
Abstract
Aims: The purpose of the present study was to examine the comparative vascular healing response to stents coated with permanent or biodegradable polymer and uncoated stents in a porcine model of coronary artery stenting.
Methods and results: Juvenile pigs were randomly allocated to implantation of stents coated with permanent polymer (PP, methacrylate-based, n=10), biodegradable polymer (BP, poly-lactic acid-based, n=10) or bare metal control stents (n=10), in the absence of antiproliferative drugs. At 28 days, animals were sacrificed and specimens prepared for histopathologic assessment. Endothelialisation was complete in all treatment groups. Vascular injury at 28 days was greater in PP stents as compared with uncoated stents (p=0.05) though not as compared with BP-coated stents (p=ns). PP stents showed increased inflammatory scores compared with BP-coated (p=0.03) and uncoated stents (p=0.02). There was also greater neointimal growth with PP-coated stents compared with uncoated stents (p=0.02).
Conclusions: In the absence of antiproliferative drugs, stents coated with methacrylate-based PP, but not with poly-lactic acid-based BP, provoked significant vessel wall inflammatory reactions resulting in greater vascular injury and increased neointimal growth compared with uncoated stents. Biodegradable polymer coatings may be considered preferable to facilitate drug elution with minimal vessel wall toxicity.
Introduction
Control of drug release kinetics is pivotal to the effectiveness of drug-eluting stent (DES) technology and the main reason for the use of polymer coatings in DES devices1. However, although the introduction of polymer-based DES technology has resulted in significant reductions in in-stent restenosis, this clinical advantage is attained at the collateral cost of delayed healing of the stented arterial segment2,3 and a related spectrum of clinical events, including late stent thrombosis4, late luminal loss creep5 and neoatherosclerosis6.
Although delayed arterial healing is undoubtedly multifactorial in aetiology3, over the course of the last decade considerable attention has focused on the role of permanent polymer coatings in the pathogenesis of this condition7-9. However, DES are relatively complex devices comprising specific combinations of metallic backbones, polymer coatings and antiproliferative drugs. As such, in post-mortem studies it is often difficult to isolate the specific contribution of polymer coating to the observed pathological effects. In addition, most published preclinical studies have assessed the aggregate effect of commercial devices rather than examining the individual device components in stepwise fashion10-15. Although the clinical use of biodegradable polymer DES seems promising16,17, preclinical studies directly comparing the vascular effects of permanent and biodegradable coatings are lacking, especially at a time point when biodegradable coatings undergo active degradation processes.
In an earlier study we showed that, in DES devices with identical backbone and polymer coating, varying the particular limus drug applied resulted in significant differences in vascular healing in a rabbit iliac model of stent implantation18. The aim of the current study was specifically to characterise the vascular responses to selected polymer coatings in a porcine model of coronary stent implantation using otherwise identical stents in the absence of influence from antiproliferative drugs.
Materials and methods
The study protocol was approved by the Institutional Animal Care and Use Committee of the Medstar Research Institute and conformed to the position of the American Heart Association on the use of animals in research and the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health19.
PORCINE MODEL OF CORONARY STENT IMPLANTATION
A total of 28 young adult (25-35 weeks) domestic farm pigs were included in the study. Animals were received from UpperCo Swine Inc. (Reisterstown, MD, USA), and were acclimatised for at least three days prior to use in the study. At day zero, animals were randomly assigned to one of the treatment groups, and stents were implanted in the left anterior descending, the left circumflex or the right coronary artery, with a targeted oversize of 1.2-1.3:1. After stent implantation, coronary angiography was performed to document vessel patency and absence of residual dissection.
TEST DEVICES AND GROUPING
Study devices were 3.0×13 mm cobalt-chromium (L605) stents (PRO-Kinetic Energy; BIOTRONIK AG, Bülach, Switzerland) with a strut thickness of 60 µm including a proprietary PROBIO silicon carbide coating that is aimed at reducing acute thrombotic burden after stent implantation20. The animals were randomly allocated to implantation of one of three different stent groups (Figure 1): stents coated with permanent polymer (PP, methacrylate-based, group 1, n=10); stents coated with biodegradable polymer (BP, poly-lactic acid-based, group 2, n=10); or stents with no polymer coating (group 3, n=10). The permanent polymeric stent coating was composed of a commercially available blend of poly(n-butyl methacrylate) (PBMA)/poly(ethylene vinyl acetate) (PEVA), weight percentage 22/78 with a total load of 56.9 µg/mm. The biodegradable polymer stent coating was composed of poly L-lactic acid (PLLA) with a total load of 100 μg/mm and an intrinsic viscosity of 8.0 dl/g. Inherent viscosity was measured as described previously21. The PLLA coating is expected to degrade over 36 months in man. Standard spray-coating was used for all coated stents. Study devices were otherwise identical; no antiproliferative drugs were applied.
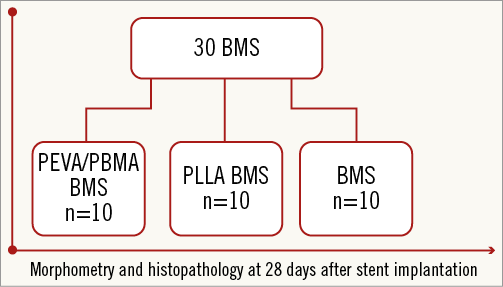
Figure 1. Study flow chart. Morphometry and histopathology performed 28 days after implantation. Random allocation of 30 PEVA/PBMA permanent polymer-coated stents (PEVA/PBMA stent) or poly L-lactide biodegradable polymer-coated stents (PLLA stent) or uncoated bare metal stents (BMS) into 28 animals.
INTERVENTIONAL PROCEDURES AND TISSUE HARVEST
Animals were sedated with a combination of ketamine (20 mg/kg), xylazine (5 mg/kg) and atropine (0.05 mg/kg) by intramuscular injection followed by intubation and mechanical ventilation. Isoflurane (1.5-3.0%) in oxygen was administered to maintain anaesthesia. A dose of 75 mg clopidogrel was administered daily for three days prior to stent implantation, followed by 81 mg aspirin and 75 mg clopidogrel daily for the remainder of the study.
Standard angiographic images of the coronary arteries were acquired with contrast media to identify appropriate locations for stent implantation. Animals were sacrificed at 28 days following implantation. Stented portions were embedded in methyl methacrylate, divided into proximal, middle and distal blocks and sectioned at 8 µm thickness. Non-stented coronary segments were embedded in paraffin. All sections were stained with haematoxylin and eosin (H&E) and Verhoeff-van Gieson (VVG).
STUDY ENDPOINTS AND DATA ANALYSIS
Vascular injury and inflammation following stent implantation were graded as described previously22. Computerised planimetry was performed (Image-Pro Plus 6.1; Media Cybernetics, Inc., Rockville, MD, USA) on all stented sections as previously described23. Fibrin was identified as intense, homogenous pink stain and semi-quantified as a score on a strut-by-strut analysis as previously described24. The cross-sectional areas (external elastic lamina [EEL], internal elastic lamina [IEL] and lumen) of each stented section were measured, as well as the luminal areas of the proximal and distal non-stented reference sections, using digital morphometry. For sections exhibiting significant artificial vessel flattening after processing, a circumference measurement was taken and the area calculated. Neointimal thickness was measured as the distance from the inner surface of each stent strut to the luminal border. Area measurements of EEL and IEL were used to calculate vessel layer areas of media, neointima and percentage area stenosis. Endothelial coverage was semi-quantified and expressed as the percentage of the lumen circumference covered by endothelium.
STATISTICAL ANALYSIS
For morphometry and histopathology assessment, three sections were obtained per stented segment. Means of the three sections were calculated for each artery (n=10) in each group. Numerical data are presented as median with 25% and 75% quartiles. Continuous variables were checked for normal distribution using the Shapiro-Wilk W-test for equal variances. Mean values with standard deviation were derived from normally distributed parameters while non-parametric data were described as median with percentiles. Statistical comparison was performed using the ANOVA test with Dunnett’s post hoc correction when data sets were normally distributed or Wilcoxon rank-sum tests with individual comparison in the event of non-parametric distribution of data; p-values ≤0.05 were considered as significant.
Results
A total of 30 stents were implanted into the three major coronary arteries of 28 pigs, comprising 10 stents coated with PEVA/PBMA, 10 stents coated with PLLA and 10 uncoated stents. No animal died during the study period and all stents could be placed within the intended coronary artery. All 30 implants were available for follow-up at day 28. There were no cases of stent migration.
HISTOMORPHOMETRIC ASSESSMENT AND ANGIOGRAPHIC MEASUREMENTS
Results of morphometric analysis are shown in Table 1. Results of angiographic oversize measurements are shown in Table 1 and Figure 2. The mean reference vessel diameter was similar across the study groups. Neointimal area at follow-up was different across the study groups. There was significantly greater neointima formation in the stents coated with permanent PEVA/PBMA polymer as compared to control uncoated stents (p=0.02). In contrast, stents coated with PLLA biodegradable polymer revealed similar neointimal growth compared with control stents (p=ns). Percent area stenosis was significantly greater in the PEVA/PBMA group compared to uncoated stents (p=0.01) but was similar between PLLA and uncoated stents (p=ns). Mean lumen area was significantly lower in the PEVA/PBMA group compared to uncoated stents (p=0.03) with no differences between PLLA and uncoated stents (p=ns).
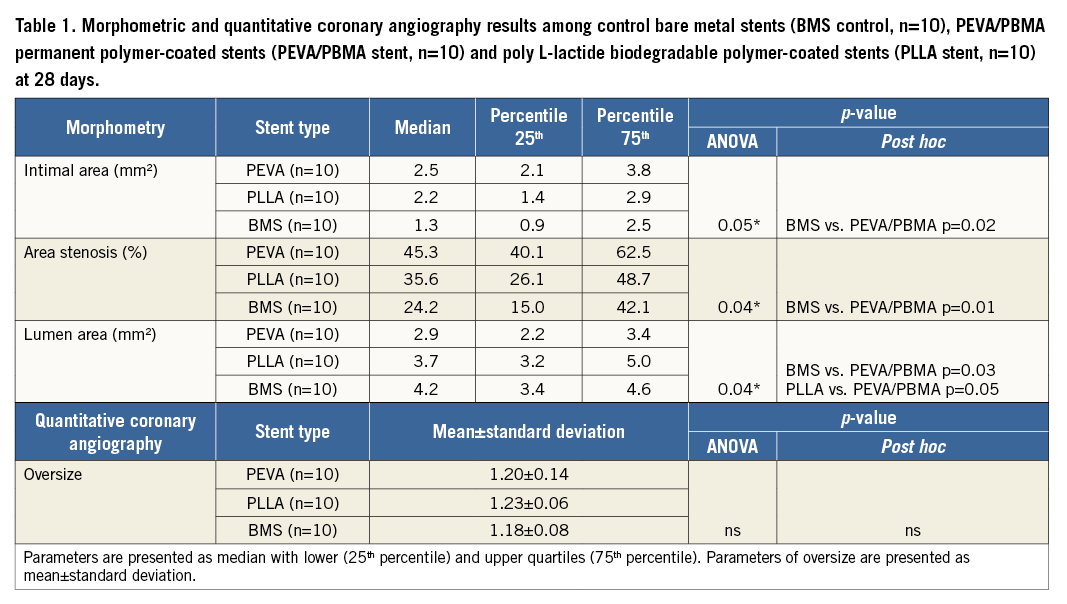
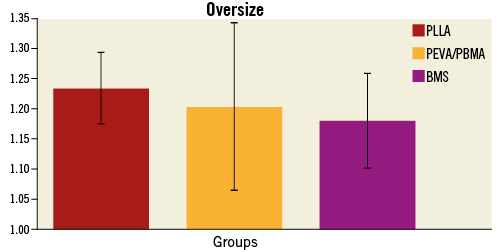
Figure 2. Results of baseline angiographic oversize measurements obtained by quantitative coronary angiography. Bare metal stents (BMS control, n=10), PEVA/PBMA permanent polymer-coated stents (PEVA/PBMA stent, n=10) and poly L-lactide biodegradable polymer-coated stents (PLLA stent, n=10). Parameters are presented as mean±standard deviation.
HISTOPATHOLOGICAL RESPONSE
Results of histopathological analysis are shown in Table 2. Scores for fibrin deposition were low in all groups without significant differences across the groups. Endothelialisation was complete in all treatment groups at 28 days. Inflammation scores were significantly greater for stents coated with PEVA/PBMA when compared to stents coated with PLLA (p=0.03). In contrast, stents coated with PLLA biodegradable polymer revealed similar inflammation scores compared with control stents (p=ns). Representative histological sections with similar injury scores are shown in Figure 3.
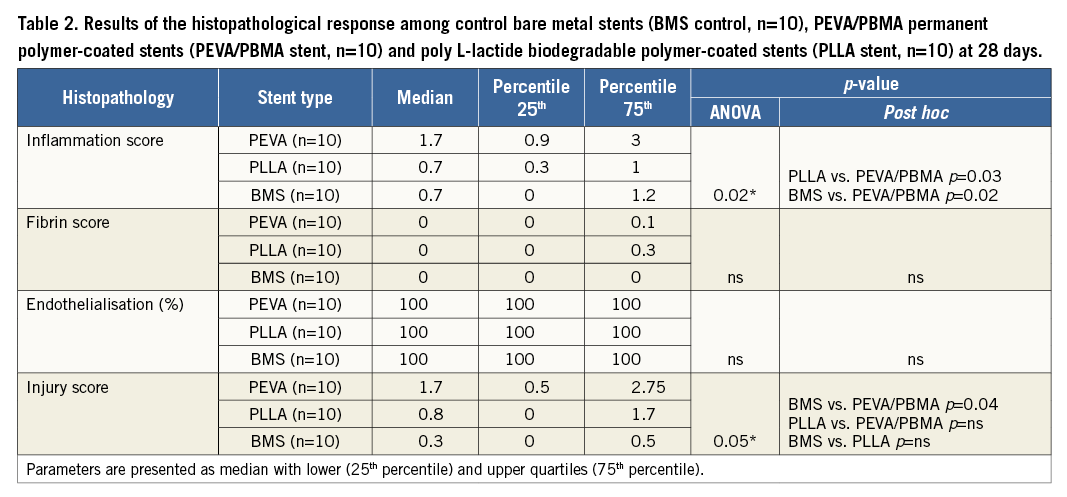
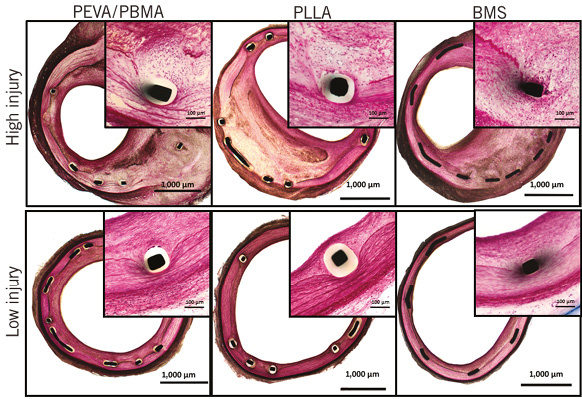
Figure 3. Representative histological images (×40 magnification, Verhoeff-van Gieson stain) and ×200 magnified images (inserts, H&E stain) showing bare metal control stents and stents with permanent PEVA/PBMA polymer coating as well as stents with biodegradable PLLA polymer coating at 28 days with similar injury scores.
Discussion
The current study examined the vascular response to different polymer coatings in the absence of antiproliferative drugs in a porcine model of coronary stenting at 28 days post implantation. The aim of the study was to characterise the specific effects of selected permanent and biodegradable polymer coatings on vascular healing using otherwise identical stent platforms without influence from drug-induced vascular effects at a time point when biodegradable polymer coatings remain intact. In this regard, the salient findings were: (i) delayed endothelialisation and increased fibrin deposition were not prominently observed after implantation of stents coated with permanent or biodegradable polymer coating in the absence of antiproliferative drugs; (ii) there was significantly greater vessel wall inflammation in stents coated with PEVA/PBMA permanent polymer but not in stents coated with PLLA biodegradable polymer when compared with uncoated stents; (iii) this increased inflammation with PEVA/PBMA-coated stents was accompanied by increased vascular injury, increased neointimal growth and reduced mean lumen area.
Delayed arterial healing is a well-recognised side effect of drug-eluting stent therapy1-3. Histopathologically it comprises a constellation of hallmark features including delayed re-endothelialisation, chronic vessel wall inflammation and persistent fibrin deposition. Although its aetiology is undoubtedly multifactorial, human autopsy studies have implicated local inflammatory response to polymer coatings as an important contributory factor. For example, one study found that patients treated with permanent polymer sirolimus-eluting stents had increased inflammatory responses compared to duration-matched bare metal stent autopsies3 . Furthermore, local hypersensitivity reactions, characterised by neointimal infiltration of eosinophils and T lymphocytes were reported in autopsy case series and implicated as a causative factor for stent thrombosis in in vivo studies using thrombus aspiration3,7,8.
Perhaps somewhat surprisingly, there is a paucity of data in the peer-reviewed literature examining the specific contribution of polymer coatings to the clinicopathological entity of delayed arterial healing. The reason for this might be that drug-eluting stents are relatively complex devices composed of specific combinations of metallic backbones, polymer coatings and antiproliferative drugs. As such, in observational autopsy studies, the ascription of any observed effect to one individual device component might be challenging. Moreover, in relation to animal data, most published studies were designed to investigate the aggregate vascular response to implanted DES devices using uncoated bare metal stents as control devices because the primary goal of these studies was to evaluate the safety of the test devices prior to clinical investigation10-15. In an earlier study we showed that, in DES devices with identical backbone and polymer coating, varying the applied limus drug resulted in differences in vascular healing in a rabbit iliac model of stent implantation18. In the present report we aimed to investigate the differential impact of selected permanent and biodegradable polymers in a dedicated experimental set-up where other device components were kept constant.
In the current study, stents coated with PEVA/PBMA permanent polymer provoked increased inflammation along with augmented vascular injury and neointimal growth compared to uncoated stents. A number of different permanent polymers have been utilised in commercially available DES devices. However, the majority of devices have used permanent polymers with methacrylate components, which was the reason for the choice of this copolymer in the current study. Early in the course of DES development, Suzuki et al examined polymer biocompatibility in porcine and canine coronary arteries10. Although the primary goal of this study was to assess the safety of a commercial sirolimus-eluting stent, a substudy examined the biocompatibility of the PEVA/PBMA coating in isolation in the absence of antiproliferative drugs. The authors found that a low-dose PEVA/PBMA coating showed excellent vascular biocompatibility in comparison to uncoated control stents, while a higher dose showed increased inflammation along with augmented neointimal growth. The findings of the current report are broadly in line with the observations of the higher dose group in that study. Notably, however, the current study utilised a considerably lower polymer load than that used in the report of Suzuki et al.
A second important finding in our study was that stents coated with PLLA biodegradable polymer showed inflammatory reactions similar to uncoated bare metal stents. This suggests that poly-lactic acid-based biodegradable polymers exhibit high biocompatibility at least in healthy porcine coronary arteries. In another preclinical study investigating the safety of a slow-release paclitaxel-eluting stent applying biodegradable polymer technology, the vascular response to stents coated with polylactide co-glycolide polymer (PLGA) was also examined in the absence of an antiproliferative drug25. In this study, polymer-coated stents also revealed similar inflammation and neointimal hyperplasia compared to uncoated control stents at 30 days. Similar high biocompatibility was shown in another study by some of the same authors assessing the safety of a novel everolimus-eluting stent using PLGA copolymer26. There was no increase in inflammation in PLGA copolymer-coated compared to uncoated control stents at 30 days of follow-up, again pointing to the improved biocompatibility of PLGA in vascular applications. However, PLLA and PLGA polymers differ substantially in their chemical composition as well as in their rate of biodegradation. While PLGA exhibits a more rapid degradation profile, there is slower breakdown in PLLA polymers due to the advanced crystalline structure of this polymer that protects from rapid hydrolysis27. As the pace of biodegradation also influences the biological behaviour of these polymers, direct comparison between PLGA and PLLA polymers should be the subject of future investigation.
A third important finding of this study is that polymer-only coated stents revealed complete re-endothelialisation 28 days following implantation in this porcine model. This is an important difference in comparison to the delay in healing observed with drug-loaded DES at this time point15. When interpreted in the context of our previous report showing a substantial delay in re-endothelialisation with limus drug-coated stents in rabbit iliac arteries compared to uncoated stents18, it is tempting to speculate that re-endothelialisation seems to be predominantly determined by the presence of an antiproliferative drug and its release kinetics18. Though different animal models might not be comparable, on the basis of our data and those from the studies of Wilson and colleagues14, the presence of polymer coating alone does not seem to impact directly on the degree of re-endothelialisation. Interestingly, this observation was independent from the type of polymer used. These findings should be reproduced in further preclinical studies across the spectrum of permanent and biodegradable polymer stents.
A fourth important finding from the current study refers to the association of chronic inflammation with higher vascular injury, which has not previously been described with current polymeric coatings. While it has been reported in human autopsy samples that greater injury results in increased inflammation and subsequently amplified neointimal growth in the setting of BMS implantation28, the current study extended these observations to polymer-coated stents in an animal model. In the absence of an antiproliferative drug, chronic inflammatory response to the polymeric coating of the struts resulted in deferred disruption of the internal elastic lamina, which is an important landmark when adjudicating vascular injury. Therefore, it is likely that chronic vascular injury resulted in greater neointimal growth in the current study. This effect was clearly more pronounced in the PEVA compared to the PLLA and BMS groups. Moreover, it can be recognised from the current study that acute injury as assessed by the overstretch ratio was greater in the PLLA compared to both the PEVA and the BMS groups. Nevertheless, chronic injury was lower in the PLLA group at 28 days, which can be attributed to decreased inflammation over time.
Limitations
The current study has a number of limitations. First, the chemical composition of a polymer is only one element of the polymer coating that might contribute to local vessel wall reactions. Production-specific processing, surface coating homogeneity and polymer degeneration (or microparticulate formation) during deployment may significantly impact on local vascular response29,30. Second, the current study examined only one particular formulation and load of PEVA/PBMA and PLLA coatings, respectively, and the results may not be generalisable to other formulations and load doses. Third, evaluation was performed at a single time point of follow-up at 28 days. Although this is a standard time point for preclinical assessment, further studies with longer-term follow-up should be undertaken, particularly as, in investigation of biodegradable polymer coatings, potential benefit may accrue first at time points where polymer degradation has completed. Fourth, the favourable vascular compatibility of PLLA biodegradable polymer in comparison to PEVA/PBMA permanent polymer in healthy porcine coronary arteries cannot be extrapolated to diseased human coronary arteries, where disease conditions and atherosclerotic plaque composition might influence polymer degradation and inflammatory responses31. Fifth, there was a trend towards increased neointimal growth between PLLA-coated and uncoated stents. The lack of significance may be related to the limited numbers in this study and should be addressed in future histopathological studies with sequential follow-up.
Summary
In summary, the current study might show that PLLA biodegradable polymer-coated stents provide improved vascular compatibility compared with PEVA/PBMA permanent polymer-coated bare metal stents in porcine coronary arteries at 28 days.
| Impact on daily practice The specific contribution of polymer coatings to the clinicopathological entity of delayed arterial healing observed in drug-eluting stent therapy has yet to be explained. Biodegradable polymer-coated stents showed improved biocompatibility compared to permanent polymers when studied in a preclinical model in the absence of antiproliferative drugs. These findings may contribute to facilitating the understanding of the promising clinical results observed with selected biodegradable polymer-based drug-eluting stents. |
Funding
This study was funded by Biotronik SE & Co. KG, Erlangen, Germany.
Conflict of interest statement
E. Wittchow, G. Bayer and T. Diener are employees of Biotronik SE & Co. KG, Erlangen, Germany. M. Joner is a consultant to Biotronik SE & Co. KG, Erlangen, Germany. T. Koppara and R. Byrne have no conflicts of interest to declare.

