Abstract
Aims: The aim of the present study was to evaluate the five-year safety and efficacy of a biodegradable polymer-coated sirolimus-eluting stent with six months dual antiplatelet therapy in daily practice.
Methods and results: Two thousand and seventy-seven daily practice patients, exclusively treated with biodegradable polymer-coated sirolimus-eluting stents (EXCEL; JW Medical Systems, Weihai, China), were prospectively enrolled in the multicentre CREATE study. Clinical follow-up was completed in 1,982 patients (95.4%) at five-year follow-up. The rates of cardiac death, non-fatal myocardial infarction (MI), target lesion revascularisation and overall major adverse cardiac events (MACE) at five-year follow-up were 3.0%, 1.5%, 3.7% and 7.4%, respectively. The rates of definite or probable stent thrombosis (ST) at five years and definite ST from one to five years were 1.1% and 0.3%, respectively. Heart failure (hazard ratio [HR]: 3.324, 95% confidence interval [CI]: 1.729-6.391, p<0.001) and prior MI (HR: 2.664, 95% CI: 1.358-5.227, p=0.004) were independent predictors of ST. Landmark analysis of a propensity score matched patient cohort showed that patients with or without clopidogrel treatment after six months had similar clinical outcomes.
Conclusions: The present study demonstrates satisfactory and sustained five-year clinical safety and efficacy profiles as evidenced by the low rates of MACE and ST for the EXCEL, a biodegradable polymer-based sirolimus-eluting stent, when patients were treated with six months dual antiplatelet therapy in daily practice.
Introduction
First-generation drug-eluting stents (DES) with durable polymers considerably reduced the risk of target lesion revascularisation (TLR) compared to bare metal stents (BMS)1,2. However, the delayed arterial healing associated with DES has led to an increase in the rates of late and very late stent thrombosis (ST) and rebound of restenosis, termed as late catch-up3. The aetiology behind the late catch-up phenomenon is multifactorial with the possibility that the durable polymer residue results in chronic arterial wall inflammation and hypersensitivity4,5. Therefore, there is increasing interest in designing a new generation of DES with a biodegradable polymer that may overcome these potential drawbacks of durable polymer DES.
The CREATE (Multi-Center Registry of EXCEL Biodegradable Polymer Drug-Eluting Stents) study, which examined the safety and efficacy of the biodegradable polymer-based sirolimus-eluting stent (EXCEL; JW Medical Systems, Weihai, China) with six months dual antiplatelet therapy (DAPT) in daily practice, has shown satisfactory low rates of major adverse cardiac events (MACE), TLR and ST up to three years6,7. The present study was aimed at determining whether the safety and efficacy of the EXCEL stent is maintained at five years, which is the longest available follow-up of biodegradable polymer stent technology.
Methods
STUDY OVERVIEW
CREATE is a post-marketing surveillance multicentre, prospective study which enrolled 2,077 daily practice patients at 59 medical centres from four countries6. All lesions were exclusively treated with the EXCEL stents. A six-month DAPT regimen with aspirin and clopidogrel was recommended. The indications for stenting were left to the operators’ discretion. Patients with device or procedural failure, who received ≥1 stent other than the protocol stent, or had contraindications for DAPT, heart function worse than New York Heart Association functional Class III or a planned upcoming surgery were excluded. Ethics committees in all participating centres approved the study protocol, and a signed informed consent was obtained from every enrolled patient. The study was registered in the National Institutes of Health website as identifier NCT00331578. The change of study protocol with prolonged clinical follow-up up to five years was approved by the ethics committees in Shenyang Northern Hospital.
DATA COLLECTION
Patients had clinical evaluations at 30 days, every six months within two years and every 12 months thereafter up to five years. All data were collected on case report forms and submitted to a data co-ordination centre (located at Shenyang Northern Hospital). The consistency and accuracy of the data, including baseline, in-hospital, and follow-up outcomes, were audited by an independent contract research organisation (China Cardiovascular Research Foundation, CCRF, Beijing, China). An audit check was performed at five years which included 53% of all enrolled patients (including all patients lost to follow-up). All adverse clinical events were reviewed and adjudicated by an independent clinical events committee.
OUTCOMES AND DEFINITIONS
The safety and efficacy outcomes of the CREATE study were MACE, cumulative TLR and thrombotic event rates at five years after the index procedure. MACE was defined as a composite of cardiac death, non-fatal myocardial infarction (MI), and TLR. All deaths were considered to be cardiac unless a non-cardiac origin could be clearly established by clinical and/or pathological study. The diagnosis of MI was based on either the development of new pathological Q-waves in ≥2 contiguous electrocardiogram leads and/or level elevation of creatine kinase myocardial band isoenzyme >3 times the upper normal limit after the procedure during index hospitalisation, or cardiac enzyme level elevation >2 times the upper normal limit thereafter. TLR was defined as any repeat intervention inside the stent that had been implanted during the index procedure or within the 5 mm proximal or distal segments to the stent. ST was classified as definite, probable, and possible according to the definitions proposed by the Academic Research Consortium8. It was stratified as acute (≤24 h), subacute (24 h to 30 days), late (30 days to one year), and very late ( ≥1 year).
STATISTICAL ANALYSIS
Comparisons between continuous variable data, expressed as mean ± standard deviation, were performed with the t-test, while the chi-square or the Fisher’s exact test was used for categorical data, expressed as percentages. The last observation carried forward (LOCF) method of missing observations was the prospectively defined statistical method. Event-free incidence or cumulative incidence of events was calculated according to the Kaplan-Meier method. Survival curves were constructed with Kaplan-Meier estimates and compared by log-rank tests. A “landmark survival analysis” with a landmark set at six months was performed to determine the impact of six-month clopidogrel treatment on late ST and MACE. Multivariate predictor analyses were performed with a Cox proportional hazards model. All available variables considered potentially relevant were included: age, gender, hypertension, diabetes mellitus, hyperlipidaemia, heart failure, prior MI, family history, acute MI, multivessel disease, prior cerebral and peripheral vascular disease, multi-stent implantation and duration of DAPT. Results are presented as adjusted hazard ratio (HR) with 95% confidence interval (CI). To compensate for the non-randomised design of the present study, propensity score matching, based on a custom dialogue in SPSS with R plug-in9, was performed to screen cases with comparable baseline characteristics. Statistical analyses were performed with the SPSS 20.0 software (SPSS, Inc., Chicago, IL, USA). A two-sided p-value of <0.05 was considered statistically significant.
Results
BASELINE CHARACTERISTICS
The baseline clinical and procedural characteristics were previously reported6. In brief, the mean age of the cohort was 60.6±11.1 years, 73.6% were men, and 21.2% were diabetes mellitus cases. The majority of the patients (89.8%) were admitted with acute coronary syndromes, including 386 acute MI (18.6%). A total of 3,080 target lesions were treated, including 26 (0.8%) unprotected left mains, 667 (21.7%) bifurcations, 76 (2.5%) chronic total occlusions, 69 (2.2%) in-stent restenoses, and 1,309 (42.5%) diffusely diseased lesions. Multistenting was performed in 26.9% of the patients and 84.5% of enrolled patients had at least one off-label indication for DES implantation. A total of 3,748 EXCEL stents were implanted at index procedure (1.8 stents per patient) with an average diameter and total stent length of 3.05±0.44 mm and 26.7±13.0 mm, respectively. The average duration of clopidogrel treatment was 199.8±52.7 days and 80.5% of discharged patients discontinued clopidogrel at six months.
CLINICAL EVENTS
Clinical follow-up was completed in 1,982 (95.4%) patients at five years. There were 127 (6.1%) patients who died during the follow-up period, including 63 (3.0%) cardiac deaths and 64 (3.1%) non-cardiac deaths. MACE occurred in 154 (7.4%) patients, consisting of 63 (3.0%) cardiac deaths, 31 (1.5%) non-fatal MIs and 76 (3.7%) TLRs (Table 1). The five-year MACE-free survival curve is depicted in Figure 1.
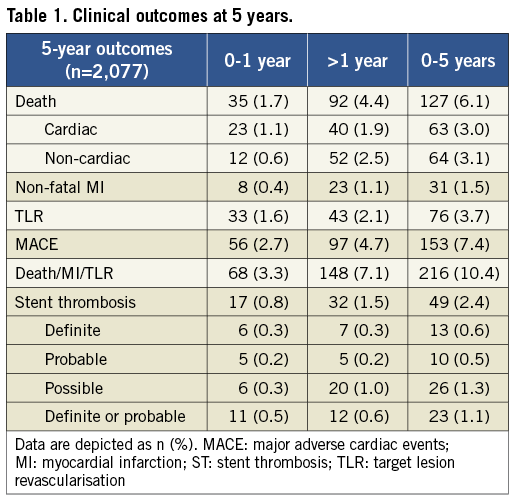
Figure 1. Kaplan-Meier curve of cumulative MACE-free survival over 5 years.
Stent thrombotic events developed in 49 (2.4%) patients up to five years, consisting of 2 (0.1%) acute, 8 (0.4%) subacute, 7 (0.3%) late and 32 (1.5%) very late ST. Among these, 23 (1.1%) were identified as ARC definite or probable ST, including 11 (0.5%) which developed within the first year and 12 (0.6%) which developed between one to five years (Table 1).
PREDICTORS OF ADVERSE CLINICAL EVENTS
Multivariate analyses showed that older age, diabetes mellitus, heart failure, prior MI, and acute MI were independent predictors of all-cause death. Older age, male gender, diabetes mellitus, heart failure, prior MI, and acute MI were independent predictors of MACE. Heart failure and prior MI were independent predictors of ST. Details are demonstrated in Figure 2.
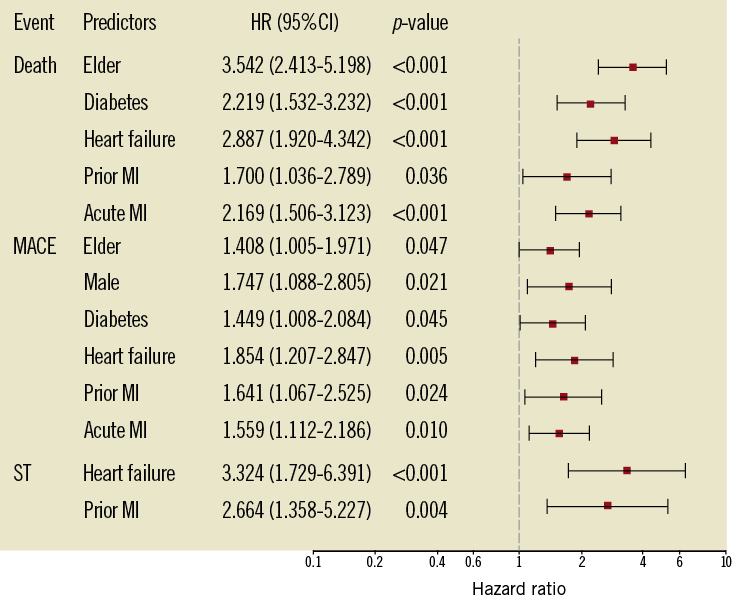
Figure 2. Multivariate predictors of adverse clinical events at 5-year follow-up. CI: confidence interval; HR: hazard ratio; MACE: major adverse cardiac events; ST: stent thrombosis
SAFETY OF SIX-MONTH CLOPIDOGREL THERAPY
A total of 2,034 patients (97.9%) survived free from MACE at six months. Among these patients, 1,626 (79.9%) discontinued clopidogrel treatment within six months, and the other 408 (20.1%) prolonged their duration of clopidogrel treatment up to 12 months. The clinical baseline and percutaneous coronary intervention (PCI) results of the two patient cohorts are listed in Table 2. Patients treated with clopidogrel after six months were older than those who discontinued clopidogrel treatment at six months (61.7±10.9 years vs. 60.3±11.1 years, p=0.03) and had a tendency of a higher rate of prior MI (14.0% vs. 10.6%, p=0.057). Landmark analysis showed that the incidences of MACE and ST from six months to five years were similar between the patients with or without clopidogrel treatment after six months except that patients with prolonged DAPT had a higher risk of cardiac death compared with their counterparts (3.9% vs. 1.8%, p=0.012). After propensity score matching, the baseline characteristics were well matched and no significant differences in clinical outcomes were observed between the two groups (Table 2 and Table 3). Kaplan-Meier curves showed that prolonged clopidogrel therapy (>6 months) did not produce beneficial effects in terms of reducing the cumulative hazards of MACE or ST between six months and five years (Figure 3).
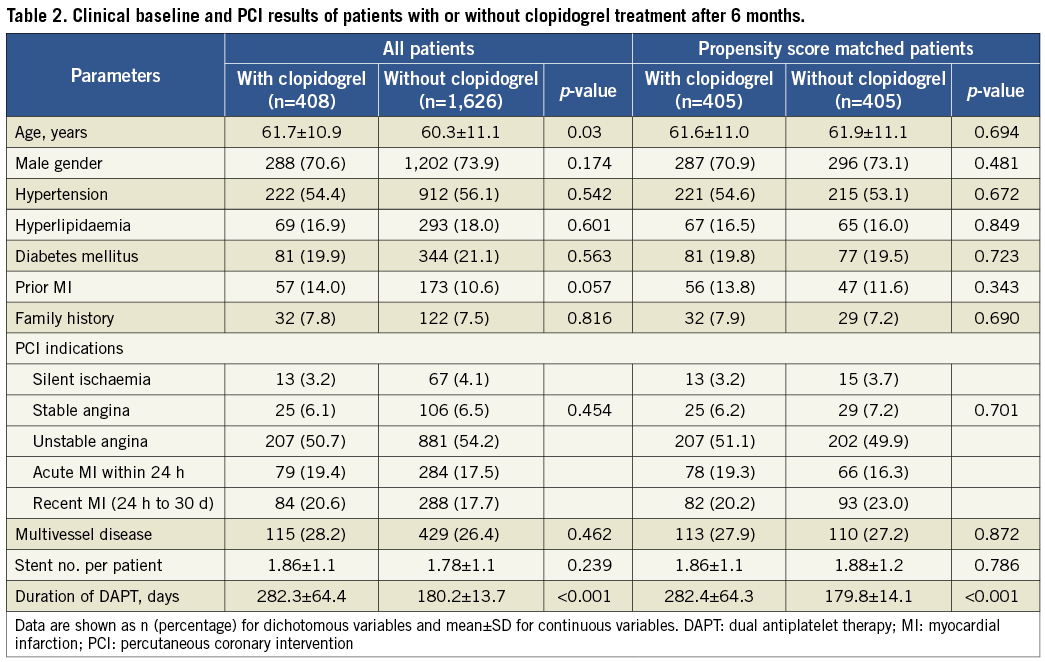
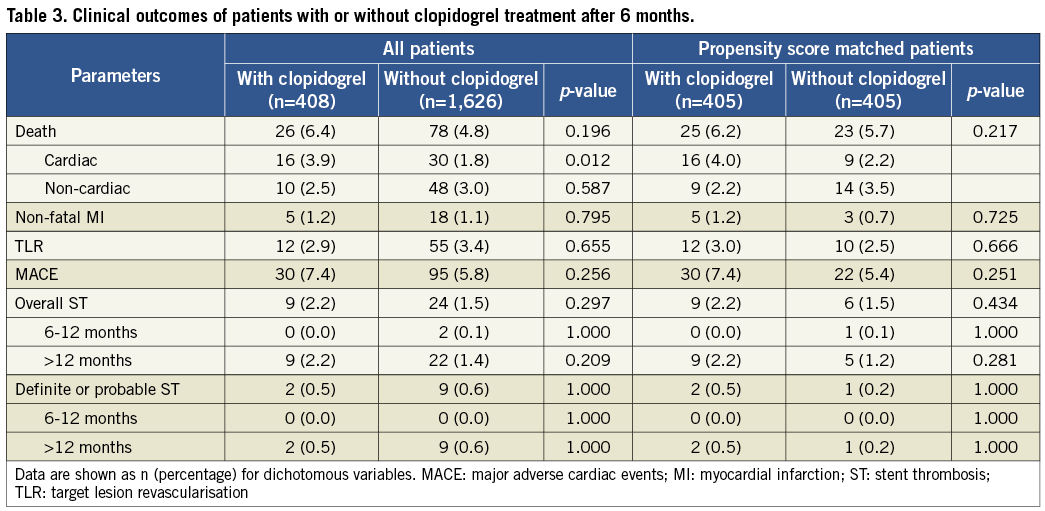
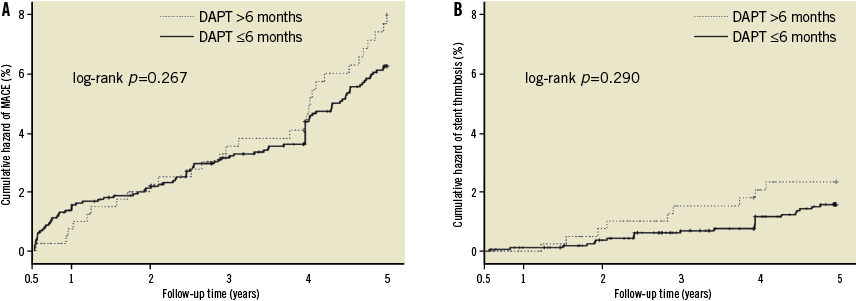
Figure 3. Landmark analysis of the impact of prolonged clopidogrel treatment (>6 months) on cumulative hazards of MACE (A) or stent thrombosis (B) between 6 months and 5 years. DAPT: dual antiplatelet therapy; MACE: major adverse cardiac events.
COMPARISON WITH OTHER DURABLE POLYMER DES
The five-year clinical outcomes of the CREATE study were compared with those from pivotal studies of durable polymer DES10-12. As illustrated in Figure 3, the overall rates of MACE (composite of all-cause death, MI and TLR) and TLR of the CREATE study were numerically lower compared with other DES (Figure 4).
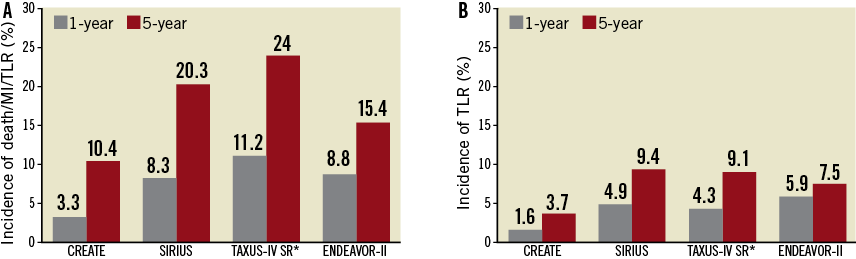
Figure 4. Comparison of clinical outcomes between the EXCEL stent and other drug-eluting stents with durable polymer. A) incidence of composite endpoints of death, MI, and TLR at 1 year (grey) and 5 years (red). B) incidence of TLR at 1 year (grey) and 5 years (red). *Kaplan-Meier estimated death/MI/TVR. MI: myocardial infarction; TLR: target lesion revascularisation; TVR: target vessel revascularisation
Discussion
The CREATE study, which included 2,077 patients with 3,080 lesions after implantation of 3,748 new-generation biodegradable polymer drug-eluting stents, demonstrated satisfactory and sustained five-year clinical safety and efficacy profiles as evidenced by the low rates of MACE and ST with six months DAPT in daily interventional practice in China and some East Asia countries.
The development of DES is a milestone in the history of PCI. Randomised studies have demonstrated that DES are effective in reducing the rates of TLR and MACE compared with BMSs. However, the benefits are attenuated after three years or later, especially with first-generation DES in complex lesion subsets, which has been termed as the late catch-up phenomenon13. A study with serial angiographic follow-ups reported that the DES with durable polymer was associated with a significantly increased rate of late lumen loss at two years versus that at six to eight months, but the phenomenon was not observed in polymer-free DES14. Those results indicated that durable polymers may play an important role in late catch-up after DES implantation15,16. Previous studies have shown that the remnants of polymers may cause a series of chronic inflammatory responses17,18 such as delayed arterial healing, the persistence of fibrin, platelet deposition, flow dynamics alteration, local thrombus, or even vessel remodelling, which may lead to poor long-term clinical outcomes, especially in complex cases and high-risk patients19,20. Biodegradable polymer, which would clear itself out after six to 12 months, may have a potential to reduce the persistence of durable polymers related to long-term adverse events21,22. The randomised LEADERS studies showed that biodegradable polymer stents reduced the risk of cardiac events associated with very late ST, which might improve long-term clinical outcomes compared with durable polymer sirolimus-eluting stents23,24.
In the present study, although the patient demographics and lesion characteristics were more complex than those of most randomised pivotal DES studies, the mid-term angiographic follow-up results showed that the EXCEL stent had similar anti-restenosis efficacy, with 0.21 mm in-stent late lumen loss and an in-segment binary restenosis rate of 6.7%, compared to the other DES6. At five-year follow-up, as pooled analyses reported, DES with durable polymer (CYPHER™; Cordis, Johnson & Johnson, Warren, NJ, USA; TAXUS®; Boston Scientific, Natick, MA, USA; and Endeavor® Medtronic, Inc., Minneapolis, MN, USA) are associated with significantly increased risks of TLR (7.5%-9.4%) and overall major adverse events (15.4%-24%) compared with early results (Figure 4)10-12. However, in the present study, the rate of TLR (3.7%) and overall major adverse events (death/MI/TLR, 10.4%) were still maintained at a low level at five-year follow-up. The above numeric differences in long-term clinical events between the present study and other DES studies imply there are potential benefits of biodegradable polymer-based DES in reducing late catch-up, which need further confirmation by randomised comparisons.
Publications including long-term follow-up of randomised trials and registries as well as some meta-analyses have indicated that first-generation DES might be associated with an increased risk of very late ST, an approximately 0.4% increment per year of definite ST25. Therefore, prolonged DAPT for at least one year after DES implantation was recommended to reduce the risk of late ST26. New-generation DES with bioabsorbable polymer coatings or even bioabsorbable stent struts are designed to overcome the potential pro-thrombosis of DES with durable polymer. A pooled analysis of the ISAR TEST-3, ISAR TEST-4 and LEADERS randomised trials showed that DES with biodegradable polymers were associated with a significantly lower rate of very late definite ST from one to four years compared with DES with durable polymers (0.2% vs. 1.3%, OR = 0.22, p=0.004)27, indicating the rationality of shortened DAPT after biodegradable polymer DES implantation. CREATE is the first post-marketing study with a large sample size which had a prespecified objective to evaluate the feasibility and safety of six-month DAPT after biodegradable polymer-based DES implantation. In this study, although approximately 80% of patients discontinued clopidogrel therapy within six months after the index procedure, the cumulative incidences of definite and definite/probable ST from 1-5 years were 0.3% and 0.6%, respectively, which, consistent with the results derived from pooled analysis of the ISAR TEST-3, ISAR TEST-4 and LEADERS randomised trials27, were lower than those of first-generation DES (definite: 0.9%-1.1%, definite/probable: 1.4%)10,11. The landmark analysis also showed that patients who received six months of DAPT had a similar occurrence of MACE compared with those who received prolonged (>6 months) DAPT. These results suggest that a six-month DAPT strategy after EXCEL stent implantation might be safe. Currently, several randomised trials have reported that prolonged DAPT up to one or two years is not any better than six-month DAPT after DES implantation28,29, which supports the evidence of our conclusion. Bearing this in mind, further confirmation by randomised studies is needed.
Compared with other trials evaluating the efficacy of biodegradable polymer DES, such as the LEADERS trial, the rates of long-term MACE, TLR and mortality in the present study are numerically lower. There may be several reasons for this: first, follow-up angiography and routine cardiac biochemical marker surveillance were not mandatory in the present study, which may have caused the under-reporting of MI and TLR. Second, patients with device failure, which may influence the long-term prognosis, were excluded from the present study. Third, several studies had reported relatively lower incidence of stent thrombosis in Asian populations30-32, suggesting the potential role of ethnic specificity. Fourth, compared with the LEADERS study23, the baseline clinical characteristics of the present study were less complex, represented as lower incidences of diabetes, hyperlipidaemia, history of MI, prior coronary revascularisation (PCI or bypass surgery), family history and acute MI. These distinctions between the two studies, although both enrolled all-comers, may reflect the different daily practice patient cohort and PCI indications between China and other countries, which may result in some differences in clinical outcomes.
Limitations
A few limitations of the present study must be addressed. First, patient enrolment was not randomised. Second, approximately 20% of patients received clopidogrel for a prolonged time period (>6 months), which was not prespecified or randomised, thus increasing the risk of a selection bias and the probability of type II error even after propensity score matching. Third, follow-up angiography and routine cardiac biochemical marker surveillance were not mandatory in the present study, which may have caused under-reporting of MI and TLR. However, asymptomatic MIs and restenosis are typically associated with lesser clinical significance than symptomatic events, suggesting that the under-reporting of ischaemic events in the present study has a limited impact on the conclusions.
Conclusions
The use of biodegradable polymer-based sirolimus-eluting stents demonstrated persistent superior safety and efficacy in terms of the low incidence of ST and MACE when treated with six months DAPT in daily practice.
Acknowledgements
This study was supported by the National Key Technology R&D Program in the 12th Five-Year Plan of China (No.2011BAI11B07), Major High-Tech Clinical Army Projects (No.2010gxjs001).
Conflict of interest statement
The authors have no conflict of interest to declare.

