Atherosclerotic lower extremity peripheral artery disease (PAD) is one of the leading causes of cardiovascular morbidity, with more than 230 million people affected worldwide1. The high prevalence of symptomatic disease has led to an increased focus on research and innovation aimed at improving procedural success and long-term outcomes among patients with PAD. Traditionally, endovascular treatment of lower extremity PAD consisted primarily of plain balloon angioplasty, which provided short-term results but was plagued by high long-term failure rates from restenosis. Drug-coated balloons (DCB) were conceived to address this issue, delivering antiproliferative agents directly to the arterial wall to limit neointimal proliferation, but without the requirement of a scaffold. Since their introduction almost a decade ago, DCB have become a cornerstone of the management of femoropopliteal PAD. While DCB have demonstrated their superiority over uncoated balloons in a number of randomised controlled trials (RCT)2345, patients with complex disease were often excluded. In real-world practice, however, a significant proportion of patients have diffuse disease with heavy calcification and prior stents, for whom data remain limited.
In this issue of EuroIntervention, Zeller et al present the five-year results of the IN.PACT Global Study, a prospective, single-arm, international study of the safety and efficacy of paclitaxel-coated balloons for obstructive femoropopliteal PAD6. The analysis included 946 patients (of the 1,406 patients in the original clinical cohort) with claudication or rest pain and builds upon previously presented results at 3 years7. The primary efficacy endpoint of freedom from clinically driven target lesion revascularisation (CD-TLR) was 69.4% at 5 years, an expected decrease compared with the 3-year results of 76.9%. Nonetheless, when taking into context the broader patient population that was included in this registry, overall freedom from CD-TLR at 5 years was within range of previously reported clinical trials (74.5% in the IN.PACT SFA trial4 and 79.2% in the THUNDER Trial5), and superior to comparator uncoated devices (Figure 1). Reflective of the more complex lesions included in the IN.PACT Global Study, the median lesion length was 12.1±9.5 cm (markedly longer than reported in prior randomised DCB trials), 18% of lesions had in-stent restenosis (ISR), and 10% had severe calcification. These features are all often excluded from pivotal trials. Other supportive data of the conclusions of the study include analyses demonstrating that ISR, severe calcification, and longer lesions were independently associated with an increased risk of CD-TLR, while larger reference vessels and lack of popliteal involvement were associated with a reduced risk. Importantly, rates of major adverse events, amputation, and all-cause death were high, but again comparable to previously reported data. Moreover, despite conflicting evidence surrounding the efficacy of DCB in women, this study did not find a difference in CD-TLR between men and women.
While the data presented are compelling and add to the collective evidence available for DCB, some limitations should be highlighted. Although complex calcified lesions may require the use of adjunctive atherectomy, this was not allowed in this study. Additionally, provisional stenting was used in 1 out of 5 patients. Despite not being independently associated with CD-TLR, the inclusion of this subset of patients adds a layer of complexity when interpreting the outcomes. The single-arm nature of the study also limits the ability to draw major conclusions. The authors reference previous trials and historical controls to provide some insights, but comparisons are limited due to the differences in populations studied, and conclusions about these differences are speculative. Furthermore, there is a significant proportion of missing data at 5 years (~15%), which is to be expected with a registry study, but may add bias to the reported findings.
The five-year results of the IN.PACT Global Study demonstrate that the IN.PACT Admiral paclitaxel drug-coated balloon (Medtronic) has acceptable safety and efficacy in real-world participants with complex femoropopliteal disease and help validate the findings from the IN.PACT SFA trial in a broader patient population. These data provide continued support for the first-line indication of DCB in femoropopliteal artery revascularisation. Moreover, real-world registries of PAD patients are essential to better understand long-term outcomes with novel therapies, particularly among underrepresented individuals, and the investigators and sponsor should be applauded for performing this study.
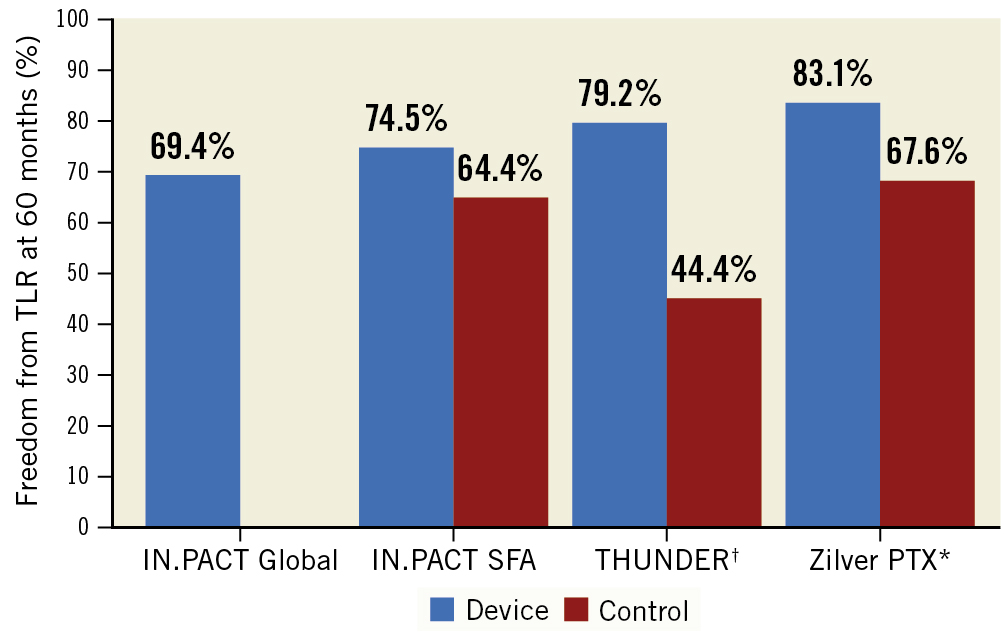
Figure 1. Freedom from clinically driven target lesion revascularisation at five years from femoropopliteal artery intervention studies. †CD-TLR not reported; results reported are TLR. * Randomised trial of paclitaxel-coated stent; results are from a per protocol population. SFA: superficial femoral artery; THUNDER: Local Taxan With Short Time Contact for Reduction of Restenosis in Distal Arteries; TLR: target lesion revascularisation
Conflict of interest statement
E. Secemsky has been a consultant for Abbott, Bayer, BD, Boston Scientific, Cook, CSI, Medtronic, Philips, and VentureMed; has been on the speakers bureau of BD, Boston Scientific, Cook, CSI, Inari, Medtronic, Philips, Shockwave, and VentureMed; is on the advisory board of Abbott, Bayer, BD, Boston Scientific, Cook, CSI, Medtronic, Philips, and VentureMed; and has received grants (to his institution) from NIH/NHLBI K23HL150290, the Food & Drug Administration, BD, Boston Scientific, Cook, CSI, Laminate Medical, Medtronic, and Philips. S. Korjian has no conflicts of interest to declare.

