Abstract
Aims: The time-dependent changes in endothelial and healing properties of coronary arteries implanted with a biodegradable polymer-based Biolimus A9-eluting stent (BioPol-BES) have not been investigated. We evaluated the short-term and the long-term in vivo response of BioPol-BES, as compared to a permanent polymer-based sirolimus-eluting stent (PermPol-SES), and a bare metal stent (BMS).
Methods and results: Overlapping stents were placed in 33 swine (n=11 for BES, SES, and BMS, respectively) for two and four weeks and single stents in 30 miniature pigs (n=18 for BES, n=9 for SES, n=3 for BMS) for three, nine and 15-month evaluations. The vessel patency, arterial healing and endothelialisation were assessed by angiography, histopathology and scanning electron microscopy. At four weeks, the endothelialisation at overlapping stent regions was greater with BioPol-BES (87.8±3.7%) and BMSs (98.0±0.4%) than with PermPol-SES (66.4±3.2%). The inflammation score in vessels implanted with single BioPol-BES increased slightly from three to 15 months (0.00±0.00 to 0.28±0.14), while this increase was more pronounced with PermPol-SES (0.11±0.07 to 1.56±0.68). Compared to BMS moderate lymphocyte infiltration was seen with BioPol-BES, and marked granulomatous formation with PermPol-SES.
Conclusions: The level of endothelial coverage in BioPol-BES was comparable to BMS at four weeks, with no significant increase of inflammatory reaction up to 15 months.
Abbreviations
BioPol-BES: biodegradable polymer-based Biolimus A9-eluting stent
BMS: bare metal stent
D: distal
DES: drug-eluting stent
HE: haematoxylin-eosin
HPLC: high-performance liquid chromatography
LAD: the segments of left anterior descending artery
LCX: left circumflex artery
M: middle
O: overlapping
P: proximal
PermPol-SES: permanent polymer-based sirolimus-eluting stent
PLA: polylactic acid
QCA: quantitative coronary angiography
RCA: right coronary artery
SEM: scanning electron microscopy
ST: stent thrombosis
Introduction
Use of drug-eluting stents (DES) has significantly decreased restenosis and target vessel revascularisation compared with bare metal stents (BMS)1. However, increased use, extended indications and the longer follow-up time window of first-generation DES have led to the emergence of late stent thrombosis (ST), which was not initially observed in controlled clinical trials. Although infrequent, this adverse event results in abrupt occlusion of the implanted artery, with a high risk of sudden cardiac death or myocardial infarction2,3. Autopsies of patients who died due to DES thrombosis at >30 days post-implantation suggest pathological mechanisms involving delayed neointimal healing, incomplete tissue coverage of the stent strut, and chronic inflammatory reactions to polymers coated on the stent strut as drug carriers2,4-6.
The biodegradable polymer-based Biolimus A9™-eluting stent (BioPol-BES) (Terumo Corporation, Tokyo, Japan), coated only abluminally with a matrix of polylactic acid (PLA) and the drug, has demonstrated similar efficacy and safety to other contemporary DES in clinical trials7, and also showed better preserved endothelium-dependent vasomotion of stented arteries when compared to the permanent polymer-based sirolimus-eluting stent (PermPol-SES)8. Although these data indicate a satisfactory clinical course, the assessment of neointimal healing and endothelialisation of BioPol-BES-implanted vessels is important to understand better the value of this innovative concept. Several studies have shown that animal models can be used to predict the human response to DES implantation9. They also observed a more intense biological response in overlapping segments compared to single-stented segments due to the doubled effect of the drug and polymer10.
The main purpose of our study was to assess the healing properties of porcine coronary arteries treated with overlapping BioPol-BESs compared to those of the PermPol-SESs and BMSs. The second aim was to evaluate long-term local response to a single stent and possibly to identify the underlying mechanism which has an impact on endothelial function.
Methods
The study protocol was reviewed and approved by the site animal committee and the experiments were conducted according to the “Basic Guidelines for Conduct of Animal Experiments” published by the Ministry of Health, Labour and Welfare, Japan.
PROCEDURE
Thirty-three farm swine, 28-36 kg in weight, underwent placement of overlapping stents for healing assessment at two and four weeks, and 30 Clawn miniature pigs, 26-44 kg in weight, were implanted with single stents for long-term evaluation. We selected different pig species because farm pigs have coronary arteries suitable for stent implantation and there are ample historical data for short-term study; on the other hand, miniature pigs are commonly used for long-term implantation because of their smaller size and slower growth rate compared to farm pigs. The number of animals implanted with BioPol-BES (Nobori™, length 14 mm, diameter 3.0 mm, total stent thickness 135 µm; Terumo Corporation, Tokyo, Japan), BMS (length 14 mm, diameter 3.0 mm, stent thickness 120 µm; Terumo Corporation) or PermPol-SESs (Cypher™, length 13 mm, diameter 3.0 mm, total stent thickness 152.6 µm; Cordis, Johnson & Johnson, Warren, NJ, USA) is listed in Table 1. All stents were made of 316L stainless steel. Biolimus A9™ (Biosensors International, Newport Beach, CA, USA) is a more lipophilic analogue of sirolimus. Once PLA has degraded, BioPol-BES remains as a stainless steel stent coated with a durable parylene C polymer whose biocompatibility has been proven. All animals were treated daily with acetylsalicylic acid (330 mg po) and ticlopidine (200 mg po) for three days prior to and eight weeks after operation. After being anaesthetised with medetomidine (0.04 mg/kg im) and midazolam (0.2 mg/kg im), they were supplied with 2-4% sevoflurane inhalation under 4L/min oxygen flow. Heparin (10,000 U iv) was administered via the left carotid artery through a 7 Fr sheath. The stent delivery catheter was inserted through the sheath and was advanced over the 0.035’’ guidewire to the left or right coronary artery orifice. By quantitative coronary angiography (QCA), the segments of left anterior descending artery (LAD), left circumflex artery (LCX) or right coronary artery (RCA) were selected to match available stent sizes. A single stent was implanted in LAD or LCX for long-term evaluation, and two homogenous stents in RCA with a 6-7 mm target overlap for healing assessment, without pre- or post-dilatation. During implantation, balloons were inflated for 30 seconds. The pressures were determined to achieve appropriate sizes (balloon-artery ratio=1.1 to 1.2), following compliance charts from manufacturers. Post-angiography revealed that stent-to-artery ratio was between 1.1 and 1.2. After the procedure, the animals were recovered and then returned to routine care. At the end of each study period, follow-up angiography was performed in all animals under anaesthesia which was the same as during stent placement. Immediately following euthanasia by exsanguinations, the hearts were harvested and processed for scanning electron microscopy (SEM), histopathology and residual PLA measurement. Time points and analyses are indicated in Table 1.
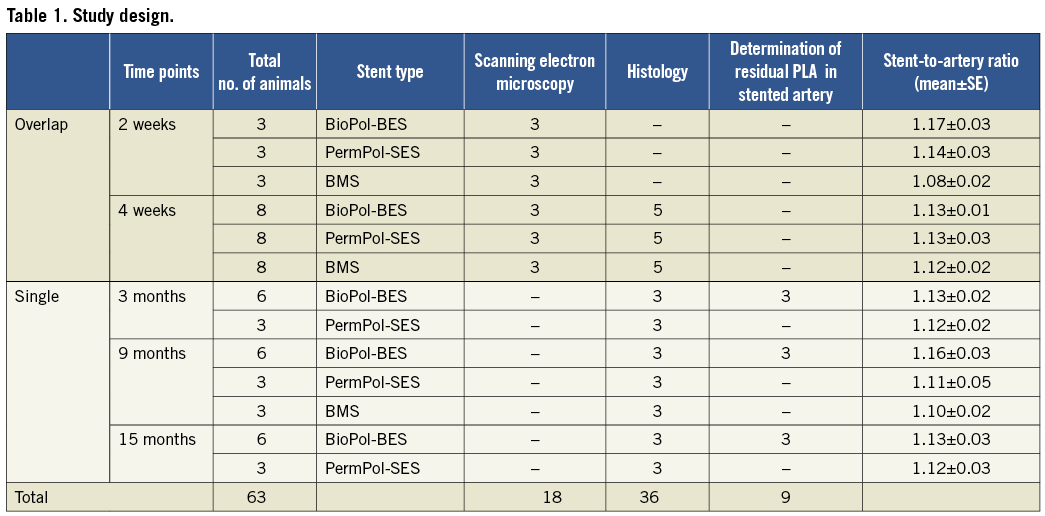
SEM ANALYSIS
Hearts were fixed by pressure perfusion with phosphate-buffered 2% paraformaldehyde and 1.25% glutaraldehyde, and then immersed in phosphate-buffered 10% formalin. Stented vessels were dissected, trimmed, carefully opened longitudinally, and post-fixed by immersion in phosphate-buffered 1% osmium tetroxide. The samples were dehydrated in ascending concentrations of ethanol, freeze-dried in t-butanol, and then sputter-coated with platinum. The interior surface of the bisected halves of each stent pair was examined by S-3400N SEM (Hitachi, Tokyo, Japan). Serial SEM images were assembled using Photoshop CS3 (Adobe Systems, San Jose, CA, USA) to make a complete picture of the stented artery. The area of endothelial coverage above and between the struts of a single stent zone was marked and quantified using WinROOF (Mitani Corporation, Tokyo, Japan) by investigators blinded to treatment groups. The area covered with fibrin or basal lamina was not considered as endothelialised. Since the luminal side strut was buried beneath the endothelium , it was difficult to trace the edge of overlapping struts. Thus, the % endothelialisation within the overlapping zone was calculated as [(endothelialised luminal area/whole luminal area) ×100], without discrimination of the areas above and between the strut.
HISTOPATHOLOGY
Hearts were perfused with saline and perfusion-fixed with 10% buffered formalin. The stented vessel segments were dissected and processed with a Kulzer Histo-Technique 8100 Kit (Heraeus Kulzer GmbH, Wehrheim, Germany) according to the manufacturer’s instructions. After polymerisation, 3 mm sections were cut from the proximal (P), overlapping (O) and distal (D) portions of each overlapping stent or the P, middle (M) and D portions of each single stent. Sections of 3 μm thickness were cut using a tungsten knife, and stained with haematoxylin and eosin (HE) or elastin-HE. Sections stained with HE were evaluated for the extent of vessel inflammation, injury and fibrin content11 by light microscopy. All parameters were scored using the above published methods by the independent pathologist blinded to treatment groups. The cross-sectional lumen area and in-stent area were measured using the 2-D image analysis software WinROOF on the sections stained with elastin-HE. The % area of in-stent stenosis was calculated as [100 × (1 – [lumen area/in-stent area])].
Measurements of residual PLA in BioPol-BES-stented arteries: the amount of residual PLA in the stented artery was estimated by high-performance liquid chromatography (HPLC) measurement of lactic acid obtained after hydrolysis of PLA. At three, nine and 15 months after implantation, the BioPol-BES -stented artery was harvested. PLA was extracted separately from the trimmed artery with acetone and converted to lactic acid by alkalisation, then redissolved in phosphate buffer solution (pH 2.2). An aliquot of the solution was injected onto the HPLC column to determine the amount of lactic acid. The non-stented right coronary artery was subjected to the same procedure and used as blank to correct for the residual amount of lactic acid in non-stented arteries.
STATISTICAL ANALYSIS
Values are shown as mean±standard error (S.E.). Data were compared among device groups at each time point on a segment-by-segment basis (P, O, D or P, M, D). Continuous parameters (% endothelialisation and % in-stent stenosis) were compared by the parametric method: Tukey’s test for four-week and nine-month three-device groups; t-test for three-month and 15-month two-device groups. Ordinal parameters (injury score, inflammation score and fibrin content score) were compared by the nonparametric method: Steel-Dwass test for four-week and nine-month three-device groups; Wilcoxon rank-sum test for three-month and 15-month two-device groups. A p value <0.05 was considered statistically significant. SAS v. 7.5.0 (SAS Institute Inc., Cary, NC, USA) was used for all analyses.
Results
All animals survived the procedure and remained healthy until the planned follow-up times. All arteries were angiographically patent and showed no evidence of filling defects, aneurysm formation, dissection and side-branch occlusion at the predefined time points.
ENDOTHELIAL COVERAGE
There was significantly lower endothelial coverage of PermPol-SES struts as compared to BMS struts, whereas there was no difference in endothelialisation between BioPol-BES and BMS over the whole luminal surface at two and four weeks (Figure 1E and Figure 1F, Table 2). A similar trend was noted above the non-overlapping strut, with significantly lower % endothelialisation for PermPol-SES compared to BMS, and no difference between BioPol-BES and BMS (Figure 1G and Figure 1H). The areas between the single struts showed >90% surface area coverage for all three stent types at two and four weeks (data not shown). Although we were unable to calculate the extent of endothelial coverage above overlapping struts, it was apparent from SEM images (Figure 1A, Figure 1B and Figure 1C) that the stent struts of BioPol-BES and BMS were almost completely buried beneath the smooth endothelial layer. Complete endothelial coverage by spindle-shaped, longitudinally oriented cells was observed on the surface of BioPol-BES- and BMS-implanted vessels, in contrast to uneven coverage by pavement-shaped cells on the surface of PermPol-SES-implanted vessels (Figure 1D).
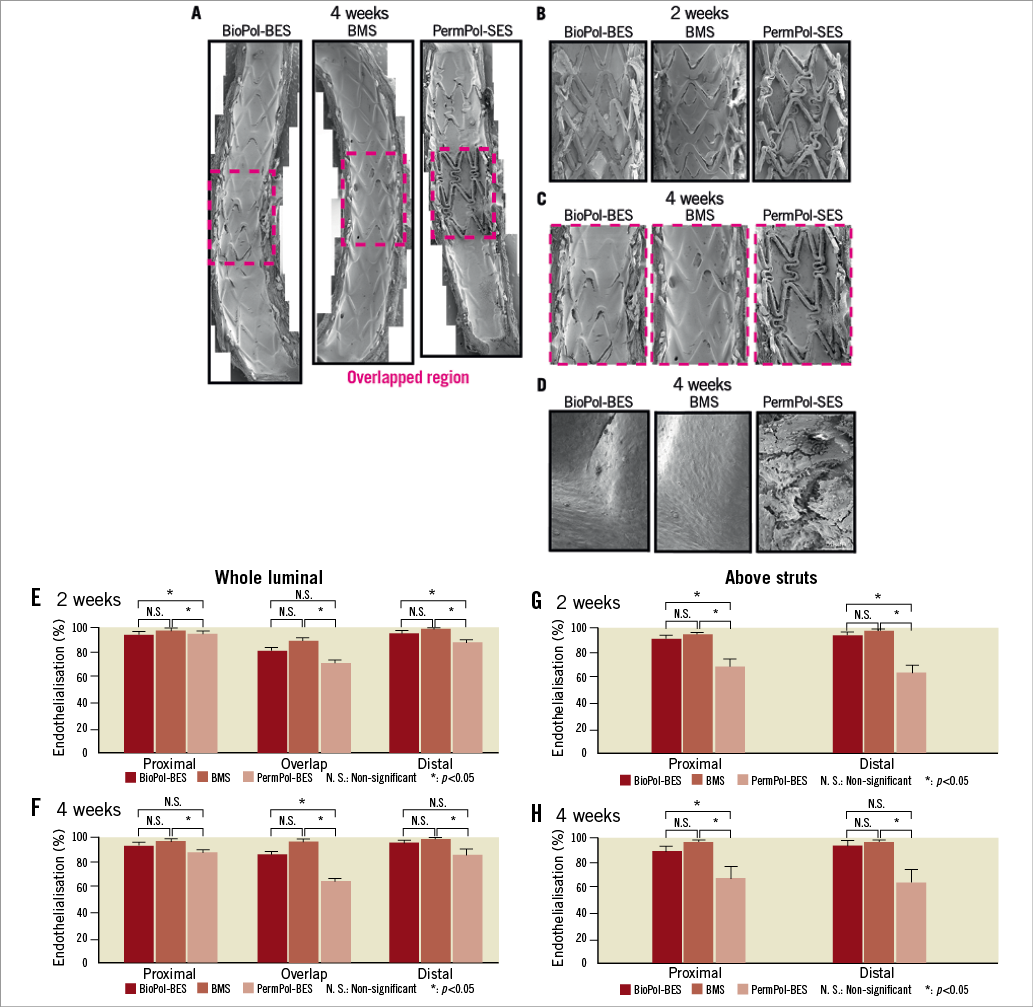
Figure 1. Endothelialisation evaluated by scanning electron microscopy (SEM). Representative SEM images of whole vessels at 4 weeks (A); enlarged image of overlapping segments at 2 weeks (B) and 4 weeks (C); higher magnification (x 200) of an overlapping strut and leukocytes at 4 weeks (D). Percentage endothelialisation of whole vessels at 2 weeks (E) and 4 weeks (F) and in the region above struts at 2 weeks (G) and 4 weeks (H).
HISTOPATHOLOGICAL EVALUATION OF ARTERIES WITH OVERLAPPING STENTS AT FOUR WEEKS
Histopathological analysis showed similar injury scores for cross-sections in arteries with BMS, BioPol-BES and PermPol-SES, and no stented arteries had an injury score >1 (Table 3). The extent of inflammation seen with BioPol-BES was comparable to that with BMS. Vessels implanted with PermPol-SES had higher inflammation scores compared to those with BMS in all sections, reaching statistical significance only in the proximal region (p<0.05) (Table 3). Representative images of overlapping and single sections at four weeks are shown in Figure 2. In all groups, inflammatory cells consisting of foreign body giant cells, macrophages and lymphocytes were observed (Figure 2A, Figure 2B and Figure 2C). Mild infiltration of eosinophils into the intima and adhesion of leukocytes to vessel walls were identified at the non-overlapping site with both DES, and granuloma formation was observed in the non-overlapping proximal and distal segments with PermPol-SES (Figure 2D and Figure 2E). These biological responses were attenuated at overlapping DES sites. The mean fibrin score tended to be greater with DES than with BMS for all sections, with a significantly higher score for BioPol-BES vs. BMS in the proximal region (Table 3).
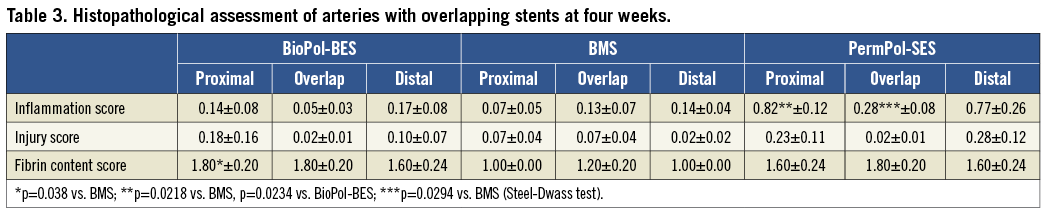
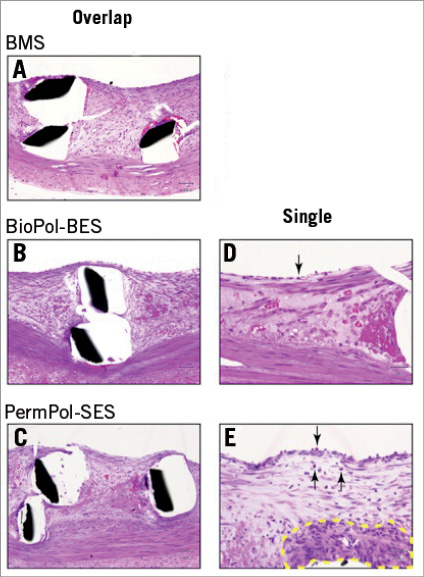
Figure 2. Representative histopathological images of arteries with overlapping stents at 4 weeks. The overlapping struts of bare metal stents (BMSs) (A), BioPol-BESs (B) and PermPol-SESs (C). In higher magnification images of non-overlapping single struts of BioPol-BESs (D) and PermPol-SESs (E), mild eosinophil infiltration into intima and leukocytes adhesion to vessel walls were identified (arrow). Granuloma formation was observed in a single strut of the PermPol-SES (E) (broken yellow line). BioPol-BES: biodegradable polymer-based Biolimus A9-eluting stent; PermPol-SES: permanent polymer-based sirolimus-eluting stent.
Degradation of PLA in BioPol-BES-stented arteries: the amounts of residual PLA in arteries implanted with BioPol-BES were 70.6±6.4%, 44.8±3.7% and 3.8±1.0% at three, nine and 15 months, respectively. The intact BioPol-BES stent contained 209.2 µg of PLA, which gradually degraded after implantation and was almost completely eliminated by 15 months post-implantation.
Histopathological evaluation in long-term single-stent implantation: the % in-stent stenosis in middle sections at four weeks, three, nine and 15 months for BioPol-BES and PermPol-SES, and at four weeks and nine months for BMS, are shown in Table 4 (middle section at four weeks corresponds to the overlapped region, and data for proximal and distal sections are not shown because they showed a similar tendency to that of the middle section). The % in-stent stenosis remained stable at around 40-50% from three to 15 months in all three sections with BioPol-BES. Stenosis with BMS was lower than in those with DES at nine months. Up to 15 months, the increase of the inflammation score with BioPol-BES was mild and remained similar to BMS at nine months, while it was higher with PermPol-SES (Table 5A and Figure 3). Representative images of single sections are shown in Figure 4. Macrophages were predominantly observed in all vessels implanted with stents until 15 months. At three and nine months, both DES had infiltration of lymphocytes into the adventitia, which was not seen for BMS at nine months. However, few lymphocytes apparent in the adventitia of BioPol-BES-implanted vessels at nine months had faded away at 15 months. Marked granuloma formation was observed in PermPol-SES-implanted arteries and remained from three to 15 months. The mean injury scores for each DES increased from three to nine months and remained at about 1.0 beyond nine months, which was greater than that for the BMS at nine months (Table 5B). At all time points, the mean fibrin scores were 1.0±0.0 for all sections of vessels with each type of stent (Table 5C).
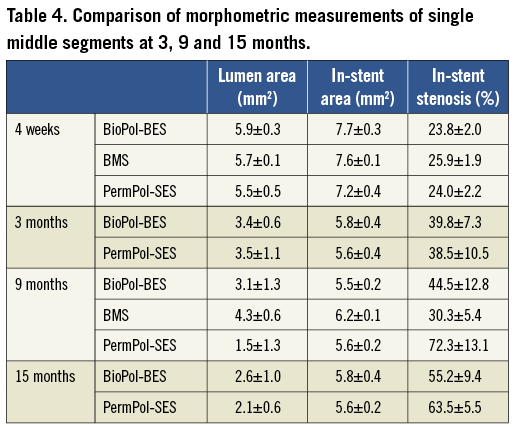
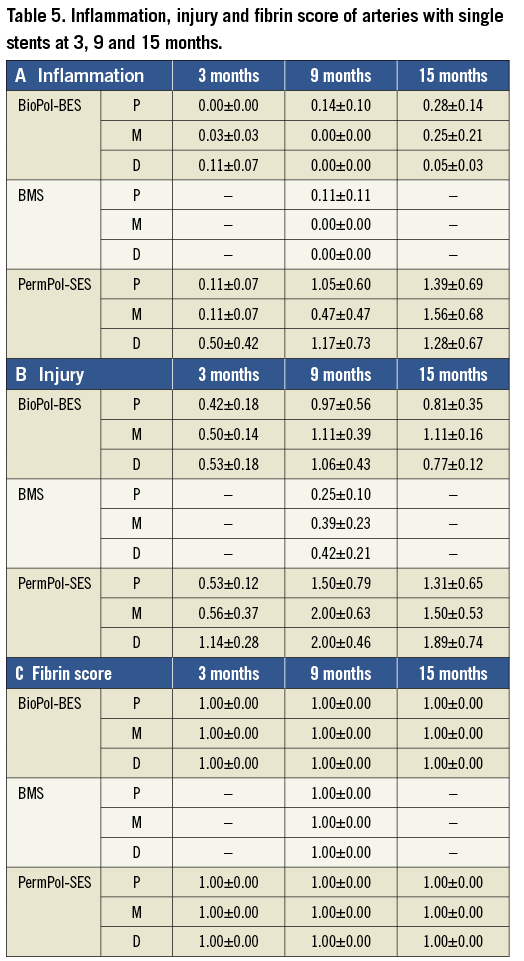
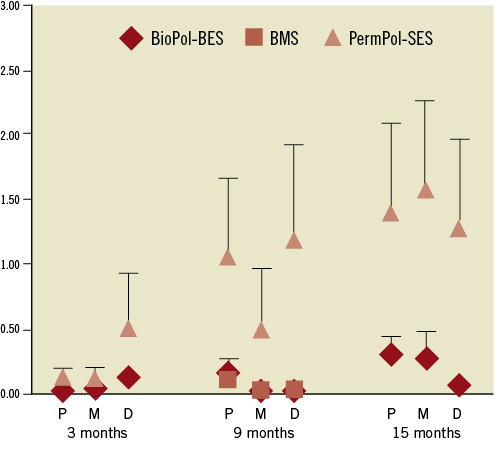
Figure 3. Inflammation score at 3, 9 and 15 months. BioPol-BES: biodegradable polymer-based Biolimus A9-eluting stent; PermPol-SES: permanent polymer-based sirolimus-eluting stent; BMS: bare metal stent; P: proximal; M: middle; D: distal
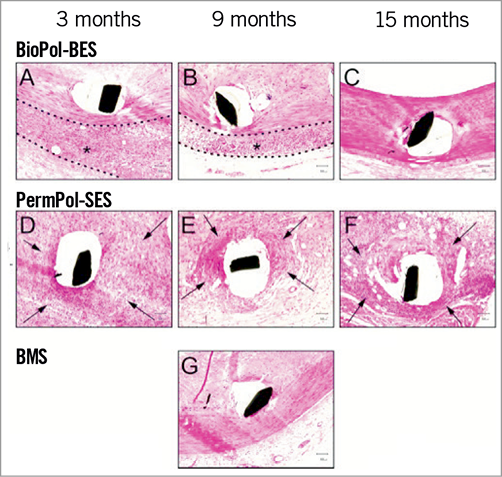
Figure 4. Representative photomicrographs of neointima surrounding single stents at 3, 9 and 15 months. (A, B, C) For BioPol-BES, a few macrophages were observed around stent struts and lymphocytes had slightly infiltrated the adventitia (*) at 3 months (A) and 9 months (B). After degradation of the PLA polymer (15 months, C), slight macrophage infiltration persisted, but lymphocyte infiltration of the adventitia was not seen. (D, E, F) For PermPol-SES implanted vessels, inflammatory cells (macrophages, lymphocytes, eosinophilic leukocytes, foreign body giant cells) had infiltrated around stent struts (arrow) at all-time points. These cells formed granulomatous tissue and the inflammatory reaction extended to the adventitia. (G) With the bare metal stent (BMS), only macrophages were observed slightly around the struts at 9 months. All micrographs show the middle portion of the vessels. BioPol-BES: biodegradable polymer-based Biolimus A9-eluting stent; PermPol-SES: permanent polymer-based sirolimus-eluting stent.
Discussion
Autopsy studies of patients implanted with first-generation DES suggest that late ST is frequently associated with incomplete endothelialisation of stent struts and vessel walls. It has been speculated that direct exposure of strut surfaces to the bloodstream may result in ST2,5. In particular, uncovered struts of malapposed stents are implicated in blood flow disturbance, which may increase the risk of ST12,13. The fully functional endothelial layer is known to exhibit antithrombotic and anticoagulant effects via secretion of factors inhibiting platelet aggregation, such as nitric oxide or prostacyclins14,15. Several questions remain to be answered such as whether coverage seen over the stented segments is truly endothelium, and if yes whether this endothelium is functional. Hamilos et al found better preserved endothelium-dependent vasomotion at adjacent stent segments nine months after implantation of BioPol-BES as compared to PermPol-SES, presumably because implantation of BioPol-BES had a less damaging impact on functional regeneration of endothelial cells8. SEM analysis in our study showed that implantation of a first-generation DES, PermPol-SES, significantly delayed endothelialisation at all segments compared to a BMS, and that this tendency was markedly increased above struts. The endothelial cells covering PermPol-SES were pavement-shaped with poorly formed cell junctions, similar to the SEM images described in human coronary arteries treated with this stent16. In contrast, BioPol-BES had a smooth surface covered mostly by spindle-shaped endothelial cells which are considered to be morphologically mature at four weeks, and endothelialisation scores did not differ from those of BMSs. Simon et al suggested that the endothelialisation is more impaired on metal with a thickness larger than 75 µm in vitro17, and Joner et al showed that endothelial coverage of rabbit arteries implanted by DESs having a stent thickness over 100 µm was uniformly poor. Our finding, however, showed that this did not apply to the BioPol-BES, which has a total stent strut thickness of 135 µm. It could be speculated that endothelial coverage on the luminal surface of the stent struts was achieved without direct contact with the drug layer which is coated only on the abluminal surface of the strut. Also the less inflammatory polymers or the stent strut design of BioPol-BES could have contributed to these findings.
With regard to fibrin deposition, due to the effects of the drugs, both DES showed a tendency for delayed healing at four weeks as compared to the BMS. The significantly higher fibrin score in BioPol-BES as compared to BMS at four weeks could be related to the initial burst of the drug shortly after stent implantation. Nonetheless, beyond three months fibrin depositions were minimal with all types of stent.
NEOINTIMAL FORMATION
Both DESs failed to reduce neointimal formation over 30 days after implantation. This phenomenon is generally observed in the current non-injured porcine models implanted both with PermPol-SES18 and BioPol-BES19. Although all DESs could not completely prevent late neointimal growth at the long term in humans, the extents vary among the types of DES20,21. The attribution of these differences among DESs is unidentified22 and there is no predictive model using animals.
INFLAMMATION
Consistent with previous results in porcine models at 30 days23,24, we also found a tendency for PermPol-SE-stented arteries to show more intense inflammation compared to BM-stented arteries at four weeks, in both non-overlapping and overlapping zones. This tendency did not disappear at long-term evaluation, but gradually increased from three to 15 months with fading away of the immunosuppressive agent. This chronic inflammation in first-generation DESs is considered to be one of the pathological mechanisms leading to late ST25. In contrast, minimal inflammation was observed with the BioPol-BES at a level roughly similar to that with the BMS at nine months. The inflammation did not increase significantly even after disappearance of the drug, which is completely released from the polymer coating at six months after implantation (unpublished data from Terumo). Regarding the difference in inflammation reactions between BioPol-BES and BMS, we observed minor to moderate infiltration of lymphocytes into the adventitia of BioPol-BE-stented vessels at three and nine months. This did not occur with BMS at nine months, even in vessels stented with an excessively expanded BMS (stent/artery ratio 1.2-1.3:1, data not shown). One possible mechanism of lymphocyte infiltration in BioPol-BE-stented vessels is a biological reaction against PLA degradation products. This hypothesis comes from the observation that lymphocytes faded away at 15 months after implantation of BioPol-BES, coinciding with the time point of PLA disappearance. We speculate that lymphocytes migrate into vessel tissue to eliminate PLA degradation products or protein denatured by acidic degradation products. However, although biodegradable polymers are reported to be associated with a risk of evoking inflammation, in this in vivo study we did not identify any cause for serious concerns. The BioPol-BES was also coated with parylene C coating, which was exposed to the vascular cavity for up to 15 months and covered stably with smooth endothelium and neointima without inducing inflammation.
Study limitations
Vessel repair in swine occurs very rapidly and the stents were deployed in normal non-atherosclerotic arteries, which may limit direct extrapolation of the results to patients. Also, the non-injured porcine models have the design limitation of predicting the long-term efficacy in clinical use. Second, the study was limited to BioPol-BES, PermPol-SES and BMS, and did not include stents coated with polymer but without drug. Therefore, the effect of the polymers themselves cannot be separated from the influence of the drugs. Moreover, the polymer degradation dynamic is influenced by the surrounding environment and therefore the PLA degradation time observed in our animal model cannot be extrapolated to humans. Also, we did not perform histopathological observation of arteries stented with BMSs at three and 15 months, which prevented comparison of the effects of the three types of stent at this time point under the same experimental conditions. Finally, due to the different design of the stents used in this study, complete blinding of the assessors cannot be guaranteed.
In conclusion, we assessed biocompatibility and the short- and long-term healing process of BioPol-BES, PermPol-SES and BMS. The BioPol-BES led to recovery of endothelial coverage to a level comparable to BMS at four weeks, with no significant increase of inflammatory reaction up to 15 months. These findings support the favourable outcomes with BioPol-BES previously demonstrated in clinical trials.
Conflict of interest statement
H. Hagiwara, Y. Hiraishi, H. Terao, T. Hirai, A. Sakaoka, M. Sasaki, S. Murota and J. Kimura are all employees of Terumo Corporation, Kanagawa, Japan. K. Inoue is an advisory board member of Terumo Corporation.

