
Abstract
Aims: The aim of this study was to assess whether the nine months of cytostatic inhibition by crystalline sirolimus has a beneficial effect in the two-year follow-up in an all-comer population undergoing percutaneous coronary intervention.
Methods and results: The DESSOLVE III study (n=1,398) is a prospective, all-comer, multicentre, randomised controlled study (NCT02385279) allocating 703 patients to receive the MiStent drug-eluting stent with a fully absorbable polymer coating containing and embedding a microcrystalline form of sirolimus into the vessel wall, and 695 patients to receive the XIENCE durable polymer everolimus-eluting stent. At two years, the device-oriented composite endpoint (cardiac death, target vessel myocardial infarction [TV-MI], and clinically indicated target lesion revascularisation [TLR]) occurred in 8.7% and 8.6% (p=0.958) of patients, and the patient-oriented composite endpoint (all deaths, all MI, and all revascularisations) was observed in 18.5% and 19.6% (p=0.598) of patients in the MiStent and XIENCE arms, respectively. The frequency of TV-MI and clinically indicated TLR was also comparable for both stent types. The rate of definite/probable stent thrombosis was not different in the two arms (0.9% vs 1.3%, p=0.435).
Conclusions: In an all-comer population, at two-year follow-up, the use of the MiStent sirolimus-eluting bioabsorbable polymer-coated stent was at least as safe and efficacious as the XIENCE durable polymer stent. The MiStent’s potential long-term clinical benefit will be further elucidated after five years of follow-up.
Introduction
Permanent polymers on drug-eluting stents (DES) have been linked to late stent thrombosis (ST) and DES failure due to inciting a late inflammatory response1. Bioabsorbable polymer-based DES were developed to address this limitation. Results have supported their use as safe and efficacious alternatives to permanent polymer DES2.
The MiStent® sirolimus-eluting absorbable polymer coronary stent system (MiStent SES; Micell Technologies, Durham, NC, USA) combines crystalline sirolimus, polylactide-co-glycolic acid (PLGA) biodegradable polymer and a cobalt-chromium stent platform3,4. The controlled release system of crystalline sirolimus provides the benefit of protection from an initial burst release followed by rapid drug degradation and allows a targeted delivery of the active ingredient into the surrounding tissue with continuous and predictable drug elution throughout the affected artery following stent implantation up to nine months. The theoretical advantage of MiStent is the longer inhibition of neointimal hyperplasia due to the sustained presence of microcrystalline sirolimus, and thinner struts which promote earlier neointimal coverage.
Previously, the DESSOLVE III all-comer randomised trial showed non-inferiority of the MiStent SES as compared to the XIENCE everolimus-eluting stent (EES: Abbott Vascular, Santa Clara, CA, USA) in terms of the device-oriented composite endpoint (DOCE) at 12 months5. Considering the fact that the polymer in the MiStent SES disappears at three months while the drug is eluted up to nine months post implantation, longer-term follow-up is needed to confirm the benefit of the early disappearance of the absorbable polymer and late elution of the drug. So far, in the DESSOLVE I single-arm trial (n=30), no patients receiving the MiStent had target lesion failure (TLF) up to five years6. In DESSOLVE II (n=184), TLF was not significantly different between the MiStent group and the Endeavor® (Medtronic, Minneapolis, MN, USA) group at five years (9.2% vs 8.5%)6. However, it is unclear whether this is still the case in an all-comer population at midterm follow-up as there is a potential concern that the longer duration of low-level drug might be associated with a rebound of neointimal hyperplasia once the upregulation of p27 has subsided7. Therefore, we aim to present the two-year evaluation after implantation of the MiStent sirolimus-eluting bioabsorbable polymer-coated stent as compared to the XIENCE durable polymer everolimus-eluting stent in the DESSOLVE III all-comer trial.
Methods
STUDY DESIGN AND POPULATION
The trial design and methods, as well as the study population, have been described in the previous report5. In brief, the DESSOLVE III all-comer trial is a prospective, randomised, 1:1 ratio, controlled, single-blind, multicentre study (NCT02385279). Twenty sites in Europe participated in the study and the enrolment period was from March to December 2015. There were minimal inclusion/exclusion criteria. The primary endpoint of the study was a non-inferiority comparison at 12 months of a DOCE (commonly described as TLF, a composite of cardiac death [including death of unknown cause or no information on cause of death], target vessel myocardial infarction [TV-MI]), and clinically indicated target lesion revascularisation [TLR]) of the MiStent SES group as compared to the XIENCE EES group. All patients provided written informed consent to participate in the study, which was approved by the ethics committee of each enrolling site. The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practices (GCP).
Two-year follow-up was planned in the trial protocol. The predefined two-year endpoints are listed in Supplementary Table 1. Event adjudication by a clinical events committee (CEC) and additional quantitative coronary angiography (QCA) were performed by Cardialysis (Rotterdam, the Netherlands). For all revascularisations and stent thrombosis events, the angiogram was sent to the angiographic core laboratory, regardless of whether target or non-target vessel (non-TV) revascularisation was performed.
DEVICE DESCRIPTION
MISTENT SES
The metallic platform of the MiStent SES is a cobalt-chromium alloy with a thin 64 µm strut thickness. The stent coating (approximately 5 μm thick on the luminal and 15 μm thick on the abluminal stent surfaces)4 consists of PLGA loaded with microcrystalline particles of sirolimus. It has been shown that the polymer is fully biodegraded and resorbed within three months post implantation while the drug remains in the tissue surrounding the stent for up to nine months in order to continue to control the growth of neointimal tissue3.
XIENCE EES
The control XIENCE EES is a cobalt-chromium alloy device with a strut thickness of 81 µm and a 7.8 µm thick durable polymer coating. The durable polymer is made of polyvinylidene fluoride-hexafluoropropylene loaded with everolimus.
EXPLORATORY ANALYSES
In an attempt to explain the difference in non-TV revascularisation at two years, post hoc paired QCA analyses at baseline (post procedure) and before revascularisation (at the time of event) were performed in lesions with non-TV revascularisation. Quantitative flow ratio (QFR) was further analysed in revascularised non-TV at baseline and before revascularisation whenever technically feasible (methods and details of the feasibility of QFR analysis are provided in Supplementary Appendix 1 and Supplementary Figure 1).
STATISTICAL ANALYSIS
Details of the statistical analysis are provided in Supplementary Appendix 2.
Results
STUDY SUBJECTS
A study flow chart is shown in Figure 1. Two-year follow-up information was available in 98.4% (692/703) of patients in the MiStent arm and in 99.1% (689/695) of patients in the XIENCE arm of the initial cohorts. Three patients withdrew consent in the MiStent arm and eight and six patients were lost to follow-up in the MiStent and the XIENCE arms, respectively.
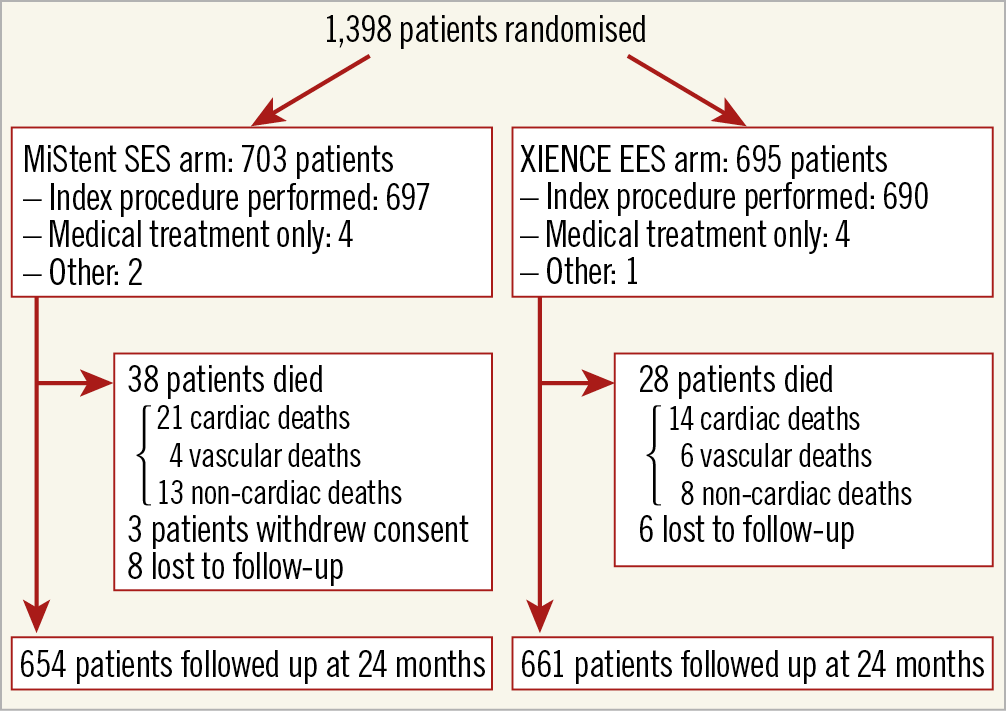
Figure 1. Study flow chart. EES: everolimus-eluting stent; SES: sirolimus-eluting stent
TWO-YEAR CLINICAL ENDPOINTS
Comparisons of clinical endpoints as well as the individual components of the composite endpoints are presented in Table 1.
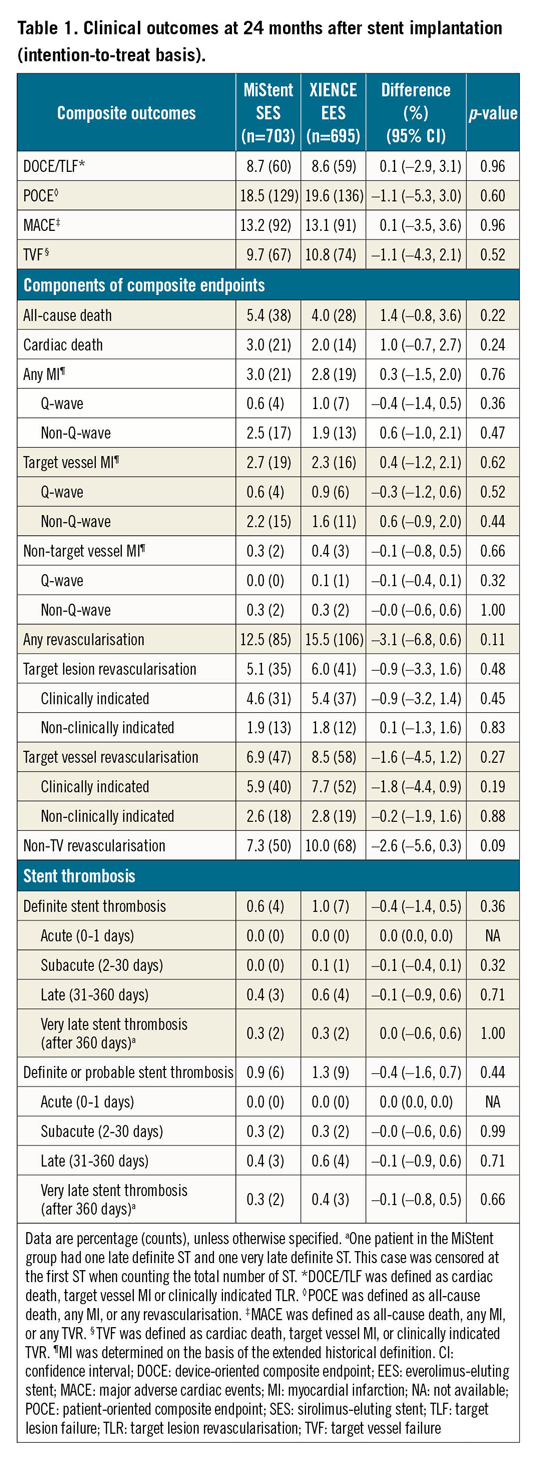
At two-year follow-up, DOCE occurred in 60 (8.7%) patients treated with MiStent and 59 (8.6%) patients treated with XIENCE (difference 0.1%, 95% confidence interval [CI]: –2.9 to 3.1, p=0.958) (Figure 2A). Frequencies of all death (38 [5.4%] vs 28 [4.0%], difference 1.4%, 95% CI: –0.8 to 3.6, p=0.220), cardiac death (21 [3.0%] vs 14 [2.0%], difference 1.0%, 95% CI: –0.7 to 2.7, p=0.239), TV-MI (19 [2.7%] vs 16 [2.3%], difference 0.4%, 95% CI: –1.2 to 2.1, p=0.621), and clinically indicated TLR (31 [4.6%] vs 37 [5.4%], difference –0.9%, 95% CI: –3.2 to 1.4, p=0.447) were not significantly different for both stent types (Figure 2B-Figure 2D).
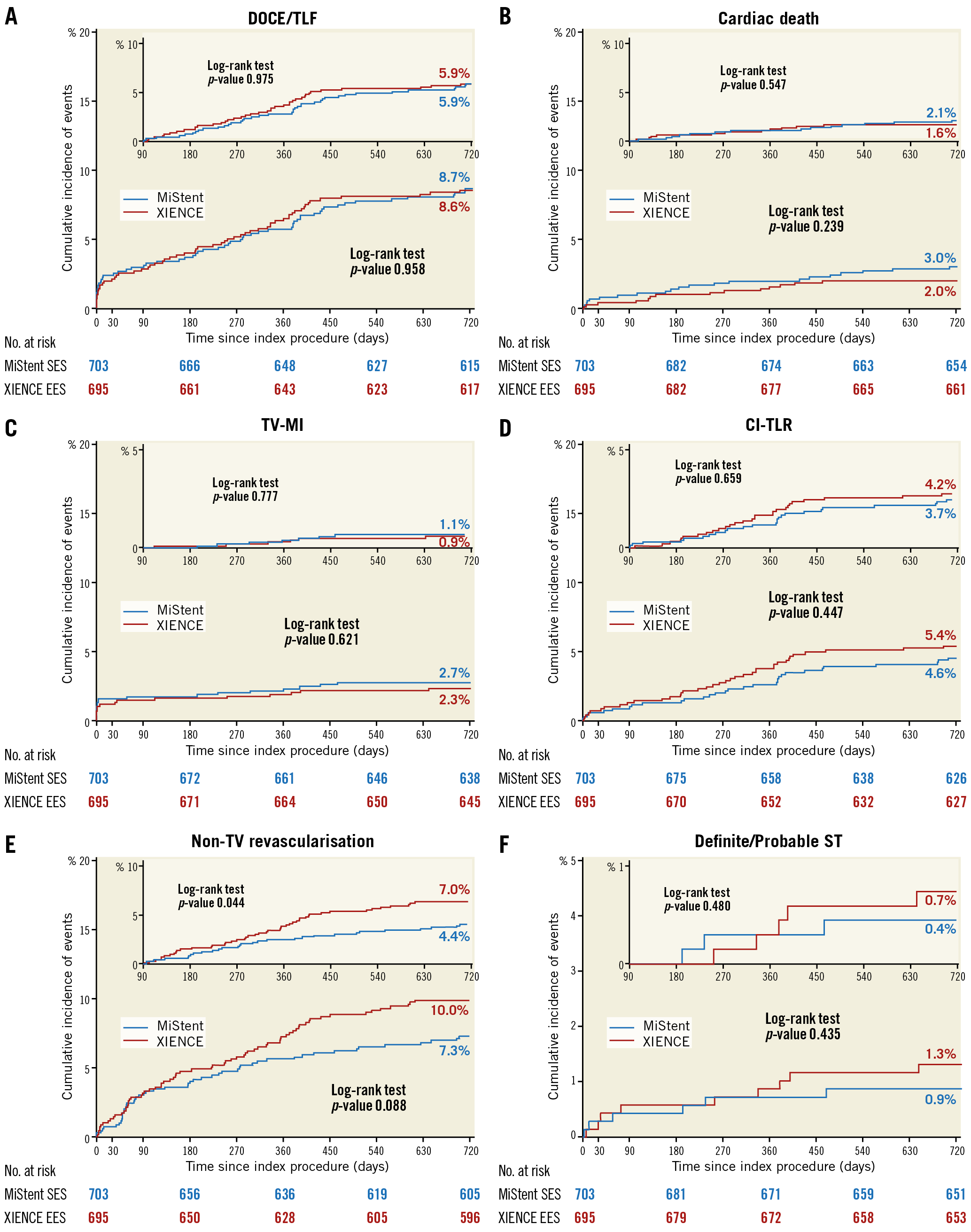
Figure 2. Kaplan-Meier estimates for the device-oriented composite endpoint (DOCE), its components, non-target vessel revascularisation, and stent thrombosis over 720 days of follow-up. Kaplan-Meier curves show the cumulative incidence of DOCE - a composite of cardiac death, target vessel myocardial infarction (TV-MI), or clinically indicated target lesion revascularisation (CI-TLR) (A); cardiac death (B); TV-MI (C); CI-TLR (D); non-target vessel revascularisation (non-TV revascularisation) (E); and definite/probable stent thrombosis (ST) (F).
The patient-oriented composite endpoint (POCE: defined as all deaths, all MI and all revascularisations) was observed in 129 patients (18.5%) and 136 patients (19.6%), respectively, in the MiStent arm and the XIENCE arm (difference –1.1%, 95% CI: –5.3 to 3.0, p=0.598) (Figure 3). Any revascularisation at two years tended to be less frequent in the MiStent arm than in the XIENCE arm (85 [12.5%] vs 106 [15.5%], difference –3.1%, 95% CI: –6.8 to 0.6], p=0.106), largely driven by a difference in the rate of non-TV revascularisation (50 [7.3%] vs 68 [10.0%], difference –2.6%, 95% CI: –5.6 to 0.3, p=0.088) (Figure 2E).

Figure 3. Kaplan-Meier estimates for the patient-oriented composite endpoint (POCE) and its components over 720 days of follow-up. Kaplan-Meier curves show the cumulative incidence of POCE - a composite of all-cause death, any myocardial infarction (any MI), or any revascularisation (A); all-cause death (B); any MI (C); and any revascularisation (D).
At two years, the rate of definite/probable ST was not different in the MiStent and XIENCE arms (6 [0.9%] vs 9 [1.3%], difference –0.4%, 95% CI: –1.6 to 0.7, p=0.435) (Figure 2F).
LANDMARK ANALYSIS AT 90 DAYS
Landmark analyses after 90 days, the time point when the biodegradable polymer of the MiStent was supposed to be completely absorbed, are presented in the upper right corner of each panel of Figure 2. The rate of DOCE, as well as its components after 90 days, was not significantly different between the two arms. However, the rate of non-TV revascularisation was significantly lower in the MiStent arm after 90 days (29 [4.4%] vs 46 [7.0%], p=0.044) (Figure 2E).
SUBGROUP ANALYSIS
The treatment effect in DOCE was not different across the stratified analyses for diabetes, acute coronary syndrome (ACS), ST-elevation myocardial infarction (STEMI), gender, renal insufficiency, small vessel, long lesion, bifurcation, left main, bypass lesion, restenotic lesion, multivessel, and overlapping stent (Figure 4).

Figure 4. Stratified analyses of the device-oriented composite endpoint at 24 months across subgroups. Hazard ratio with 95% CI and p-value results were from Cox proportional hazards analysis. Renal insufficiency is defined as serum creatinine >2.5 mg/dL or creatinine clearance ≤30 mL/min. ACS: acute coronary syndrome; CI: confidence interval; STEMI: ST-segment elevation myocardial infarction
Discussion
The first report of the two-year follow-up of a randomised comparison between MiStent and XIENCE in an all-comer setting showed that the rate of DOCE at two years was not significantly different in the two groups and was low. The rate of very late stent thrombosis did not differ between groups and was low in both treatment arms. The efficacy and safety of the MiStent is further supported by a recent meta-analysis showing that newer-generation ultra-thin strut DES (60~65 µm), including the MiStent, were associated with a 16% reduction in TLF driven by less MI with lower rates of any stent thrombosis8.
In the present study, no significant differences were documented between the two treatment groups in the individual components of the primary endpoint as well as the secondary clinical endpoints, except for non-TV revascularisation after 90 days, the rate of which was lower in the MiStent arm than in the XIENCE arm.
CAUSE OF DEATH
All-cause death occurred in 5.4% (38) in the MiStent arm and 4.0% (28) in the XIENCE arm (HR 1.36, 95% CI: 0.83-2.21, p=0.22). This difference came mainly from the difference in cardiac death (3.0%21 vs 2.0%14, p=0.24), although the differences in both all-cause death and cardiac death were not statistically significant. Since cardiac death includes unexplained death, it would be difficult to elaborate further on the exact cause of the differences in cardiac death. The differences were less in vascular death (0.6%4 vs 0.9%6, in the MiStent and XIENCE arms, respectively [p=0.53]) and non-cardiovascular death (1.9%13 vs 1.2%8, in the MiStent and XIENCE arms, respectively [p=0.27]).
COMPARISON WITH OTHER STUDIES
So far, several randomised controlled trials comparing biodegradable polymer (BP)-DES and durable polymer (DP)-DES have been reported (LEADERS, BIOSCIENCE, EVOLVE II, CENTURY II)9,10,11,12. However, overall results do not suggest superiority of BP-DES.
The subgroup analysis in the present study showed that DOCE rates were not significantly different across the predefined patient subsets. In contrast, the BIOSCIENCE trial comparing Orsiro BP-SES (Biotronik, Berlin, Germany) and XIENCE DP-EES showed that the Orsiro BP-SES had a significantly lower rate of DOCE at two years in STEMI patients (5.4% vs 10.8%; risk ratio [RR]: 0.48, 95% CI: 0.23 to 0.99; p=0.043), with a significant interaction p-value compared with patients without STEMI (RR: 1.15, 95% CI: 0.86 to 1.53; p=0.86; p for interaction=0.026). Previously, two-year clinical outcomes were compared in a large population acute myocardial infarction (AMI) registry (n=3,559)13, including biolimus-eluting stents (the BioMatrix Flex™ stent; Biosensors, Morges, Switzerland, or the Nobori® stent; Terumo Corporation, Tokyo, Japan) as BP-DES. The two-year MACE rate was 10.7% and 9.9% (p=0.679) and the incidence of ST was 0.8% vs 0.9% (p=1.0) in the BP-DES group and second-generation DP-DES group, respectively. These results suggest at least similar efficacy of BP-DES and DP-DES in AMI patients.
The observed rate of the primary endpoint in XIENCE at two years (8.6%) was almost identical to the expected one-year rate of DOCE (8.0%) in a sample size calculation based on the XIENCE arm of the RESOLUTE All Comers trial, which was published in 2010. In the two-year report of the RESOLUTE All Comers trial14, the rate of DOCE was 10.7%, the rate of cardiac death was 2.2%, the rate of TV-MI was 4.5%, and the rate of ischaemia-driven TLR was 5.1% in the XIENCE arm. The difference in DOCE between the XIENCE arm in the DESSOLVE III trial and that in the RESOLUTE All Comers trial was mainly driven by the difference in TV-MI. Although both trials were based on the World Health Organization (WHO) (extended) definition of MI, periprocedural MI occurred in 1.2% of patients in the XIENCE arm in DESSOLVE III and 3.1% of patients in the XIENCE arm in the RESOLUTE All Comers trial15. In DESSOLVE III, cardiac enzyme sampling after the index procedure was available in 92.3%, 94.2%, and 93.1% in CK-MB, troponin, and CK, respectively5, whereas 92.5% had an analysable data set for either biomarker in RESOLUTE All Comers. Thus, potential underreporting of periprocedural MI should be excluded. Regarding difference in baseline characteristics, the XIENCE arm of DESSOLVE III had more patients presenting with stable angina (41%) than that of the RESOLUTE All Comers trial (36%). Notably, there are several differences in the protocols of the two trials: 1) only XIENCE V was used in the RESOLUTE All Comers trial, whereas XIENCE V®, XIENCE PRIME®, or XIENCE Xpedition® (all Abbott Vascular) was allowed in the DESSOLVE III trial (Supplementary Table 2); 2) only clopidogrel was used as P2Y12 inhibitor in the RESOLUTE All Comers trial, while ticagrelor and prasugrel were also available and preferred in case of ACS in the DESSOLVE III trial. In addition, as there has been accumulating evidence showing benefit of a physiology approach to define the appropriateness of revascularisation based on the presence of ischaemia16,17, as compared to the era when the RESOLUTE All Comers trial was conducted, frequent use of physiological assessment might have contributed to better outcome in the DESSOLVE III trial, although none of the information regarding physiology assessment was captured in the electronic case report form (eCRF) of the trial. Although it is not to be expected that all consecutive patients will be included in an all-comer trial18, it is important to note that we were only able to enrol 1,398 (16.6%) patients out of 8,423 patients treated with PCI during the study period in the enrolling sites5, whereas the Thoraxcenter in the Erasmus MC (Rotterdam, the Netherlands) reported that 579 patients (48%) were actually included out of 1,242 consecutive PCI patients treated during the inclusion period of the LEADERS and RESOLUTE All Comers trials18. This raises the question as to whether we enrolled a similar population to other all-comer trials, which may explain the observed low DOCE and TV-MI rates.
EXPLORATORY QFR ANALYSES AND INSIGHTS INTO OBSERVED DIFFERENCE IN NON-TV REVASCULARISATION
(Supplementary Appendix 3, Supplementary Table 3, Supplementary Figure 2-Supplementary Figure 4).
Limitations
Further long-term follow-up data are necessary to demonstrate the implications of biodegradable polymer as compared to durable polymer. The omission of events occurring earlier than the landmark was among the recognised limitations of landmark analysis. The study did not have adequate power to undertake a statistical comparison for any itemised endpoints due to the relatively small sample size. Indication (i.e., clinically driven or non-clinically driven) of non-TV revascularisation was not adjudicated by the CEC. The post hoc hypothesis-generating QFR analysis in non-TV had low feasibility due to the absence of a pre-specified angiographic acquisition protocol.
Conclusions
In an all-comer population, use of the MiStent sirolimus-eluting bioabsorbable polymer-coated stent was related to sustained efficacy and safety at two-year follow-up, as compared to the XIENCE durable polymer stent. The MiStent’s potential long-term clinical benefit will be further elucidated up to a total of five years of follow-up.
|
Impact on daily practice In an all-comer population, use of the MiStent sirolimus-eluting bioabsorbable polymer-coated stent was related to sustained efficacy and safety at two-year follow-up, as compared to the XIENCE everolimus-eluting durable polymer stent. This study adds a promising option of a drug-eluting stent to the armamentarium to be used in our daily practice. It remains to be elucidated whether the MiStent has a beneficial effect at the planned medium-term follow-up up to five years. |
Appendix. Study collaborators
Gillian A.J. Jessurun, MD, PhD; Department of Cardiology, Treant Zorggroep, Emmen, the Netherlands. Karel T. Koch, MD, PhD; Amsterdam University Medical Centre, Amsterdam, the Netherlands. Roland P.T. Troquay, MD; VieCuri Medical Centre for Northern Limburg, Venlo, the Netherlands. Bas J.B. Hamer, MD; Meander Medisch Centrum, Amersfoort, the Netherlands. Ton Oude Ophuis, MD, PhD; Department of Cardiology, Canisius Wilhelmina Ziekenhuis, Nijmegen, the Netherlands. Krzysztof P. Milewski, MD, PhD; Oddzial Kardiologii Inwazyjnej, Elektrofizjologii i Elektrostymulacji PAKS, American Heart of Poland S.A., Tychy, Poland. Sjoerd H. Hofma, MD, PhD; Medisch Centrum Leeuwarden, Leeuwarden, the Netherlands.
Guest Editor
This paper was guest edited by Alec Vahanian, MD, PhD; Department of Cardiology, Hôpital Bichat-Claude Bernard, and University Paris VII, Paris, France.
Funding
The trial was sponsored by the European Cardiovascular Research Institute (ECRI) and supported with unrestricted grants from Micell Technologies, Durham, NC, USA, and Stentys, Paris, France.
Conflict of interest statement
Y. Onuma, J.J. Piek, and P.W. Serruys have served as members of the advisory board of Abbott Vascular. S.H. Hofma declares an unrestricted research grant to the institution by Abbott. W. Wijns has received speaker fees from Abbott Vascular, Biotronik, MicroPort, and Terumo; he is a scientific advisor for Rede Optimus Research and a co-founder of Argonauts, an innovation accelerator. The other authors have no conflicts of interest to declare. The Guest Editor is a consultant for Edwards Lifesciences.

