- drug coated balloon
- paclitaxel
- peripheral stent
- porcine
Abstract
Aims: Despite recent abundance of data on drug-coated balloon technology, the biological effects of paclitaxel coated balloon (PCB) treatment followed by bare metal stent (BMS) implantation in peripheral arteries (simulating bail-out stenting, a common clinical scenario), have not been published.
Methods and results: PCB technology containing a paclitaxel-iopromide coating and identical iopromide-coated controls (without paclitaxel) were used in 16 porcine ilio-femoral arteries. The biological effects of inflating one (PCBx1) or two sequential (PCBx2) paclitaxel coated balloons before BMS implantation were compared to the single application of a control balloon (CCBx1; contrast coated balloons). At 30 days PCBx2 displayed significantly reduced late lumen loss by angiography (58% reduction vs. CCBx1; p=0.04) and neointimal area by histomorphometry (35% reduction vs. CCBx1 and 30% vs. PCBx1; p=0.02). Similarly, percent area stenosis in the PCBx2 group was reduced by 45% as compared to CCBx1 and PCBx1 (p=0.04). At this time, all parameters of vessel wall healing (including injury score, inflammation, and endothelialisation) following drug coated balloon treatment were comparable to the control group.
Conclusions: Paclitaxel delivery to porcine ilio-femorals using PCB followed by BMS implantation effectively decreased neointimal proliferation. More extensive and prolonged proliferative response of the vessel after stenting (necessitating higher drug dose) could potentially explain the undetectable effect of PEBx1 relative to CCBx1 in this pilot study. Histological analysis confirmed the safety and biocompatibility of PCB technology.
Introduction
Despite recent advances in endovascular therapy, the optimal treatment for atherosclerotic superficial femoral-popliteal artery (SFPA) disease is still undetermined1-3. Meta-analysis of randomised controlled trials comparing routine stenting with percutaneous transluminal angioplasty for symptomatic SFPA disease suggests that the stenting approach is not associated with a significant reduction in the rate of restenosis or total vessel revascularisation1. In addition, attempts to reduce restenosis rates in the SFPA using self-expanding nitinol stents eluting sirolimus (SIROCCO trials) and everolimus (STRIDES trial; unpublished data) have failed to demonstrate inhibition of neointimal proliferation4,5. The first positive results of the SFPA treatment using stent technology were demonstrated recently based on the multinational, prospective and randomised study comparing safety and effectiveness of the Zilver PTX self-expanding nitinol stent eluting paclitaxel6.
An angioplasty balloon technology containing a specific paclitaxel-iopromide formulation (Cotavance™, Paccocath® technology; Bayer Schering Pharma / MEDRAD Inc, Indianola, PA, USA) has been demonstrated to significantly reduce late lumen loss and rate of target vessel revascularisation (TVR) in patients with de novo atherosclerotic SFPA disease as compared to conventional uncoated balloon catheters7,8. A common clinical scenario is the necessity for balloon pre-dilatation followed by bare metal stent (BMS) implantation (“bail-out“ stenting) While early studies have described the combination of paclitaxel-coated balloon (PCB) followed by BMS in coronary arteries, their biological profile in peripheral vascular segments is uncharacterised9. In the present study, we aimed to assess via angiographic and histological analysis the efficacy and local tissue effects of dilatation with either one PCB or two PCBs, or a control iopramide-coated balloon preceding implantation of a self-expandable BMS in the ilio-femoral territory of domestic swine.
Methods
Study design
A total of 16 peripheral sites in four healthy domestic swine were treated, including eight superficial femoral arteries (SFA) and eight external iliac arteries (EIA). There were two treatment groups and one active control group: group 1= PCBx1 (single PCB treatment followed by BMS placement, n=6 treated sites), group 2= PCBx2 (double PCB treatment followed by BMS placement, n=6 treated sites) and group 3=CCBx1 (single contrast coated balloon control followed by BMS placement, n=4 treated sites). All animals received all three treatments. The balloons were inflated to achieve a 1.3:1.0 balloon to artery ratio. All animals were followed up for 30 days and then control angiography and histological analysis were performed at termination.
Device description
The Cotavance™ PCB catheters (Paccocath® technology; Bayer Schering Pharma / MEDRAD Inc, Indianola, PA, USA) used were 6 to 7×40 mm balloons coated with paclitaxel (3µg paclitaxel/mm2 of balloon surface) solubilised in contrast medium (Iopromide, Ultravist®; Bayer Schering Pharma). The CCBx1 catheters (active control) were identical in design to the PCB, but were coated only with Iopromide. The stents implanted after balloon inflation were self-expanding nitinol “LifeStent®” devices (Bard Peripheral Vascular, Inc., Tempe, AZ, USA) approved for the treatment of occlusive disease in native SFPA in humans. The stents used were 6 to 7×40 mm and were selected according to target vessel size.
Experimental procedures
All study procedures were conducted in accordance with the Guide to Care and Use of Laboratory Animals by National Academy of Science and animal welfare regulations. The study protocol was approved by the institutional animal care and use committee. All animals were pre-treated with 325mg of aspirin and 150mg of clopidogrel bisulphate administered one day before the procedure. Following anaesthesia induction with Telazol, Xylazine and Glycopyrrolate, they were intubated and general aesthesia was maintained with inhaled isoflurane (1-3%). A vascular access sheath was placed in the common carotid artery by cut-down. Before catheterisation, heparin was injected to maintain an activated clotting time of >250 seconds. An 8Fr guiding catheter was advanced into the external iliac artery and then peripheral angiograms with quantitative vascular angiography (QVA) measurements were performed to select optimal treatment sites (randomised and evenly distributed). To accurately implant the devices, the profunda femoris artery was used as an anatomical marker and the distance from its origin to the balloon or marker stent was measured using a marker wire. All balloons were inflated for 60 seconds (±1) using pressure to achieve the balloon to artery ratio of 1.3:1.0 (30% overstretch). In cases where a second balloon was planned (PEBx2 group), the procedure was repeated using another balloon having identical dimensions. After the balloons had been delivered the study stents were implanted. Following the procedure, the sheaths were removed, the access vessel ligated and the surgical incision repaired. All animals received aspirin (81mg) and clopidogrel (75mg) per os daily until the last day of the study. Upon completion of the 30 days survival period, follow-up angiography of the treated vessels was performed and then the animals were euthanised, the peripheral vasculature completely exposed and after the precise identification of the stent location the arterial tree containing stents was extracted, flushed and immersed in normal buffered formalin 10% for further histological analysis.
Angiographic evaluation
QVA analysis was performed using QAngio XA Software™ version 7.1.14.0 (Medis Medical Imaging Systems®, Leiden, The Netherlands). The baseline and 30-days follow-up minimal lumen diameters (MLD) within the treated segments were measured from non-superimposed and non-foreshortened views using the guiding catheter as a standard for calibration. Reference vessel diameters (RVD) were taken from the proximal and distal portion of the vessel at treated sites. The balloon-to-artery ratio was calculated. Percent diameter stenosis (%DS) at follow-up was calculated as: [1-(MLD/RVD])×100% and the late lumen loss (LLL) was calculated as a difference between MLD after procedure minus MLD at follow-up.
Histological analysis
Histology analysis was conducted by an independent pathology laboratory (CVPath Institute, Inc., Gaithersburg, MD, USA). For light microscopy, all stented vessel segments were dehydrated in a graded series of ethanol and embedded in methylmethacrylate plastic. After polymerisation, two to three millimetre segments were sawed from the stented area of each vessel at the proximal end, mid portion and distal end of each stent for a total of three sections. These segments were ground to a thickness of 43-50 microns using Exakt Linear Grinding technology. Ground sections were polished and stained with Toluidine Blue and Basic Fuchsin stains. Light microscopic examination included note of inflammation, neointimal formation, vessel wall injury and endothelial coverage. Endothelial coverage was semi-quantified and expressed as the percentage of the lumen circumference covered by endothelium. Morphometric measurements were performed using the morphometric software (IP Lab for Mac OS X; Scanalytics, Rockville, MD, USA). The cross-sectional areas (external elastic lamina [EEL], internal elastic lamina [IEL] and lumen) of each section were measured. Neointimal thickness was measured as the distance from the inner surface of the stent struts to the luminal border. The neointimal area (NA), the media area and % stenosis were calculated. Ordinal data collected on each stent section included fibrin deposition, strut-associated giant cells and haemorrhage around the stent struts and were expressed as a percentage of the total number of struts in each section. An overall vessel wall injury, neointimal inflammation and fibrin value were scored for each section10-12.
Statistical analysis
Statistical analysis on angiographic data was performed using MATLAB software (R2008A; The MathWorks, Inc., Natick, MA, USA).Statistical analysis on pathology data was performed using JMP Statistical Software (Version 5.0; SAS Institute, Cary, NC, USA) for Macintosh OS X. All parameters were found to be normally distributed by the Lilliefors test and homoscedastic by Levene’s test.One-way ANOVA was performed to determine within-group differences. Upon detection of a difference, comparisons between test subjects and the control were performed using Dunnett’s test, and between test subjects using the Tukey-Kramer method. Ordinal score data, including injury, fibrin, neointimal and adventitial inflammation were compared using a Kruskal-Wallis test. If a significant omnibus effect was seen for any scored parameter, a Mann-Whitney test was then used to compare between each group, with a value of p<0.05/3 (i.e., Bonferroni correction was used) per pair-wise comparison considered statistically significant. Values are expressed as mean±standard deviation.
Results
Quantitative angiographic analysis
A summary of QVA results are shown in Table 1. Baseline overstretch ratio and reference vessel diameters at baseline, post-stent and at 30 days were not significantly different between treatments. Omnibus testing did not detect any effect of treatment on %DS (p=0.21), but did detect an effect of treatment on late lumen loss (p=0.04). Pair-wise comparisons detected a difference between PCBx2 and CCBx1 (p<0.05 by Dunnett’s test), corresponding to a 58% reduction in lumen loss in PCBx2 relative to CCBx1 (Figure1, sample angiogram in Figure 2).
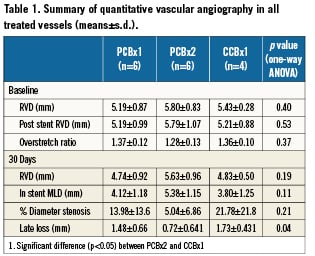
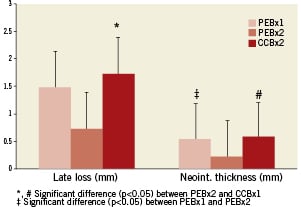
Figure 1. Comparison of the angiographic and histologic parameters representing device effectiveness.
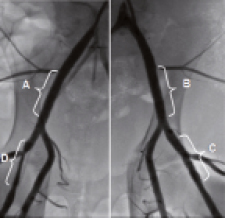
Figure 2. Representative images of iliofemoral arteries assessed by angiography at 30 days: A. Right external iliac – PCBx2+stent.; B.Left external iliac artery – PCBx1+stent.; C. Left superficial artery – PCBx2+stent; D. Right superficial artery – CCBx1+stent
Histology analysis
Histomorphometric analysis (summarised in Table 2) detected that the PCBx2 group significantly reduced neointimal area relative to PCBx1 and CCBx1 (by 30% and 35%, respectively; p=0.02). Similarly, percent area stenosis in the PCBx2 group was reduced by 45% as compared to CCBx1 and PCBx1 (p=0.04). The results of the histological comparison of vessel injury and healing as well as inflammatory response are presented in Table 3. Injury scores were similar among all studied groups. There were no significant differences in any of the scored fibrin parameters between the groups. Inflammation was mild overall with no significant differences between the groups. Adventitial inflammation was minimal in all groups. Luminal surfaces were largely covered with endothelium with all groups (>90% coverage). Notably, granulomas and giant cells were not observed in the groups employing PCB technology (Figure 3).
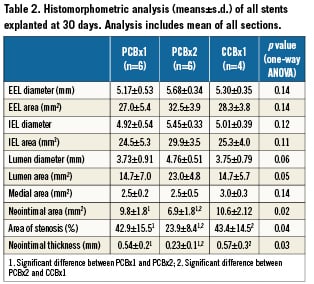
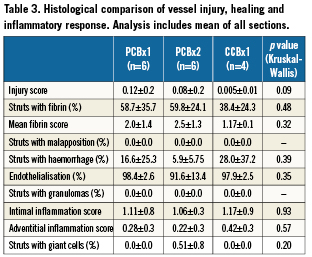
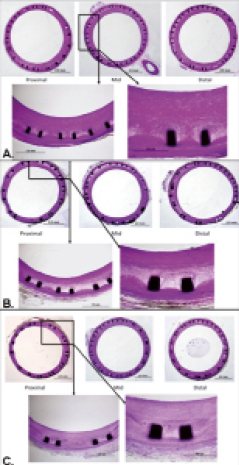
Figure 3. Representative microscopic sections (CVPath Institute, Inc.) of the vessels treated with CCBx1 (A), PCBx1 (B) and PCBx2 (C) [Basic Fuchsin stains].
Discussion
Drug coated balloons are emerging as a promising technology for the treatment of peripheral atherosclerotic vascular disease. A specific balloon technology based on paclitaxel-iopromide formulation has been demonstrated to be effective in reducing clinical restenosis in the peripheral vascular territory7,8. However, the use of self-expanding metallic stents in conjunction with PCB has not been sufficiently explored in the limited clinical experience. Until the present study, the biological effects of the complementary use of both technologies in peripheral vasculature have not been described in current literature at the pre-clinical level. The present study design mimics a frequent clinical situation in which stent placement follows balloon angioplasty in a relevant vascular territory to secure acute angiographic patency. By using the model of iliofemoral injury of healthy domestic swine we assessed the efficacy and local tissue effects of PCB catheters when followed by stent implantation. Although the historical data in domestic swine for the evaluation of peripheral stents are not as abundant as for the coronary stents, we chose the ilio-femoral territory, as the size, distribution and morphology is very similar to those of human vessels. More-over, this vascular territory seems to have a histological composition comparable to human peripheral arteries13.
Antirestenotic efficacy
The results of our study follow previous experimental observations by Albrecht et al14, in which bare metal stents mounted on balloons coated with contrast medium and paclitaxel (in doses of 1.2 and 1.8µg/mm2) reduced neointimal formation after five weeks follow-up compared to stents crimped on uncoated balloons in porcine peripheral arteries (superficial femoral and popliteal arteries). However, in the Albrecht’s study no histological analysis has been done and their conclusions were derived solely from angiographic results (angiographic LLL; 0.9 and 0.7mm for PEB versus 1.6mm for stents crimped on uncoated balloons; p<0.05).
In this study, the use of a PCB containing paclitaxel in a dose of 3µg/mm2 led to a significant reduction of the neointima proliferation. The PCBx2 displayed significantly reduced LLL as assessed by angiography and neointimal area as assessed by histomorphometry compared with CCBx1 and PCBx1. This is an important finding as the dose required to overcome the injury caused by the permanent stent prosthesis may be higher than the dose required to decrease restenosis, when PCB is applied in segments with a single injury from angioplasty balloons only. The lack of efficacy observed in the PCBx1 group may be explained by a more extensive and prolonged proliferative response of the vessel after stent implantation necessitating higher drug dose to sufficiently inhibit neointimal proliferation15,16. However, this theory comes only from experimental data which may not translate into clinical practice, and may be influenced by specific proliferative response pattern inherent to juvenile normal porcine vasculature.
The concept of drug delivery from a balloon surface in the treatment of femoro-popliteal disease has been already shown in clinical studies. The first published randomised peripheral study (THUNDER Trial) using Paccocath technology included 154 patients with de novo stenosis or occlusion of a femoro-popliteal artery8. At six months, PCB catheters significantly reduced late lumen loss and target lesion revascularisations as compared to control uncoated balloons. The second published trial (FemPac Trial) using Paccocath technology included 87 patients with femoro-popliteal disease who were randomised to PCB versus conventional balloon angioplasty7. The trial also demonstrated significant reductions in LLL and TLR rates after six months. However, in both of these studies the effects of additional stent implantation following balloon based drug delivery were not primarily analysed. In fact, in both these studies approximately 11% of patients needed bail-out stenting due to sub-optimal results from angioplasty alone. Clearly, we believe our results shed some light in regard to the potential of combining both technologies and indicate that the effective dose required for bail-out stenting might be higher than the dose required for balloon angioplasty only.
Local tissue effects
The mean injury scores were minimal and not significantly different between treatments indicating homogeneity of the methodology of the stent implantation procedure and similar biocompatibility of the tested technology. This also shows that the initial drug transfer does not particularly affect the patterns of vascular healing following stent implantation. This is in contrast to drug eluting stent technology in which the injury score can be influenced not only by mechanical injury but also by drug toxicity due to its prolonged release kinetics and the inflammation that can be caused by the polymer17. In the present study, the biocompatibility of the PCB technology is also confirmed by the low inflammation scores (almost identical in all tested groups), the lack of granulomas, and giant cells. In addition, the stent luminal surfaces of all tested groups were similar and largely covered with endothelium (>90% coverage). Fibrin depositions, typical for paclitaxel presence in the tissue17,18 were not significantly different between the groups. This probably reflects the biological effect of the relatively short time of paclitaxel transfer because by 30 days most of the fibrin deposits were already absorbed. By contrast, in the studies with paclitaxel eluting stents, peri-strut fibrin deposits are commonly found even beyond 90 days and far exceed those that can be found in the bare metal stent control groups. This is a hallmark of delayed vessel healing18,19 which can act as a substrate for excessive neointimal formation at later time points20. Therefore, it is possible, that by accelerating the process of paclitaxel absorption, the process of vascular healing could be improved following stent implantation.
Study limitations
The main limitation is a small number of animals. For this reason the efficacy signals observed in our pilot study should be more precisely scrutinised in larger studies. Although healthy domestic swine are a well-established model for understanding the safety of interventional coronary devices, their utility for predicting device efficacy, especially in the peripheral vascular territory is not well established13,21. This study focused in assessing the short-term efficacy and the local tissue effects, thus long-term effects are currently not available. In the present study we did not confirm the pharmacokinetic behaviour of paclitaxel in the presence of a stent following PCB delivery. However, similar data have already been published for the PCB technology22. Also, there is a lack of a negative control group consisting of uncoated balloons. However, several studies performed by our group in the same vascular territory have shown comparable results to what has been presented here within the CCBx1 group (data not published). Also, while study was positive for the PCBx2, it is possible that the PCBx1 would also have been effective with larger sample sizes. Furthermore, this pilot study design does not allow assessing the relative contribution of the increased drug dose in the PCBx2 group versus the effect of repeated balloon inflation (the repeated balloon inflation may also have helped more drug to penetrate into the arterial wall).
Conclusion
Paclitaxel delivery into iliofemoral arteries of the normal swine using a PCB catheter followed by nitinol bare metal stent implantation effectively decreased neointimal proliferation when compared to the CCBx1 group. More extensive and prolonged proliferative response of the vessel wall after self-expanding stent implantation (necessitating higher drug dose) could explain the lack of effect of the PCBx1 catheters in the normal porcine peripheral vessels. After 30days, all parameters of vessel wall healing following drug coated balloon treatment were comparable to the control group, indicating acceptable biocompatibility. This novel approach when used in clinical practice may increase long-term patency post endovascular interventions in the superficial femoral artery, however further clinical investigation is necessary.
Conflict of interest statement
MEDRAD Interventional/Possis (Indianola, PA, USA) provided financial support and interventional devices for this study. Mark Stenoien is a full-time employee of MEDRAD Interventional/Possis. Krzysztof Milewski, Renu Virmani, Juan F. Granada and Greg L. Kaluza have performed contract research services for MEDRAD Interventional/Possis during the conduct of this study. The other authors have no conflict of interest to declare.

