- peripheral artery obstructive disease
- drug-eluting balloon
- restenosis
- angioplasty
Abstract
Aims: To evaluate safety and results of the Freepac™ drug-eluting balloon (DEB) technology for the treatment of chronic femoropopliteal steno-occlusions.
Methods and results: In a multicentre registry we enrolled 66 patients with symptomatic femoropopliteal stenosis and/or occlusion <15 cm (Rutherford stages 2 to 4). Patients were treated first with an undersized uncoated balloon and then with an appropriate sized DEB. In case of unsatisfactory results, nitinol stents were implanted. Clinical evaluations and echo-duplex were performed at baseline, at discharge, and at three months after intervention. Procedural success was 100%. Stents were implanted in 10.8% of the patients. At three months follow-up, the mean ankle-brachial index (ABI) significantly improved from 0.58±0.13 to 0.82±0.25, thus resulting in a Rutherford class improvement (p <0.01) and amelioration of the claudication distance (102±87 vs. 403±160 meters) (p<0.001). No serious adverse events occurred during the follow-up.
Conclusions: The results of this registry demonstrate the safety of mediated paclitaxel elution for the prevention of restenosis in the superficial femoral and popliteal arteries after angioplasty. This technique was associated with encouraging results in terms of clinical improvement as well as a lack of adverse events, including target lesion revascularisation, at three months.
Introduction
Endovascular techniques have improved during the last decade becoming reliable alternatives to open surgery for revascularisation of patients with peripheral artery obstructive disease (PAOD). Percutaneous transluminal angioplasty for recanalisation of the superficial femoral artery has a satisfactory initial technical success rate of more than 95%1. However, the rate of restenosis after superficial femoral artery (SFA) angioplasty and/or direct stent implantation remains high compared with other vascular districts. After one year, restenosis occurs at least in 40% to 60% of the treated vessels2-4. The patency rates in the SFA ranges between 22% and 61% after bare metal stent implantation1. The beneficial effect of stenting for preventing restenosis seen in the coronary circulation has to be confirmed in the infra-inguinal vessels4-7. Furthermore, clinical trials with drug-eluting stents also failed to indicate restenosis inhibition in the SFA8,9. The anti-cancer agent paclitaxel (PTX) is an effective anti-restenosis agent on drug-eluting stents, primarily due to growth inhibition of coronary artery smooth muscle cells across a wide dose range. Paclitaxel was identified as the primary drug for DEB because of its rapid intracellular uptake, lipophilic characteristics , and prolonged retention. This new promising technology for peripheral angioplasty may have the following advantages: the homogeneous drug transfer to long segments of arterial wall during a single long inflation; the balloon catheter delivers a short burst dose of paclitaxel that linearly decreases within 72 hours; the secondary increase of intratissue levels of the drug that persists up to 28 days10. Recent studies on DEB use for SFA percutaneous transluminal angioplasty (PTA) shows very encouraging results on the reduction of late lumen loss and restenosis11,12.
The purpose of this multicentre, prospective registry was to assess safety and efficacy of the Freepac paclitaxel Eluting Balloon (DEB) technology for the treatment of chronic steno-occlusions of the SFA. Furthermore, the impact of the DEB technology in reducing the number of implanted stents after SFA angioplasty was evaluated.
Methods
The study is a prospective, multicentre registry performed at six Italian centres – Maria Eleonora Hospital (Palermo), Montevergine Clinic (Mercogliano), Maria Cecilia Hospital (Cotignola), Villa Azzurra (Rapallo), Citta’ di Lecce (Lecce), Anthea Hospital (Bari)– with the intention to investigate the safety and efficacy of the IN.PACT Admiral DEB (Medtronic-Invatec, Roncadelle [BS], Italy) on restenosis after angioplasty of stenotic or occluded superficial femoral or popliteal arteries. Furthermore, the number of implanted stents has been considered as well.
Population
Eligible patients were older than 18 years and had symptomatic peripheral artery disease (Rutherford class 2 to 4). Angiographic inclusion criteria were:
– SFA target vessel with reference diameter between 3 and 7 mm;
– lesion and/or occlusion <15cm (a maximal length of 15 cm has been included in the study due to the fact that at the study beginning the longest DEB available was 8 cm and investigators chose to use no more than two DEBs. Long diffuse stenoses or multiple adjacent lesions or occlusions with cumulative length of less than 15cm were cumulatively considered and treated as a single lesion);
– adequate run-off with evidence of at least one patent crural vessel to the foot either pre-existing or successfully established, prior the target SFA, by below the knee artery angioplasty.
– de novo lesions and restenoses reducing at least 70% of vessel diameter in the superficial femoral artery, the popliteal artery (including the first and the second segment) entered the study.
Exclusion criteria included in-stent restenosis, poor inflow, absence of at least one patent crural artery, duration of symptoms less than two weeks, pregnancy, life expectancy of less than one year, and contraindications to required medication, creatinine clearance <30ml/min.
All patients gave written informed consent. The study treatment was not different from the standard care of the institution involved in the study.
Technology
The IN.PACT Admiral is a 0.035” paclitaxel-eluting over-the-wire (OTW) peripheral balloon catheter, specifically designed for PTA in atherosclerotic obstructed femoropopliteal vessels.
Applied to the balloon surface is a proprietary hydrophilic drug-coating formulation called Freepac, which allows for several functions:
– separates paclitaxel molecules
– balances hydrophilic and lipophilic proprieties
– facilitates paclitaxel elution into the vessel wall
Paclitaxel, is an anti proliferative drug whose safety and effectiveness has been shown in thousands of patients, can be given intravenously in amounts of up to 300mg. The maximum amount on a balloon (6x120mm) is 8mg. Freepac uses urea as a hydrophilic spacer. Urea is naturally produced by the body, synthesised in the liver (18-35g per day) and used by the body to detoxify and excrete nitrogen derived from proteins. The maximum amount of urea on a balloon is 1.1mg, 20,000 times less than what the body produces in a single day.
All these components work when the balloon contacts the vessel wall (30 sec) and the drug is released into the vessel wall during the inflation. This allows an effective transfer of drug controlled by how the drug is loaded on the balloon, that inhibits tissue growth within the artery, afactor that leads to the re-narrowing of the arteries.
During the inflation of the balloon, the drug is almost completely released13. Experiments in animals show that 10% to 20% of the drug is taken up by the vessel wall.
Procedure
All patients underwent an initial, selectively performed diagnostic angiography of the target leg to confirm their candidacy for the study.
After placement of the sheath in the common femoral artery, all patients received an initial bolus of 5,000 IU heparin. The intervention was performed according to the usual procedure, preferably with crossover access. After successful crossing of the lesion with a conventional guidewire (in most of the cases a J-shaped hydrophilic 0.0035” guidewire was used, alternatively a 0.018’’ or a 0.014’’ guidewire was used depending on lesion characteristics), the lesions were treated with an undersized uncoated balloons (1 mm smaller in diameter than the indicated DEB) using a 2minute long inflation at 6-12 atm. After a control angiography, the appropriate size and length of the DEB was chosen referring to a ruler located behind the patient’s leg. The DEB was gently handled in order not to damage the balloon surface. The SFA was dilated 10 mm beyond both ends of the total lesion length, and sizing of the balloon was 1:1.1 vessel-balloon ratio. In case requiring two DEBs, a 1 cm overlapping area was considered optimal. The inflation time was 3 minutes at 6-12 atm. All study balloons were inflated only once. If angiography after the DEB inflation showed residual stenosis of more than 30%, a long inflation with a conventional non-study balloon was repeated. In case of unsatisfactory results, due to refractory suboptimal results after multiple and prolonged inflations or to flow limiting dissection, self-expanding nitinol stents were implanted.
Patients not already taking aspirin and clopidogrel were administered a loading dose of 300 mg of each drug 12 hours before the procedure. All patients received aspirin (100 mg daily) indefinitely and clopidogrel (75 mg daily) for four weeks after the intervention.
Follow-up and endpoints
Clinical evaluations were performed at baseline, at discharge and at three months after intervention. The evaluations included evaluation of the Rutherford class, measurement of the ankle–brachial index, clinical laboratory testing and assessment of adverse events as well as measurement of free walking interval. Echo-duplex evaluation consisted of a biplane imaging of treated SFA segment and colour Doppler assessment of the restenosis degree, if present. All duplex scans were assessed in a blinded fashion by an independent operator. The primary endpoint was the restenosis rate (defined as incidence of stenosis >50%) in the treated lesion, target lesion revascularisation (TLR), target vessel revascularisation (TVR), and amputation. Secondary endpoints were changes in mean ankle-brachial index and Rutherford class at baseline and 3-month visit. Safety endpoints included thrombotic complications of the target vessel and clinical adverse events.
Statistical analysis
Descriptive statistics
Categorical variables were represented as frequencies and percentage and continuous variables with mean and standard deviation. Age was represented with median and 25th, 75th percentiles.
Inferential statistics
The baseline data are compared across the study using the Kolmogorov-Smirnov (and re-tested with Wilcoxon-Mann-Whitney) for Rutherford classification and T-test for the three peak systolic velocities.
Results
Patient and lesion characteristics
Patient enrolment began in September 2009 and ended in March 2010. The study centres enrolled 66 patients (74 lesions) for de novo or recurrent lesions (Table1). Population demographics are presented in Table 1.
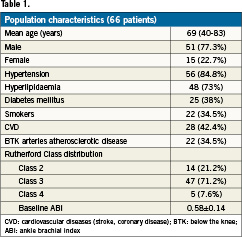
The vast majority of the patients had a Rutherford class 3 (71.2%) before the intervention, and the mean ankle–brachial index was 0.58±0.14. Lesion characteristics are presented in Table2.
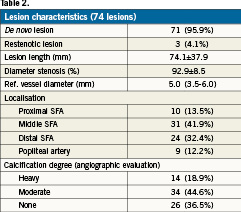
Angioplasty
The procedural data are displayed in Table3. Puncture of the contralateral common femoral artery was the default strategy, followed by placement of a crossover sheath ending in the external iliac artery or common femoral artery of the leg to be treated.
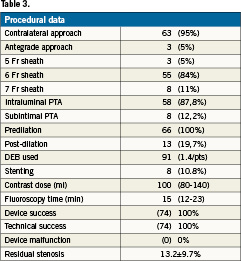
Procedural success — defined as completion of the procedure with less than 30% residual stenosis of the target lesion (after prolonged dilatation and stenting, if necessary) — was estimated by the investigators and achieved in 100% of the cases.
Technical success, defined as completion of the procedure with less than 30% residual stenosis of the target lesion without adjunctive treatment, was achieved in 89.2% of the cases. Self-expanding nitinol stents were implanted in only 10.8% of the patients because of vessel wall recoil or a flow limiting dissection.
The average residual stenosis at the end of the intervention was 13.2±9.7% (Table 3). Lesions longer than 8 cm could be treated by using a maximum of two coated balloons including an overlapping areas. Ninety-one DEBs were used, with a per patient DEB usage of 1.4.
No difference in terms of study adverse events were observed among patients treated with one or more DEBs.
No technical problems or adverse events –assessed as at least possibly related to the drug coating– were observed during in-hospital stay.
Clinical follow-up
Sixty-five patients (73 lesions) completed clinical follow-up assessment at three months.
The overall mean ankle-brachial index improved from 0.58±0.13 before the intervention, to 0.82±0.25 at 3-month follow-up (p <0.01) (Figure 1), thus resulting in a Rutherford class redistribution, significantly improved in all the patients (p <0.01) (Figure 2) and a significant amelioration of the claudication distance (102±87 vs. 403±160 meters; p < 0.001) (Figure 3).
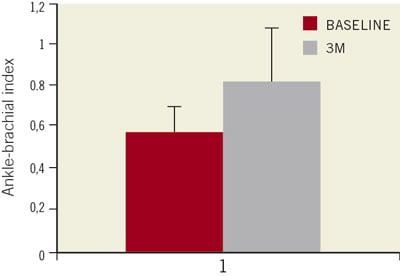
Figure 1. Ankle-brachial index (ABI) improved significantly at three month follow-up in treated patients with respect to the baseline value (p<0.001). Values are displayed as mean±standard deviation(SD)
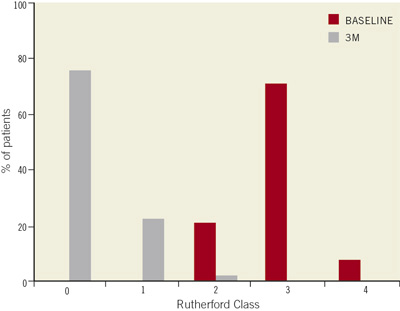
Figure 2. At baseline most of the patients were in Rutherford class 3. At three month follow-up, most of them shifted to class 0 or 1.
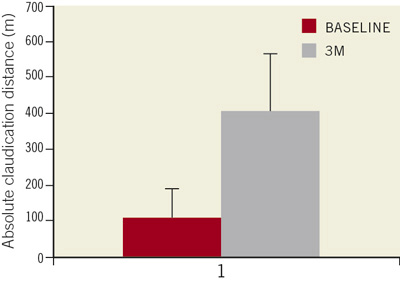
Figure 3. Absolute claudication distance significantly improved from 102±87 meters at baseline to 403±160 meters at three month follow-up (p<001). Values are displayed as mean±standard deviation.
No serious adverse events (death, amputation) occurred during the follow-up. No clinical, haematologic, or biochemical results suggested systemic effects of paclitaxel over the follow-up period.
During the three months of follow-up no TLR was detected. In the eight patients requiring bail-out stenting, no reocclusion or restenosis occurred. All the patients, but one, presented for the scheduled follow-up at three months.
Discussion
In the present registry, the safety and efficacy of a new drug-coated balloon catheter for the prevention of restenosis after treatment of atherosclerotic femoropopliteal lesions was evaluated.
The results of this registry demonstrate:
– Safety of a DEB mediated by local paclitaxel elution for the prevention of restenosis in the superficial femoral and popliteal arteries after PTA.
– The use of paclitaxel-coated angioplasty balloons was associated with encouraging results in terms of clinical improvement a and lack of adverse events, including TLR, at three months.
– A very limited use of stents (10.8%).
The beneficial effect of stenting for preventing restenosis seen in the coronary circulation has not been consistently and definitively showed in the infra-inguinal vessels4-7. Cell culture experiments show that a brief contact between vascular smooth cells and lipophilic taxane compounds is sufficient to inhibit proliferation of the cells for a long period14. Current concepts of the prevention and therapy of restenosis after angioplasty or vascular stenting are based on sustained antiproliferative drug release into the vessel wall. DES showed an adjunctive benefit in term of restenosis reduction in coronary circulation. Conversely, clinical trials failed to indicate restenosis inhibition in the SFA8,9.
The use of eluting balloons is an alternative method of drug delivery using standard angioplasty catheters as carriers. Whereas stents have a number of drawbacks, including the risk of fracture and induction of local inflammation in the long run, the concept of balloon-mediated short-time contact, offers the chance to combine drug administration for the prevention of restenosis and optional stent placement if needed for lesion stabilisation. A preclinical study with paclitaxel-coated balloons in coronary and peripheral arteries in swine indicated that a high concentration of the drug significantly reduces neointimal proliferation, even when there is only a short exposure of the vessel wall to the drug14.
With the coated-balloon approach, the vessel can be left in its native state if effective dilatation can be achieved without stent implantation. The efficacy of paclitaxel-coated balloons in preventing restenosis of the superficial femoral and popliteal arteries has been reported in recent studies using the Paccocath technology11.
In the Local Taxan with Short Exposure for Reduction of Restenosis in Distal Arteries (THUNDER) multicentre trial, Tepe et al11 showed the efficacy of DEB over standard balloon angioplasty for SFA PTA. At six months, the mean late lumen loss, the primary endpoint, was significantly less with paclitaxel-coated catheters as compared with control or contrast medium groups (p<0.001). The rate of revascularisation of target lesions at six months was significantly lower in paclitaxel-coated balloon angioplasty group than in control groups.
In the Femoral Paclitaxel (FemPac) pilot trial, Werk et al12 randomly assigned 87 patients with femoropopliteal peripheral artery disease to uncoated or paclitaxel-coated catheters.
The primary endpoint of angiographic late lumen loss at six months was significantly reduced in patients treated with the paclitaxel-coated balloon (31 of 45). Patients treated with the paclitaxel-coated balloon also had a significant reduction in TLR at six months, which was maintained up to 24 months.
In our study, the paclitaxel-coated balloons used were standard angioplasty balloons covered with FreePac, a proprietary coating that balances hydrophilic and lipophilic properties and allows paclitaxel to adhere to the balloon until it is expanded.
The use of paclitaxel-coated angioplasty balloons was associated with no significant restenosis, as detected by duplex scan. No TLR occurred. The number of stents implanted for refractory recoil or a flow limiting dissection was very low, and no reocclusion was noticed in stented population at three month follow-up. There was asignificant clinical benefit in patient populations, as clearly demonstrated by the improvement in free walking distance, ABI, and the reduction in Rutherford class.
The development of thrombosis as a result of vessel injury or delayed endothelialisation is a recognised risk of percutaneous intervention, especially when antiproliferative agents, such as paclitaxel, are used to prevent restenosis. In the present registry, thienopyridine was given for four weeks. The absence of thrombosis events following thienopyridines discontinuation demonstrates safety of short-term dual antiplatelet therapy after a DEB mediated intervention.
No adverse events occurred that could be classified as possibly related to the study drug. This was true even when the operator had to use multiple drug-eluting balloons and the total dose of paclitaxel was significantly higher. Longer follow-up based reports are necessary to demonstrate the efficacy of these techniques.
The limitation of the present study is its short-term follow-up which does not allow it to have conclusive results about middle- and long- term DEB efficacy, but still demonstrates safety as well as encouraging acute results of this new paclitaxel-eluting balloon.
Nevertheless, these data have to be confirmed in a larger randomised clinical trial, and will form the basis to calculate the appropriate power of such a trial.
Conflict of interest statement
The authors have no conflicts of interest to report.
References

