Abstract
Aims: Our aim was to evaluate the two-year clinical results of a new sirolimus-eluting stent (MiStent SES) with a bioabsorbable coating designed for rapid polymer dissolution but sustained drug delivery.
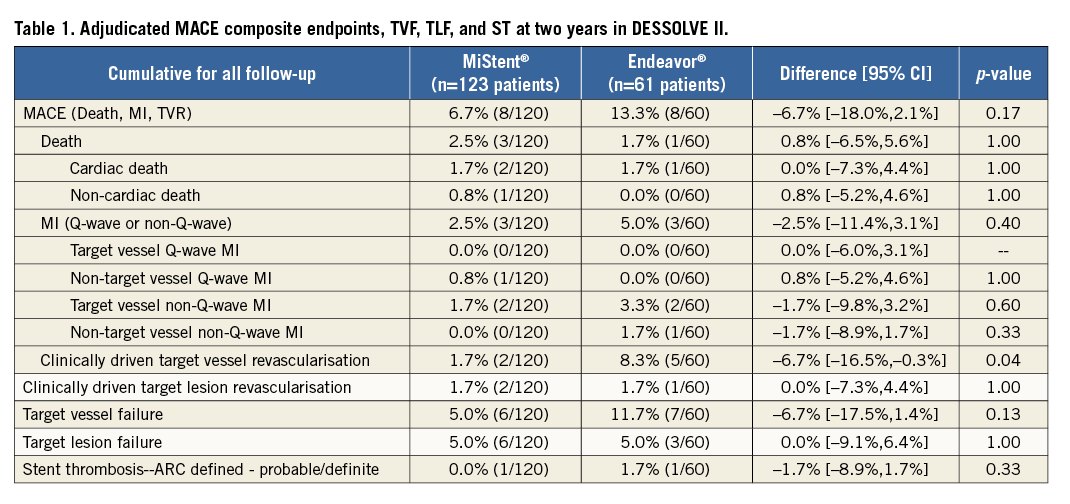
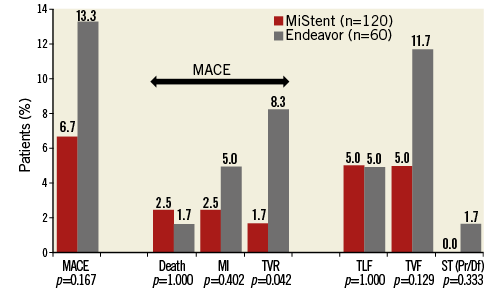
Methods and results: Major adverse cardiac events (MACE), target lesion failure (TLF), target vessel failure (TVF), and stent thrombosis (ST) at two-year follow-up are reported for the DESSOLVE I and II trials. In DESSOLVE I, the MiStent SES (n=29) demonstrated a 3.4% two-year MACE rate without TLF or TVF. In DESSOLVE II, the MiStent group had a 6.7% (8/120) two-year MACE rate compared to 13.3% (8/60) for Endeavor (p=0.167). TLF was 5.0% in the MiStent and Endeavor groups (p=1.00). TVF was 5.0% for MiStent versus 11.7% for Endeavor (p=0.129). No probable or definite ST was reported with the MiStent up to two years. The median duration of dual antiplatelet therapy (DAPT) in DESSOLVE I and II was 364 and 366 days, respectively.
Conclusions: The MiStent SES demonstrated good long-term safety and effectiveness with low two-year MACE, TLF, and TVF rates. ClinicalTrials.gov Identifiers: NCT01247428 and NCT01294748
Introduction
Permanent polymers on drug-eluting stents (DES) can produce an inflammatory response causing late stent thrombosis (ST) and DES failure1. In order to enhance their long-term safety and efficacy, biodegradable polymer-based DES were developed with results supporting their use as safe and efficacious alternatives to permanent polymer DES2,3.
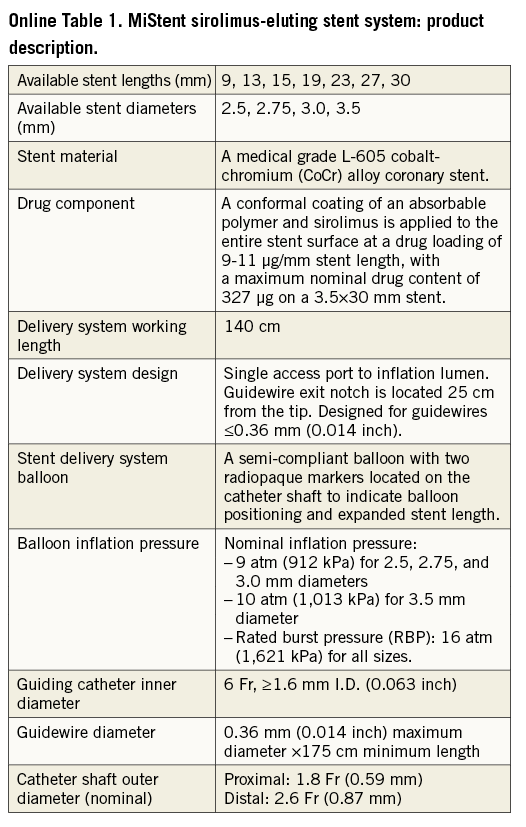
The MiStent® Sirolimus-Eluting Absorbable Polymer Coronary Stent System (MiStent SES) (Micell Technologies, Durham, NC, USA) combines sirolimus, polylactide-co-glycolic acid (PLGA) and a cobalt-chromium stent platform (Online Table 1). PLGA carries a crystalline form of sirolimus. The PLGA/sirolimus combination is cleared from the stent within 45-60 days and PLGA is fully absorbed within 90 days. The crystalline sirolimus remains in the tissue and continues to elute the drug into the surrounding tissue for up to nine months, a unique feature among DES4,5.
Methods
STUDY DESIGNS
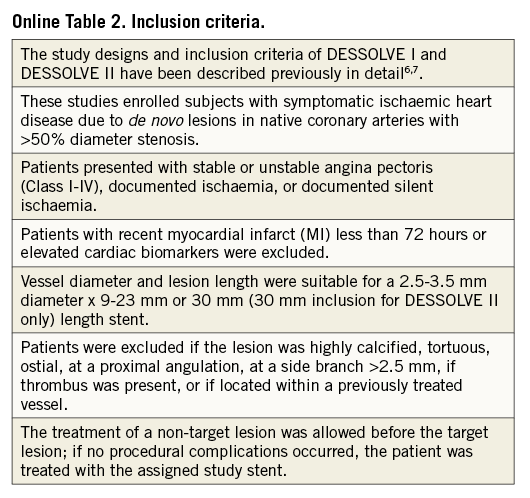
The MiStent results are derived from the DESSOLVE I first-in-human, single-arm trial, and from the DESSOLVE II 2:1 randomised trial comparing MiStent to Endeavor® Sprint DES (Medtronic, Santa Rosa, CA, USA)6,7. These studies enrolled subjects with symptomatic ischaemic heart disease due to de novo lesions in native coronary arteries with >50% diameter stenosis (Online Table 2 for inclusion criteria).
The DESSOLVE I first-in-human trial provided an initial assessment of safety and efficacy and investigated early and long-term neointimal hyperplasia (NIH) and vessel healing (n=30)6. The DESSOLVE II trial showed superiority (p<0.001) for in-stent late lumen loss (LLL) at nine months for the MiStent (0.27±0.46 mm, n=123) when compared to the Endeavor (0.58±0.41 mm, n=61)7.
Patients in both trials were contacted for clinical follow-up and reporting of adverse events at one and two years post index procedure. The minimum and maximum duration of dual antiplatelet therapy (DAPT) was per the country and/or study hospital standard-of-care recommendations.
CLINICAL ENDPOINT DEFINITIONS
Clinical endpoint definitions were identical in both DESSOLVE I and DESSOLVE II6,7. MACE was a composite of any death, myocardial infarction (MI) (Q-wave or non-Q-wave) or clinically driven target vessel revascularisation (TVR). Target lesion failure (TLF) was defined as cardiac death, MI, or clinically driven target lesion revascularisation (TLR). Target vessel failure (TVF) was defined as cardiac death, MI, or TVR.
STUDY CONDUCT
All patients provided written informed consent in accordance with the local site’s ethics committee. Participating sites and the study organisation are shown in Online Table 3 and Online Table 4. SAS, version 9.1.3 SP2 (SAS Institute, Cary, NC, USA) was used for statistical analysis.
Results
In DESSOLVE I, MACE was 0.0% (0/30) at 30 days, and 3.4% (1/29) at two years. The single event was a non-target vessel non-Q-wave MI. No patients experienced death, TVR, TLR, TVF, TLF or ST over two years. The median duration of DAPT in DESSOLVE I was 364 days (interquartile range: 328-542 days).
In DESSOLVE II, follow-up at two years was available for 97.6% in the MiStent group and 98.4% in the Endeavor group. The overall MACE rate up to two years (Figure 1) was 6.7% (8/120) for MiStent and 13.3% (8/60) for Endeavor (p=0.167). Death occurred in 2.5% of the MiStent patients and 1.7% of the Endeavor patients, with all deaths occurring later than 30 days post implantation (Table 1). The largest difference between the two stents (p=0.042) was TVR, at 1.7% with MiStent and 8.3% with Endeavor, with identical TLR at 1.7%. Probable/definite ST occurred only with the Endeavor (1.7%; 1/60). The median duration of DAPT in DESSOLVE II was 366 days (interquartile range: 341-565 days), without difference between MiStent and Endeavor (median 366 days for both arms).
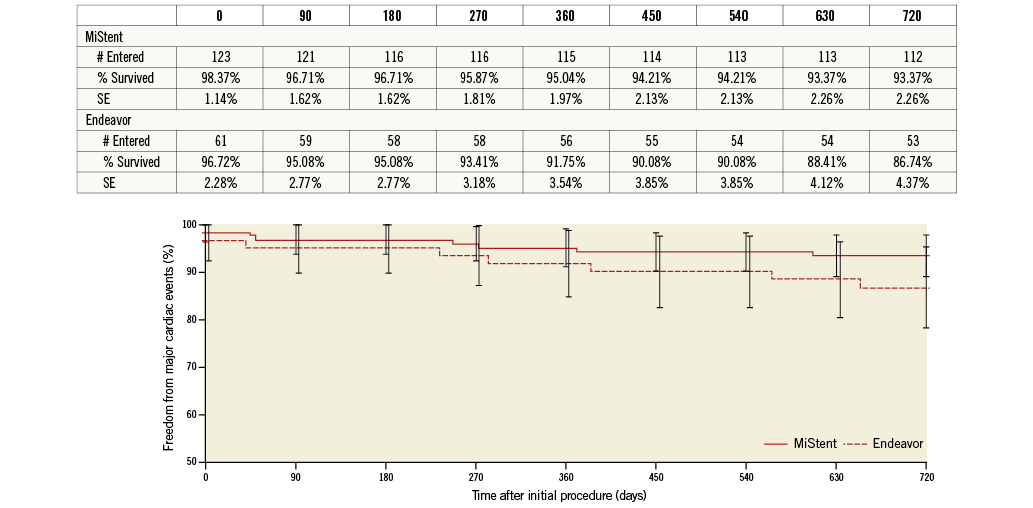
Figure 1. Survival curve - major adverse cardiac events (MACE) at two years.
Discussion
First-generation DES with permanent polymers have been associated with adverse long-term consequences8. This led to the development of second-generation DES with more biocompatible, durable coatings9,10, and third-generation DES with fully absorbable polymer coatings4.
Determination of DES efficacy is evaluated by measuring LLL, in-stent LLL ≤0.5 mm resulting in TLR <5%11. Paired angiographic results from DESSOLVE I and II revealed a plateau of NIH at 0.09 mm from six/eight to 18 months6,7. No TVR or TLR have been reported in DESSOLVE I. Low TLR rates (1.7%) have been maintained in DESSOLVE II up to two years.
Long-term DES safety requires tissue coverage of the stent struts, rapid endothelialisation and sufficient healing. Optical coherence tomography in DESSOLVE II revealed high mean rates of stent coverage (97.9%) at nine months7, and the endothelial function studies demonstrated preserved vasomotion both proximally and distally to the stent12.
Limitations
Similar to other DES trials, investigators could not be blinded to study device, and the study size does not permit comparative analysis of safety outcomes such as ST. Longer-term follow-up of the DESSOLVE I and II, in addition to larger trials and real-world experience including more complex patient populations, will provide additional insights into the performance of the MiStent SES.
Conclusion
DESSOLVE I and II have revealed a good safety profile for the MiStent SES, with limited MACE events and no ST reported up to two years. These trials were not powered to compare clinical outcomes between DES; further studies in larger populations are ongoing (DESSOLVE III).
| Impact on daily practice The sirolimus-eluting MiStent is designed for drug delivery for up to nine months while the bioresorbable polymer undergoes rapid dissolution. Clinical follow-up at two years in 152 patients shows good medium-term safety and effectiveness with low event rates, supporting clinical implementation. On the basis of these results, further evaluation in complex patient and lesion subsets is ongoing in the all-comers DESSOLVE III randomised trial. |
Funding
This trial is sponsored by Micell Technologies, Durham, NC, USA.
Conflict of interest statement
W. Wijns reports institutional grants from several device companies, including Micell Technologies and Medtronic; he is co-founder, shareholder and non-executive board member of Argonauts Partners, Cardio3 BioSciences and Genae. D. Donohoe is a consultant and C. Knape is an employee of Micell Technologies. The other authors have no conflicts of interest to declare.
Supplementary data
Online Figure 1. Major adverse cardiac events (MACE) at two years.