosis of 5% or less.
Pathophysiology of restenosis
The pathophysiology of restenosis is complex and incompletely understood (Figure 1). In the era of percutaneous transluminal coronary angioplasty (PTCA), restenosis was a major limitation with rates up to 30% to 60% within the first six months1,2. It was originally believed, based on animal models3,4 and human necropsy studies5-7, that restenosis was a result of exaggeration of the normal reparative processes after angioplas ty-induced local vessel trauma, leading to uncontrolled smooth muscle cell proliferation and encroachment of the lumen. Elegant studies published in the 90’s have challenged the traditional injury-proliferation restenosis hypothesis. These studies have shown that in patients undergoing PTCA, up to 70-80% of decrease in arterial lumen is the result of negative remodelling with arterial constriction with decrease in external elastic membrane and subsequent restenosis, with only 20-30% of the process attributable to neointimal/cellular proliferation and plaque growth8.
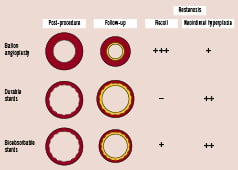
Figure 1. Pathophysiology of in-stent restenosis for bioabsorbable stents when compared to durable stents or balloon angioplasty. With balloon angioplasty alone, restenosis is largely due to recoil and partly due to neointimal hyperplasia, while that with durable stents is largely due to neointimal hyperplasia. With bioabsorbable stents there may be contribution from both stent recoil and neointimal hyperplasia.
The introduction of durable stents effectively reduced the restenosis by functioning as a mechanical scaffold that eliminates the elastic recoil and negative remodelling2. The rate of restenosis has been reduced to 15 to 30% at 12 months with the use of bare metal stents9. Long term angioscopic follow-up of patients with bare metal stents have shown that while the initial restenosis is due to neointimal proliferation and “white” plaque (arterial healing), over extended periods (4-5 years), there is transformation into “yellow” plaque (atherosclerotic plaque) and consequent restenosis10. Moreover, the metal strut elicits inflammatory response and corrodes gradually resulting in a state of chronic inflammation which contributes to the restenotic process. Newer stents made of cobalt chromium or platinum chromium with excellent anticorrosive properties are designed for higher radial strength, and improved radiopacity allowing for thinner stent struts.
Drug eluting stents (DES), with the use of antiproliferative medications, prevent/reduce neointimal hyperplasia while at the same time provide scaffolding to prevent recoil. DES have reduced restenosis risk dramatically compared to BMS, with a target lesion revascularisation rate of <10% at 12 months9. Despite the efficacy of drug-eluting stents (DES) in reducing restenosis compared with bare metal stents (BMS)11,12, there is concern that DES might be associated with higher rates of stent thrombosis – particularly beyond the first year after implantation, leading to myocardial infarction and death13. Data from animal and human studies have shown that DES can cause substantial impairment in arterial healing characterised by lack of complete re-endothelialisation and persistence of fibrin when compared with BMS. This is thought to be the primary substrate for late stent thrombosis14. Whether the presence of drug or durable polymer are culprits for these late events remains incompletely understood. Various strategies have been proposed to reduce the late adverse events after stenting, including the use of bioabsorbable polymers and fully bioabsorbable stents.
Acute gain, late loss, net gain paradigm
A clear understanding of the coronary restenotic mechanics needs a thorough understanding of the interplay of acute gain, late loss and net gain. Coronary restenosis is influenced by both acute gain provided by the intervention and the subsequent late lumen loss that ensues over the four to six months following intervention. Acute gain is defined as the increase in the luminal diameter from baseline to that immediately post-intervention (post-procedure minimal luminal diameter (MLD) – pre-procedure MLD). Late lumen loss is defined as the narrowing in luminal diameter from immediately after intervention to the follow-up period (post-procedure MLD – follow-up MLD). The net gain is thus the sum of the offsetting effects of acute gain and late lumen loss (Figure 2).
Post-procedural MLD has been shown to be an independent predictor of both late luminal diameter and percentage stenosis15. The bigger the better hypothesis was made popular in the early 90’s to explain the benefit of the stent scaffold over angioplasty alone15, suggesting that the larger the immediate post-procedure MLD, the larger the late luminal diameter and hence lower the probability of restenosis. In the era of universal use of stents for PCI, where between stents the post-procedure MLD varies minimally, late lumen loss has been used as a restenosis metric in the pilot and pivotal stent trials. Late lumen loss has been shown to be reliable metric for restenosis. However, late lumen loss is a reliable metric for comparison only when the acute gain or the post-procedure MLD is same between compared treatments. This is illustrated in Figure 2 which shows the acute gain, late loss and net gain after stenting and that after balloon angioplasty in a vessel with similar dimensions. Though the late lumen loss is lower with balloon angioplasty compared to stenting, the net gain is higher (and hence restenosis lower) with stenting compared to balloon angioplasty, largely driven by the acute gain with stenting (Figure 2). Hence, in device comparison trials, if the acute gain is not similar (usually evidenced by post-procedure MLD or residual percent diameter stenosis immediately after stent placement), the late lumen loss reflects only neointimal hyperplasia, but cannot accurately predict restenosis. In trials of drug-eluting stents versus bare metal stents, the metal scaffolding affords the same acute gain and hence late lumen loss is a good metric for restenosis probability. However, with bioabsorbable stents with temporary scaffold, the acute gain is not similar (not yet) to that with permanent scaffolds, making late lumen loss a less attractive primary angiographic endpoint. This has implications when trials are designed for bioabsorbable stents to be compared to durable stents, as outlined below.
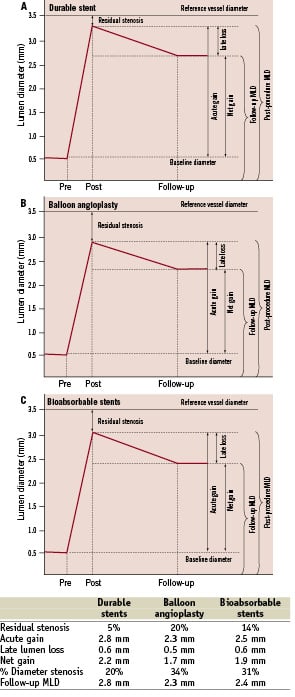
Figure 2. Late lumen loss and follow-up lumen diameter. The data is for a hypothetical patient with a reference vessel diameter of 3.5 mm and percentage residual stenosis of 5% for durable stents, 20% for balloon angioplasty and 14% for bioabsorbable stents. When the acute gain is not similar, late lumen loss is not a reliable surrogate for restenosis, and metrics that incorporate acute gain (e.g., late MLD) are more reliable. A. Acute gain, late loss and net gain for durable stents. B. Acute gain, late loss and net gain for balloon angioplasty. C. Acute gain, late loss and net gain for bioabsorbable stents.
Bioabsorbable stent and restenosis
As the stent itself represents a foreign body in the vasculature with prothrombogenic and inflammatory potential, employment of an entirely bioabsorbable or biodegradable stent appears to be a logical and attractive approach.
The potential drawback to bioabsorbable stents are the reduced radial strength compared to metal stents as the polymers are more flexible. The optimum duration for the need for scaffolding is not clear and premature stent bioabsorption can lead to recoil and subsequent restenosis. Moreover, biodegradation, in some examples, has been shown to elicit intense inflammatory reaction which can lead to an increase in the risk of restenosis16. These stents are radio-lucent, and can therefore be technically challenging for accurate placement, accurate post-dilatation and placement of additional overlapping stents without gaps or long overlap. In addition, the strut thickness of these stents is larger (150-200 µm) and vessel coverage by struts is greater compared to contemporary durable stents. For contemporary durable stents, strut thickness is a predictor of restenosis17. For bioabsorbable stents, strut thickness and vessel coverage by struts could be a problem earlier on, eliciting a greater inflammatory reaction and greater propensity for restenosis in the few months after implantation. In stents where absorption is by surface erosion, the strut thickness decreases as the stent is absorbed and the strut thickness is a short-term problem. However, in stents where absorption is by bulk erosion, absorption occurs throughout the mass of the implant and not just at the surface and the strut retains its shape until absorption is well advance.
Acute gain, late loss, net gain in bioabsorbable stent clinical trials
Assessment of restenosis using contemporary metrics of restenosis in this disappearing frame of reference is challenging. The usual ability to delineate the standard “in-stent” and “in-segment” (including the 5 mm proximal and distal to the stent margin) zones for analysis requires radio-opacity of stent material. Newer bioabsorbable stents have been designed with radio-opaque markers at either end of the stent to make it easier to visualise. The variability of acute gain between stent platforms suggests that measures that incorporate both acute gain and late loss, e.g., minimum lumen diameter or percent diameter stenosis at follow-up are the important angiographic endpoints to survey.
In the Clinical Performance and Angiographic Results of Coronary Stenting with Absorbable Metal Stents trial (PROGRESS AMS), a prospective non-randomised trial where 71 magnesium stents (no radio-opaque markers or drug) with radial strength similar to metal stents, were implanted in 63 patients. The mean acute gain was 1.41 mm but with a residual stenosis of 12% (substantially higher than the <5% residual stenosis observed with durable stents). However, complete stent absorption occurred within two months, leading to high restenosis rate at four months (50%) with target vessel revascularisation rate of 45% at one year18. It should be noted that the mean late lumen loss was 0.66 mm (similar to zotarolimus-eluting stent), but given the smaller post- procedure MLD, the restenosis rate was substantially higher than expected for approved bare metal stents.
In the Absorb trial19, the BVS everolimus-eluting stent (Abbott Vascular, Santa Clara, CA, USA) was used. The stent is made of bioabsorbable PLLA with a bioabsorbable polymer containing everolimus. The stent provides superior radial strength for months with a total absorption time of around two to three years. The stent also has two adjacent platinum radio-opaque markers at each end for enhanced visibility on fluoroscopy to guide precise post-dilation and additional stent placement. At one year, there was no late stent thrombosis with a major adverse cardiac event of only 3.3%. At 6-month follow-up, the angiographic in-stent late loss was 0.44±0.35 mm19, similar to late loss observed with contemporary durable drug eluting stents (0.39 mm for paclitaxel-eluting stents and 0.60 mm for zotarolimus-eluting stents) and significantly less that that with bare-metal stents (~0.80 mm). However, it should be noted that there was still a higher (16%) residual stenosis post- intervention (<5% with contemporary stents). The late loss with the BVS stent was mainly due to reduction in stent area, with a small contribution from intrastent neointimal hyperplasia. The loss in stent area with the BVS stent has been attributed to acute stent recoil, to non-uniform vessel support and to chronic recoil due to loss of radial strength associated with partial bioabsorption19. The 2-year substudy of this trial on 30 patients showed a late loss of only 0.48±0.28 mm, with no cases of stent thromboses, unchanged from the six month results. Additionally, the stented site and the adjacent coronary artery showed vasomotion in response to vasoactive agents, which is an important step towards normal vasoreactivity of the coronaries in response to exercise, stress and ischaemia20.
The REVA (Reva Medical Inc, San Diego, CA, USA) stent has thicker struts (200 µm) with a stent to artery ratio of 55%, much higher than that seen in contemporary durable stents. Radio-opacity is provided by impregnation with iodine. Initial results has been disappointing with higher than anticipated target lesion revascularisation rates at six months21.
Late loss and bioabsorbable stents
Clinical restenosis (ischaemia driven target lesion revascularisation) is the gold standard for the measurement of restenosis after percutaneous coronary intervention. However, clinical restenosis rates do not allow objective comparison of the restenosis propensity across studies, as the same stent may have varying propensity of restenosis (by up to four folds) in patients with different risk factors22. Various metrics have been used as surrogate measures of restenosis include percent diameter stenosis, late loss in lumen diameter (measured in millimetres) and intimal volume, measured as an absolute or relative volume by intravascular ultrasound. In-stent late lumen loss has been correlated with monotonic and incremental binary restenosis propensity and has been widely used in contemporary stent trials23. With improvement in stent technology and the introduction of drug eluting stents, rates of TLR have diminished, necessitating larger size trials to show minor differences. In this context, in-stent late loss is a more robust endpoint than TLR, particularly in early studies to evaluate efficacy, drug dose-finding studies, or evaluations of the effects of minor variations in stent design before larger trials to evaluate safety and clinical efficacy24. In a systematic evaluation based on individual patient data from 11 randomised controlled trials, late lumen loss and percent diameter stenosis were found to be reliable estimate of TLR rate for both DES and BMS9. However, just as late loss is not reliable for the comparison of mechanically different techniques – balloon angioplasty alone shows lower late loss than stent placement but higher restenosis rates, for example – in bioabsorbable stent trials, where the initial acute gain may differ from durable stents, or across bioabsorbable stents, late loss measures neointimal hyperplasia but may not be an accurate surrogate for clinical restenosis.
In the example provided in Figure 2, and based on the data on late loss with bioabsorbable stents detailed above, it is clear the late lumen loss with bioabsorbable stents is similar to contemporary drug-eluting stents. Yet, contemporary bioabsorbable stents have a higher rate of restenosis. The difference is attributable in part to post-procedure MLD or acute gain. In contemporary durable stents, the percentage residual stenosis is <5%. With bioabsorbable stents, the percentage residual stenosis is 12-16%. Hence, late lumen loss cannot be used as a reliable metric for restenosis, given the difference in acute gain. In addition, late stent recoil may also contribute towards restenosis (which is not factored in when measuring late lumen loss). Given this, metrics which are independent of acute gain such as minimal luminal diameter and percentage diameter stenosis may be better surrogates for restenosis.
Late lumen loss- role of stent recoil
For contemporary durable stents (both BMS and DES), the negative constrictive remodelling is minimal and thus late loss is a good indicator of neointimal hyperplasia (Figure 1). However, for bioabsorbable stents the negative constrictive remodelling might not be trivial, with residual stenosis of 12-16% post-procedure. In the PROGRESS-AMS trial using magnesium stents, the 4-month late loss was 0.62 mm with substantial contribution from reduction in external elastic membrane volume (negative constrictive remodelling) and neointimal hyperplasia towards restenosis18. Moreover, these stents (without radio-opaque markers) were fully absorbed within four months, making further quantitative coronary angiographic measurements challenging. Though similar angiographic projection as compared to baseline and distance from a branch vessel are used to define the stented segment, precise measurement of in-stent late loss is challenging.
Other stents like the BVS everolimus-eluting stent have radio-opaque markers at both stent edges, negating the limitations for both adequate stent placement and follow-up quantitative coronary angiography. Similar to the PROGRESS-AMS study, in a substudy of the Absorb trial with BVS everolimus-eluting stent, late absolute recoil at six months was 0.65±1.71 mm2 (compared to 0.02 mm2 with the durable everolimus-eluting stent) indicating that the stent recoil is not so trivial with bioabsorbable stents during follow-up25.
“Very” late lumen loss
A critical component to assessment of the efficacy of bioabsorbable stents is understanding the relationship between duration of polymer and drug in tissue and the neointimal response. First, the kinetics of polymer and drug must be closely matched to suppress neointimal formation, for the required period, but no longer. Second, angiographic and clinical assessment in trials must be of sufficient duration to measure any late changes in the arterial wall. In bare metal stents, a quiescent state is expected within 6-9 months after stent placement. In drug-eluting stents, it is not certain when this state is achieved, and whether it varies between stent types. In bioabsorbable stents, with variable polymer and drug content and kinetics, this period is also likely to vary. It is believed that scaffolding is needed in the first few months after stenting and is rarely required beyond that. However, the optimal duration of scaffolding for bioabsorbable stents is unknown. Furthermore, the vascular response and the changes to arterial minimal luminal diameter after the stent gets absorbed are not known. Even when drug and polymer are no longer present, it is cautious to assume that there is a lasting biologic effect from their presence. Therefore, it will remain important to monitor the late activity within the arterial wall after placement of new types of bioabsorbable stents for either remodelling or for intimal growth.
In the Absorb trial, the mean late lumen loss at six months with the BVS everolimus-eluting stent was 0.44 mm with a small contribution from neointimal hyperplasia and greater contribution from recoil. It is reassuring that the two year data showed a late loss of 0.48 mm. Similarly, in the PROGRESS AMS trial with magnesium stents, in a subset of patients (eight out of 63 patients) with long term follow-up (12 to 28 months), the in-stent late loss decreased from 0.66 mm (–0.10 to 1.66 mm) to 0.44 mm (–0.09 to 0.73 mm) with an increase in reference vessel diameter from 2.9 mm to 3.3 mm suggesting a positive remodelling after stent absorption26. This positive remodelling occurs rarely with durable stents as the vessel gets splinted with the struts. However when it does occur, it may be associated with late stent malapposition.
The angiographic efficacy of bioabsorbable stents compared to drug-eluting stents is under active investigation. Even if successful in this regard, however, one cannot assume that because the drug and polymer are absent after a given period of time, that the vessel wall remains inert, and that the absence of a durable stent yields a safer milieu. While this is an attractive concept, the clinical superiority of such stents will need to be proven with adequately powered studies driven by clinical events. Furthermore, the duration of these studies should match or exceed the time to absorption of the stent.
Summary
Bioabsorbable stents are an important advancement in stent technology providing a temporary scaffold and may be augmented with drugs to suppress neointimal hyperplasia. Though changes are being made to the design of the stents, to ensure similar rates of acute recoil, residual stenosis post-intervention remains higher when compared to contemporary durable stents. In order to achieve similar clinical restenosis outcomes, bioabsorbable stents must first show similar MLD or percent diameter stenosis at follow-up to drug-eluting stents. While variable different acute gain and post-procedure MLD for bioabsorbable stents make late lumen loss a poor marker for restenosis probability by itself, late loss can be an informative metric for neointimal hyperplasia over time in these stents. Metrics which are independent of acute gain such as minimal luminal diameter and percentage diameter stenosis may make better surrogates for restenosis (Table 1). Moreover, remodelling over time requires measurement of the vessel and lumen diameters in later follow-up. Since the natural history of the vascular response to bioabsorbable stents is in its infancy of investigation, follow-up of these stents at least beyond the duration of polymer is prudent. Finally, given a disappearing frame of reference, both careful angiographic and intravascular ultrasound assessments are helpful to delineate the varying contributions of recoil, remodelling, and neointima formation.


