
Introduction
Multiple endovascular technologies have been evaluated as stand-alone or combined therapies for atherosclerotic disease of the femoropopliteal arteries1. Amongst others, balloon catheters coated with the taxol derivative paclitaxel, a highly lipophilic antiproliferative drug, have been associated with superior efficacy compared to plain balloons in the treatment of de novo and restenotic lesions of the femoropopliteal arteries2.
Despite the large number of DCB platforms currently available on the market, little is known regarding their comparative effectiveness in preventing the risk of repeat revascularisation. Against this background, we performed a meta-analysis of randomised trials comparing DCB versus plain balloon angioplasty, and an indirect comparison of DCB platforms grouped by paclitaxel density.
Methods
We updated the literature search from a previous systematic review2. The final search was performed in May 2018. Randomised trials comparing DCB versus plain balloon angioplasty as intended stand-alone therapies for de novo or restenotic disease of the femoropopliteal arteries were included, without restriction of language or publication status. Aggregated data from selected studies were analysed according to the intention-to-treat principle. The primary outcome was target lesion revascularisation (TLR) at 12-month follow-up. For the pairwise meta-analysis, the risk ratio (RR) with 95% confidence intervals (95% CI) was obtained using the Mantel-Haenszel fixed-effects model and the Hartung-Knapp random-effects model, by grouping DCB platforms according to paclitaxel density (2, 3 and 3.5 µg per mm2 of balloon surface, respectively). We accounted for the dependency of the subgroups of trials in the total effect by calculating the risk for TLR with the robust variance estimation method (package robumeta). A χ2 test for subgroup by treatment interaction was used to evaluate the difference in treatment effect across subgroups. The I2 statistic3 was used to quantify the heterogeneity between trials: values around 25%, 50% and 75% were suggested to indicate low, moderate or high heterogeneity, respectively. In addition, we estimated the between-study variance (τ2) and the 95% prediction interval of the pooled estimate4. Publication bias was investigated according to Peters et al5. For the adjusted indirect comparison, subgroups of DCB platforms were compared assuming the plain balloon angioplasty arm as the common (adjusted) comparator. According to the method proposed by Song6 and Bucher7 we generated RRs (95% CI) and z scores for DCB with lower versus higher paclitaxel densities, finally providing the relative p-values. A requirement of adjusted indirect comparison is that compared trials are similar: for this reason, a random-effects meta-regression analysis tested the interaction of relevant baseline features of DCB-treated patients (proportion of diabetes, critical limb ischaemia and occluded vessels, lesion length and vessel size) and DCB generation (early or “PACCOCATH-like”8 versus new) with the observed treatment effect. All analyses were performed using R, version 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria).
Results
A total of 20 randomised trials with 3,038 participants were studied. Follow-up data up to 12 months were available for 1,723 patients allocated to DCB and 1,158 patients allocated to plain balloon angioplasty. A summary of trials, main characteristics of participants and description of DCB platforms are reported in Supplementary Table 1.
TLR occurred in 498 patients (17.2%) (Figure 1A). The risk for TLR was significantly reduced in patients treated with DCB versus plain balloon angioplasty (9.1% versus 29.4%; RR [95% CI]: 0.33 [0.25; 0.45], p<0.01). The results did not change after accounting for the dependency of the subgroups of trials in the total effect (0.33 [0.24; 0.43], p<0.01). Although the 95% prediction interval remained below the null (0.13; 0.85), there was evidence of high heterogeneity (I2=54%, p<0.01) due to a significant difference in treatment effect within subgroups, unrelated to publication bias (p=0.69) (Figure 1B). Similarly, there was no modification of the risk estimate for TLR according to the proportion of diabetes (p=0.79), critical limb ischaemia (p=0.83), occlusive lesions (p=0.74), lesion length (p=0.83), vessel size (p=0.97) or DCB generation (p=0.14) across treated patients. In contrast, at indirect comparison (Figure 1C), the risk for TLR related to DCB platforms with a paclitaxel density of 2 µg/mm2 was significantly higher as compared to platforms with 3.5 µg/mm2 (2.54 [1.23; 5.22], p=0.011) and trended higher as compared to platforms with 3 µg/mm2 (1.86 [0.94; 3.71], p=0.077). There was no difference in treatment effect across DCB platforms with a paclitaxel density of 2 µg/mm2 (p=0.14). DCB platforms with paclitaxel density of 3 µg/mm2 or 3.5 µg/mm2 displayed a comparable risk for TLR (1.36 [0.68; 2.71], p=0.38).
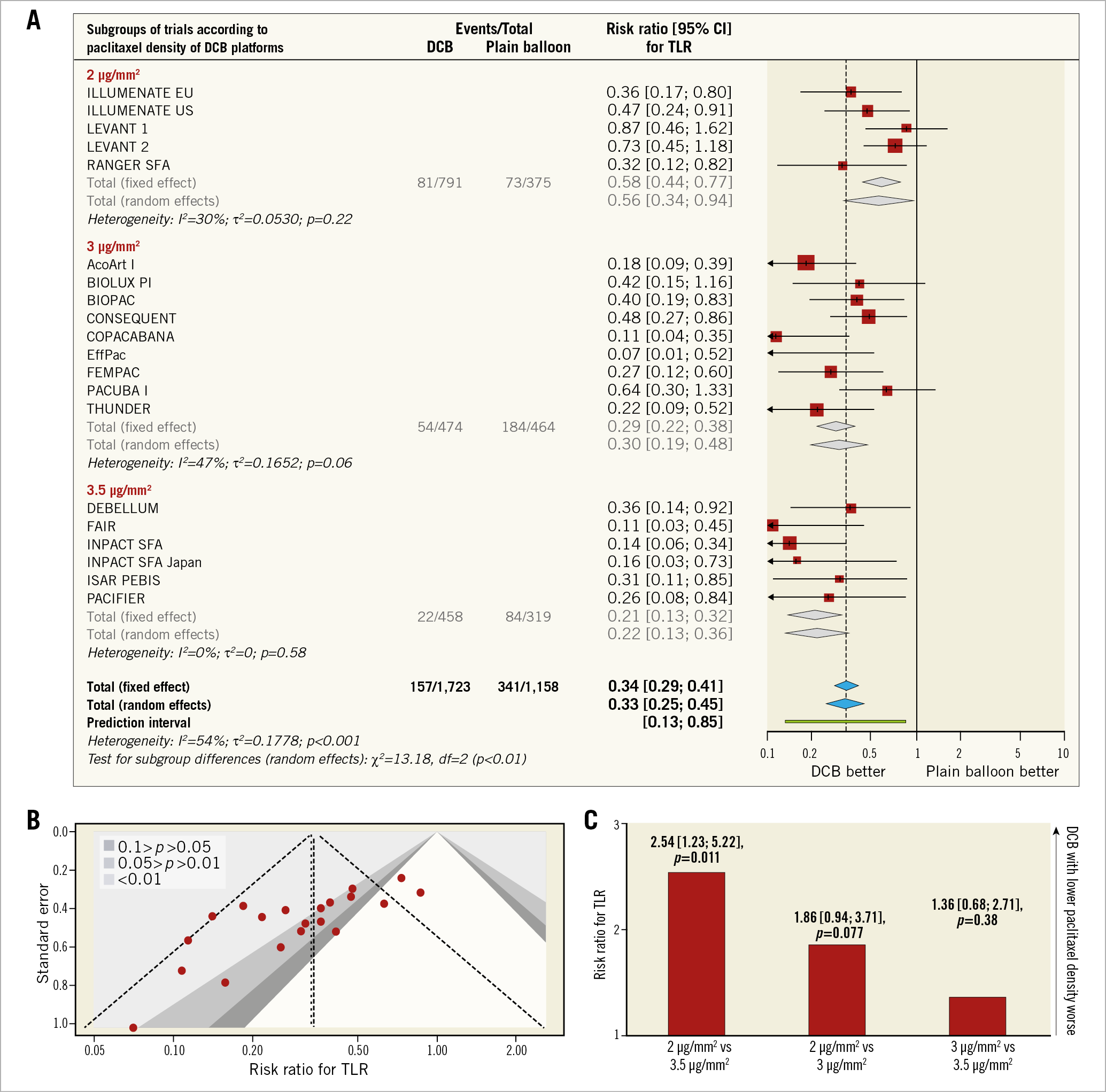
Figure 1. Plots of target lesion revascularisation with DCB versus plain balloon angioplasty. A) Risk estimates for target lesion revascularisation (TLR) with DCB versus plain balloon angioplasty. Forest plots of risk ratios for TLR associated with DCB versus plain balloon angioplasty. The squares and the diamonds indicate the point estimates and the left and the right ends of the line the 95% confidence intervals. B) Contour-enhanced funnel plot for TLR with DCB versus plain balloon angioplasty. Contours are plotted for a range of significance levels defined by p-values of 0.01, 0.05, and 0.1. C) Risk estimates for TLR at adjusted indirect comparison of DCB platforms with different paclitaxel density. Risk ratios (bar graphs display the point estimate) for TLR associated with DCB platforms grouped by paclitaxel density.
Discussion
This is the largest meta-analysis of randomised trials comparing DCB and plain balloon angioplasty for femoropopliteal artery disease performed so far. In addition, this study presents the first indirect comparison of DCB platforms grouped by paclitaxel density.
The comparative clinical efficacy of DCB platforms available on the market has yet to be investigated. On the one hand, this meta-analysis lends support to a gradient in the magnitude of clinical efficacy of DCB angioplasty in patients with femoropopliteal artery disease. In fact, DCB versus plain balloon angioplasty was associated with a relative reduction in the risk of TLR of between 78% and 44%. At indirect comparison, DCB platforms with lower paclitaxel density had up to a 2.5-fold increase in the risk of TLR as compared to DCB with higher paclitaxel densities. On the other hand, this analysis remains hypothesis-generating. Indeed, despite the lack of statistical interaction between the observed treatment effect and baselines features of participants, some heterogeneity persisted within DCB groups, suggesting that factors other than paclitaxel density affect the risk for TLR.
However, the results of this study have important clinical implications for the design of future trials. Indeed, it has become evident that plain balloon angioplasty represents a weak comparator to provide a reliable measure of effectiveness of DCB therapy. In this respect, head-to-head comparisons powered for clinical outcomes between different DCBs are urgently needed to obtain a better insight into the clinical performance of these devices.
Study limitations
First, this meta-analysis relies on aggregate data. A meta-analysis based on individual participant data would provide better evidence for the comparative efficacy of different DCB platforms in specific subgroups of patients. Second, we cannot exclude that unmeasured confounders are responsible for the gradient of efficacy of DCB platforms observed in the present study. Finally, a 12-month follow-up is certainly inadequate to address the durability of treatment effect associated with the different DCB platforms included in this report.
Conclusions
In patients with femoropopliteal artery disease, DCB angioplasty reduces the risk of repeat revascularisation at one year as compared to plain balloon angioplasty, with evidence of a gradient of clinical efficacy across DCB platforms depending on paclitaxel density.
|
Impact on daily practice In patients with femoropopliteal artery disease, drug-coated balloon angioplasty reduces the risk of repeat revascularisation at one year as compared to plain balloon angioplasty. Our study lends support to a gradient of clinical efficacy across DCB platforms depending on paclitaxel density. The comparative efficacy of different DCB platforms should be tested in head-to-head comparisons powered for clinical outcomes. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

