Abstract
Peripheral arterial disease is the third leading cause of cardiovascular morbidity after coronary artery disease and stroke. Lower limb peripheral arterial disease commonly involves infrainguinal arteries, may impair walking ability (intermittent claudication) and may confer a significant risk of limb loss (chronic limb-threatening ischaemia), depending on the severity of ischaemia. Endovascular treatment has become the mainstay revascularisation option in both the femoropopliteal and the below-the-knee arterial segments. After crossing and preparing the lesion, treatment results in these arterial segments can be enhanced by using drug-coated devices (drug-eluting stents and drug-coated balloons) that mitigate the occurrence of restenosis. As for other medical devices, the use of drug-eluting devices is based on their demonstrated safety and efficacy profiles when applied in the distinct segments of the lower limb vasculature. In this state-of-the-art narrative review we provide an overview of the safety and efficacy of drug-coated devices when used in the femoropopliteal and below-the-knee arterial segments.
Peripheral arterial disease (PAD) leading to stenosis or occlusion of the arteries supplying the lower limbs is the third cause of cardiovascular morbidity after coronary artery disease and stroke1. The global numbers of prevalent cases and deaths due to PAD have risen consistently each year since 1990, resulting in a 2-fold increase to 113 million cases (95% uncertainty interval [UI]: 99.2 to 128 million cases) and 74,100 deaths (95% UI: 41,200 to 128,000 deaths) in 20192.
Over the past decade, endovascular repair of symptomatic lower limb PAD (LLPAD) has become the preferred and recommended treatment for most PAD patients34. Since the early 2000s, the self-expanding bare metal stent (BMS) platform has been the standard scaffolding technique in the femoropopliteal segment56. However, in-stent restenosis remains a major concern after BMS implantation, with in-stent restenosis rates ranging from 20% to 50%7. From this perspective, drug-eluting devices such as drug-eluting stents (DES) or drug-coated balloons (DCBs) represent an interesting emerging technology to prevent in-stent restenosis and to improve clinical, morphological and haemodynamic outcomes.
In 2018, Katsanos reported a significantly increased risk of death with the use of paclitaxel-coated devices8. Following Katsanos’ report, in 2019, the U.S. Food and Drug Administration (FDA) notified healthcare providers of the potential late mortality safety signal and recommended restrictions in use. Finally, in a new statement in July 2023, the FDA reversed this restriction regarding the use of paclitaxel-coated devices to treat lower limb PAD, as several publications of large datasets ruled out this indication9.
In this state-of-the-art narrative review, our aim is to provide a comprehensive overview of the safety of drug-coated devices and, furthermore, to summarise the main observed treatment benefits of drug-eluting devices when used in the femoropopliteal segment and in below-the-knee (BTK) arteries, in order to inform current treatment practice when using drug-coated devices during LLPAD revascularisation procedures.
Drug-eluting stents for the femoropopliteal segment
1. DRUG-ELUTING STENTS AND THE PATHOPHYSIOLOGY OF RESTENOSIS
Recent years have seen the development of different drug-eluting stents aimed at preventing femoropopliteal restenosis (Table 1). These devices have in common a platform (stent), a drug (paclitaxel or different -limus variants) and, in some cases, a polymer to fixate and control drug release. The platform prevents recoil and fibrous constrictive remodelling. The drug targets the intimal hyperplasia (i.e., the migration and proliferation of smooth muscle cells and accumulation of extra cellular matrix)10. However, the kinetics of drug release are also important. Indeed, restenosis predominantly occurs within a year following nitinol stenting in the femoropopliteal segment7. Thus, the drug release should be sustained to match the restenosis kinetics in order to effectively prevent restenosis. For this purpose, it is the combination of the drug with a polymer that is the crucial factor, enabling a sustained release of the available drug in the target vessel wall tissue. For instance, in the absence of a polymer, a DES releases almost all the drug within 60 days11. A comparison of primary patency and freedom from target lesion revascularisation (TLR) rates for different drug-eluting devices is presented in Figure 1.
Table 1. Summary of drug-eluting stent constituents for femoropopliteal indications.
| DES brand name | Company | Platform | Polymer | Drug | Drug concentration, µg/mm² | RCT reference(s) |
|---|---|---|---|---|---|---|
| Eluvia | Boston Scientific | Innova stent | PVDF-HFP | Paclitaxel | 0.167 | 18,19 |
| Zilver PTX | Cook Medical | Zilver stent | None | Paclitaxel | 3.0 | 15, 16, 20, 2124, 25 |
| DYNALINK-E | Abbott | DYNALINK nitinol self-expanding stent | EVAL | Everolimus | 2.25 | 13 |
| Sirolimus-eluting S.M.A.R.T. stent | Cordis | S.M.A.R.T stent | Parylene, PEVA, PBMA | Sirolimus | 0.9 | 5, 12 |
| NiTiDES | Alvimedica | Nitinol self-expanding stent | None | Amphilimus (sirolimus+fatty acid) | 0.9 | 14 |
| DES: drug-eluting stent; EVAL: ethylene vinyl alcohol; PBMA: poly n-butyl methacrylate; PEVA: polyethylene-co-vinyl-acetate; PVDF-HFP: polyvinylidene fluoride co-hexafluoropropylene; RCT: randomised controlled trial | ||||||
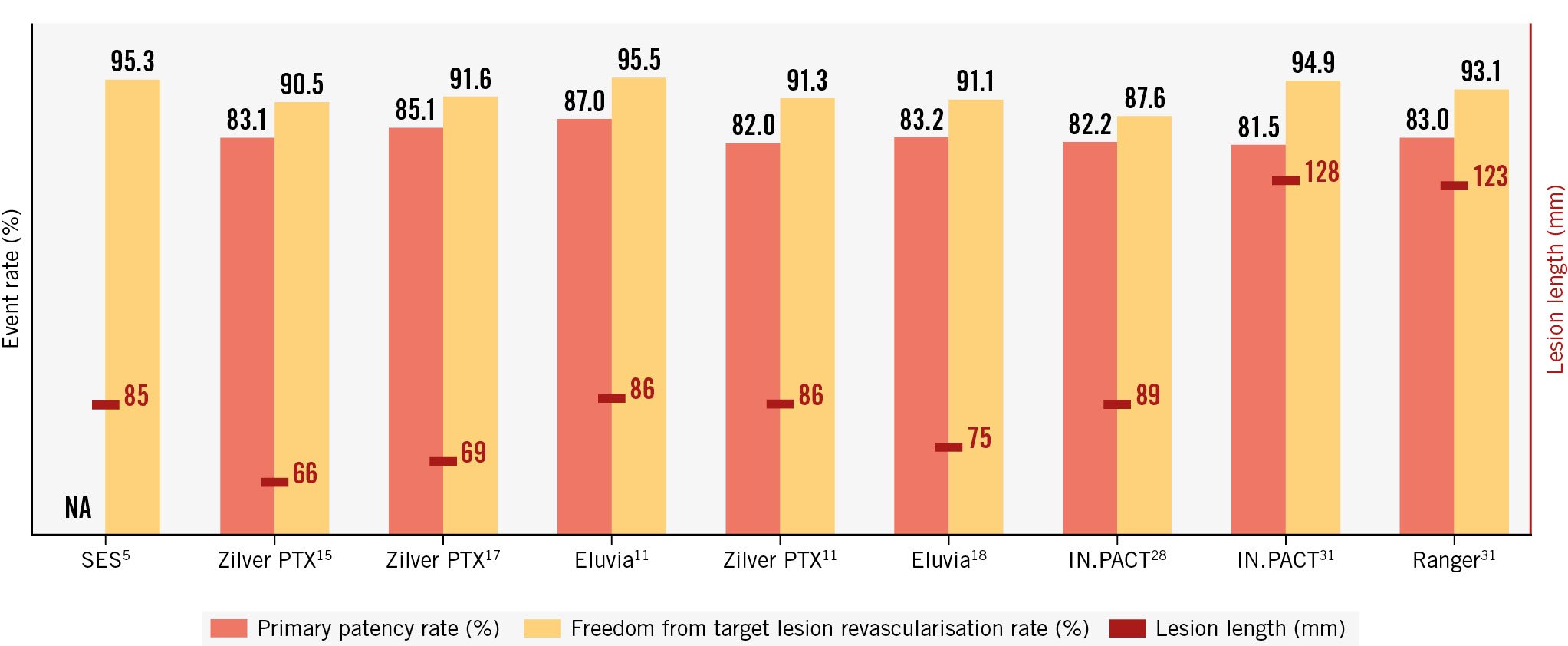
Figure 1. Primary patency and freedom from target lesion revascularisation rates in randomised controlled trials testing drug-eluting devices for femoropopliteal endovascular interventions. NA: not applicable; SES: sirolimus-eluting stent
2. -LIMUS-ELUTING STENTS
In 2002, the first randomised controlled trial (RCT) evaluating the outcomes of DES for the femoropopliteal segment was published5. The Clinical Investigation of the SIROlimus Coated Cordis SMART Nitinol Selfexpandable Stent for the Treatment of Obstructive Superficial Femoral Artery Disease (SIROCCO) included 36 patients, randomised either to a bare nitinol stent (BNS) or to a sirolimus-eluting stent (both S.M.A.R.T. [Cordis]). At 6 months, the rate of the primary endpoint, angiographic in-stent restenosis, was lower in the DES group compared with the BNS group, with a mean in-stent percentage diameter stenosis of 22.6% in the sirolimus-eluting stent group and 30.9% in the uncoated stent group. This did not reach statistical significance, probably because the sample size was too small. The subsequent SIROCCO II study included a total of 93 patients in the 2 treatment arms: 47 patients received the sirolimus-eluting S.M.A.R.T. nitinol stent, and 46 patients received a bare S.M.A.R.T. nitinol stent12. The in-stent restenosis rates were 22.9% and 21.1% for the DES and BNS, respectively. These rates were much lower than those previously reported and did not show any significant difference between the two stents for up to 2 years.
In the Abbott-sponsored European non-randomised STRIDES trial, 104 patients (106 lesions) were treated with a self-expanding everolimus-eluting stent (DYNALINK-E [Abbott])13. The everolimus-eluting self-expanding nitinol stent was successfully implanted in patients with severe PAD, with favourable outcomes and clinical improvements observed, but the overall results seemed very similar to those seen with the BNS, with a primary patency of 68±4.6% at 1 year. No further studies were performed with this DES.
Recently, a second-generation of -limus stents was developed for femoropopliteal lesions. NiTiDES (Alvimedica) is a polymer-free self-expanding nitinol DES, loaded with the amphilimus formulation (sirolimus plus fatty acid). In the absence of a polymer, the drug is contained within reservoirs on the outer surface of the stent. Consequently, the drug is eluted towards the vessel only. The entire structure, including the reservoirs, is homogeneously coated with an ultrathin film of pure carbon. At 1 and 2 years, the ILLUMINA first-in-human study showed promising primary patency rates. For instance, at 24 months, the patency and the freedom from TLR rates were 83.4% and 93.1%, respectively14.
3. PACLITAXEL-ELUTING STENTS
In 2011, Dake et al published the results of the Zilver PTX RCT15. The Zilver PTX RCT was designed to compare the Zilver PTX DES (a polymer-free, self-expanding paclitaxel-eluting stent [Cook Medical]) versus plain old balloon angioplasty (POBA). In the initial step, patients were randomly assigned 1:1 to Zilver PTX DES primary stenting versus POBA. In case of POBA failure, POBA failure patients were subsequently randomised in a second randomisation step to Zilver PTX (provisional DES) or BNS (provisional BNS). At 1 year, the Zilver PTX DES achieved its primary endpoints by showing a superior 12-month event-free survival (90.4% vs 82.6%; p=0.004) and primary patency (83.1% vs 32.8%; p<0.001). In the second randomisation step cohort, the provisional DES group exhibited superior primary patency (89.9% vs 73.0%; p=0.01) and superior clinical benefit (90.5% vs 72.3%; p=0.009) compared with the provisional BNS group. However, since the Zilver PTX RCT was powered to assess primary Zilver PTX stenting versus POBA, the observed tentative benefits of the provisional Zilver PTX versus the provisional BNS could not be considered confirmatory. In 2016, the Zilver PTX RCT co-authors released the long-term Zilver PTX RCT outcomes16. At 5 years, the Zilver PTX DES provided sustained safety and a clinical durability that superseded POBA.
The BATTLE Trial was the first RCT to compare Zilver PTX versus a BNS (MISAGO [Terumo])17. The objective of BATTLE was to demonstrate the superiority of the Zilver PTX DES in achieving a higher rate of freedom from in-stent restenosis at 12 months. In this trial, Zilver PTX failed to show superior efficacy compared with the BNS for the treatment of patients with symptomatic femoropopliteal lesions.
More recently, a polymer-based paclitaxel-eluting stent was developed (Eluvia [Boston Scientific]). The polymer allows for a sustained drug release that provides a closer match with restenosis kinetics. In the IMPERIAL study, the Eluvia DES was shown to be non-inferior to Zilver PTX at 1 year using a composite endpoint of all-cause mortality, major amputation rate and TLR11. In a post hoc analysis, the authors demonstrated the superiority of the Eluvia DES versus the Zilver PTX DES in terms of 1-year patency. More recently, the EMINENT trial included 775 patients in order to compare the Eluvia DES versus BNS (available in the European market) for the endovascular treatment of symptomatic femoropopliteal arterial lesions18. This prospective RCT showed that polymer-based DES treatment yielded superior 1-year primary patency compared with BMS treatment (83.2% vs 74.3%; p=0.0077). Recently, Tepe released the preliminary outcomes of SPORTS RCT (Tepe G. SPORTS. TCT 2023; 24 October 2023. San Francisco, CA, USA). SPORTS is a prospective, multicentre RCT designed to compare outcomes of a DES (Eluvia), BNS (all comers) and a paclitaxel DCB (SeQuent Please [Braun]) in long, complex femoropopliteal lesions. The mean lesion length was >220 mm. SPORTS showed that the Eluvia DES provides superior performance versus BNS and DCB, measured angiographically by percentage diameter stenosis and late lumen loss at 12 months. Furthermore, Eluvia provided superior outcomes versus BMS and DCB, measured by freedom from TLR up to 12 months. The DCB arm reported a 58% bailout stent rate and overall non-inferiority to BMS.
4. LIMITS OF DRUG-ELUTING STENTS
Nowadays, overall treatment safety is no longer a main concern when using drug-eluting devices. However, despite an incremental increase in available data, some limits still exist (Table 2). First, most of the patients included in the previously cited studies were primarily patients with intermittent claudication and a mean lesion length inferior to 10 cm. However, in everyday routine practice settings, the mean lesion length is closer to 15 cm and the proportion of patients presenting with chronic limb-threatening ischaemia (CLTI) is much higher1920. Regarding long femoropopliteal lesions (i.e., superior to 15 cm), a recent meta-analysis showed that high primary patency rates were obtained with drug-coated devices. However, few RCTs for long femoropopliteal lesions exist, and further studies remain necessary. Moreover, few head-to-head trials comparing endovascular therapy and surgical bypass for long femoropopliteal lesions are available. In the ZILVERPASS RCT, the Zilver PTX DES was compared to surgical bypass to determine if the Zilver PTX DES was non-inferior in terms of safety and efficacy. At 1 year, the primary patency rate was 74.5% for the Zilver PTX DES group and 72.5% for the bypass group (p=0.998), with a lower rate of perioperative complications and shorter hospital stay21.
With regard to safety, there is still a concern about the evidence of the so-called hypoechoic peristent area (i.e., halo). In 2018, Bisdas reported the presence of a hypoechoic peristent area in 5 patients, which was thought to be attributable to Eluvia DES deployment for long femoropopliteal lesions22. A recent analysis of halo prevalence and safety following BNS, Zilver PTX DES or Eluvia DES treatment of femoropopliteal lesions did not show any clinical sequelae or effect on target vessel revascularisation rates within 1 year of stent implantation23.
Lastly, few studies have compared DCB to DES, and thus the advantage of a “leave nothing behind” strategy remains uncertain, in particular for long femoropopliteal lesions (such as those studied in the aforementioned SPORTS study). DRASTICO, a single-centre RCT, compared Zilver PTX to DCBs for complex femoropopliteal lesions (mean lesion length 146 mm to 140 mm)24. At 1 year of follow-up, DCBs were not superior to the Zilver PTX DES in the treatment of complex femoropopliteal lesions in a high-risk population, yielding similar rates of restenosis and clinically driven (CD)-TLR. Furthermore, the REAL PTX RCT, comparing Zilver PTX DES to DCBs, showed similar patency rates between both treatment arms at 12 months, while a non-significant trend in favour of DES was observed up to 36 months25.
Table 2. Randomised controlled trials testing drug-eluting stents in patients undergoing femoropopliteal endovascular interventions.
| Study name | Year of publication | Exp/con arms | Primary endpoint | Number of patients enrolled | Clinical presentation: IC/CLTI, % | Mean length of treated lesions, mm | Primary endpoint met? |
|---|---|---|---|---|---|---|---|
| SIROCCO RCT5 | 2006 | Non-polymer paclitaxel-eluting stent/bare metal stent | Primary patency at 1 year | 93 |
Exp: 95/5 |
Exp: 85±44 Con: 81±52 |
No |
| Zilver PTX RCT15 | 2011 | Non-polymer paclitaxel-eluting stent/POBA | Primary patency at 1 year | 479 | 91/9 | Exp: 66.4±38.9 Con: 63.1±40.7 |
Yes |
| BATTLE RCT17 | 2020 | Non-polymer paclitaxel-eluting stent/bare metal stent | Rate of freedom from ISR at 1 year |
186 | Exp: 79/21 Con: 82/18 |
Exp: 69±35 Con: 76±41 |
Yes |
| IMPERIAL RCT11 | 2018 | Non-polymer paclitaxel-eluting stent/paclitaxel-eluting stent with polymer | Primary patency at 1 year | 465 | Exp: 96/4 Con: 94/6 |
Exp: 86.5±36.9 Con: 81.8±37.3 |
Yes |
| EMINENT RCT18 | 2022 | Paclitaxel-eluting stent with polymer/bare metal stent | Primary patency at 1 year | 775 | Exp: 96/4 Con: 97.4/2.6 |
Exp: 75.6±50.3 Con: 72.2±47.0 |
Yes |
| CLTI: critical limb-threatening ischaemia; con: control; exp: experimental; IC: intermittent claudication; ISR: in-stent restenosis; POBA: plain old balloon angioplasty; RCT: randomised controlled trial | |||||||
5. PERSPECTIVES
In 2016, Lammer released the results of the ESPRIT I clinical trial, which was the first-in-human study of a drug-eluting bioresorbable vascular scaffold for treatment of PAD involving the external iliac artery and superficial femoral artery (SFA)26. The ESPRIT drug-eluting bioresorbable vascular system (Abbott) combined a polymer backbone of poly-L-lactide coated with everolimus to form an amorphous drug-eluting coating matrix containing 100 mg of everolimus/cm2 of scaffold. In this study, system implantation was successful in all patients, and overall safety was demonstrated with sustained patency at 2 years and freedom from binary restenosis rates of 87.9% and 83.9% at 12 months and 24 months, respectively. However, this first-in-human trial was designed to treat short lesions (35.7±16.0 mm). Since this period, two studies, Efemoral I27 and DESappear (ClinicalTrials.gov: NCT02869087) have been initiated investigating the value of drug-eluting bioresorbable vascular scaffolds for PAD treatment.
Drug-coated balloons for the femoropopliteal segment
DCB technology inhibits neointimal hyperplasia by administering a single high dose of an antiproliferative agent within the vessel wall without leaving anything behind. The drug is coated on the balloon using special excipients (Table 3). The pharmaceutical agent that has been most used and investigated up to now in femoropopliteal and BTK areas is paclitaxel, owing to its lipophilic properties that can generate high local tissue concentrations. In peripheral arteries, only paclitaxel-coated balloons have proven their efficacy so far. The mode of action is different for -limus compared to paclitaxel, and therefore, these balloons might work only with appropriate coating techniques. This is now under investigation in RCTs, and the data have yet to be released. In early 2000, the first successful studies were presented that demonstrated the advantage of DCBs over non-drug-coated devices. The landmark study was IN.PACT SFA − a prospective, multicentre, 2:1 randomised single-blinded trial which included 331 subjects with symptomatic PAD (Rutherford category 2-4) and de novo femoropopliteal lesions who were allocated to treatment with the paclitaxel-coated IN.PACT Admiral DCB (Medtronic) or POBA28. The DCB arm showed significantly improved primary patency and freedom from CD-TLR, not only at 12 months but also throughout long-term follow-up up to 5 years. The freedom from CD-TLR rate was 74.5% in the DCB arm compared to 65.3% in the POBA arm. This generation of paclitaxel DCBs delivered a high dose of paclitaxel, with 3.5 μg/mm2. Subsequent DCBs that delivered lower doses (at 2 μg/mm2) later demonstrated efficacy that seemed largely comparable to the high-dose IN.PACT DCB2930.
As a consequence, a direct comparison of high-dose versus low-dose paclitaxel DCBs in the COMPARE trial was initiated, in which the high-dose IN.PACT DCB was evaluated head-to-head with the low-dose Ranger DCB (Boston Scientific)31. In this prospective, multicentre clinical trial, 414 patients with symptomatic femoropopliteal lesions (maximum lesion length 30 cm) were randomly assigned in a 1:1 ratio to endovascular treatment with either a low-dose (Ranger) or a high-dose (IN.PACT) paclitaxel DCB after stratification for lesion length. The 2-year follow-up included assessment of primary patency (defined as the absence of CD-TLR or binary restenosis with a peak systolic velocity ratio >2.4 by duplex ultrasound), safety, and functional and clinical outcomes. After 2 years, the primary patency was comparable for both groups, with a rate of 65.5% (116 of 177 lesions) in the low-dose group and 66.7% (112 of 168 lesions) in the high-dose group (p=0.91). This observation was also independent of lesion length, as shown in the subgroup analyses.
Beyond RCT settings (Table 4), the effectiveness of DCBs has also been investigated in several observational studies, including in patients with long lesions, heavily calcified lesions, in-stent restenosis and other reinterventions, and in scenarios with simultaneous in- and outflow treatment. The low-dose Stellarex 2 μg/mm2 balloon (Spectranetics/Philips) has been evaluated in the SAVER registry, which included 1,960 patients at 60 sites in Europe32. Recently, data from this complex patient/lesion cohort were presented33. Altogether, 1,084 patients (58.4% of the total registry cohort) with 1,289 complex lesions (49.3% chronic total occlusions, 31.5% long lesions [>150 mm], 29.9% with severe calcification, and 27.8% with in-stent restenosis) were included in this analysis. The primary efficacy endpoint was freedom from CD-TLR at 12 months post-procedure, which was met in 87.1% of patients overall and did not significantly differ across different lesion characteristic subgroups.
One remaining issue was to scientifically address the question of whether DCB treatment improves outcomes over a primary approach of POBA and BMS in longer lesions. The RAPID Trial was a prospective, multicentre, randomised (1:1) controlled trial that addressed this issue. Overall, 160 patients (mean age 67 years; 102 males) with Rutherford category 2-6 ischaemia were randomised to treatment with the LEGFLOW DCB (Cardionovum)+Supera stent (Abbott) or Supera stenting alone in intermediate to long SFA lesions (mean lesion length 15.8±7.4 cm vs 15.8±7.6 cm, respectively)34. With regard to primary efficacy in the per protocol analysis at 12 months, the DCB+Supera arm showed an advantage of 74.7% versus 62.0% over the Supera-alone arm; this was not confirmed at 2 years. The aforementioned approach of “leave nothing behind” was further addressed in the single-arm TOBA III trial, in which the use of the Tack system (Intact Vascular/Philips) was evaluated after treatment with the IN.PACT Admiral DCB in femoropopliteal lesions with dissections35. The 12-month Kaplan-Meier rate of freedom from CD-TLR was 97.5% in this cohort, in which all patients had dissected lesions.
One central question that is highly important to clinical practice is the overall performance of drug-eluting technologies in calcified lesions. A paper by Fanelli et al demonstrated that the higher the degree of circumferential calcium within the vessel wall, the lower the drug uptake, which in turn may precipitate higher rates of late lumen loss and loss of primary patency36. The most solid data for addressing the role of vessel wall calcium deposits in the femoropopliteal segment come from the technology of intravascular lithotripsy (IVL). This technology, which creates sonic pressure waves in a balloon-based technology, can effectively disrupt calcium while lessening barotrauma and leaving undiseased tissue unharmed. Following its demonstrated feasibility as a stand-alone technology, a prospective RCT (1:1) was initiated (Disrupt PAD III)37. In this trial, 306 patients with moderate to severe steno-occlusive femoropopliteal calcification deposits underwent vessel preparation with IVL or POBA prior to DCB implantation or stenting. Long-term outcome data up to 24 months demonstrated a statistically significant improvement in primary patency in the IVL cohort over the POBA cohort (74.7% vs 57.7%; p=0.005). However, primary patency modelled without defining provisional stenting as a failure showed similar 2-year primary patency rates between the 2 groups (IVL 79.2% vs POBA 75.6%; p=0.70).
Sirolimus DCBs are an alternative to paclitaxel DCBs. The modes of action of sirolimus and paclitaxel are different. With sirolimus, tissue absorption is low, and tissue retention is short, whereas paclitaxel tissue absorption is fast, and tissue retention is considerably longer. In patients with femoropopliteal segment lesions, only one small trial has so far been conducted and published: the single-arm, First-in-human Evaluation of the SELUTION DCB, a Novel Sirolimus Coated Balloon in Peripheral Arteries. In this prospective, non-randomised, first-in-human trial, 50 patients with symptomatic moderate to severe lower limb ischaemia (Rutherford categories 2 or 3) and femoropopliteal lesions were enrolled and treated with the SELUTION DCB (MedAlliance). Primary patency in the intention-to-treat cohort at 6 months was 88.4%, with only 1 case of CD-TLR38.
In summary, a large body of evidence supports the use of DCBs as an important adjunct during femoropopliteal revascularisation procedures, and currently, paclitaxel-coated devices have shown the best efficacy. The main head-to head comparisons for femoropoliteal procedures are shown in Central Illustration A. From a revascularisation standpoint, the most important technical issue is probably adequate vessel preparation either with POBA or other techniques such as speciality/scoring balloons. In the case of substantially calcified lesions, IVL stands out as an especially appropriate vessel preparation tool that may support an enhanced drug uptake. In case of suboptimal results of vessel preparation (like dissections or elastic recoil), the shortest scaffold possible should be used, and it remains an open question whether BMS or DES or Tack technology should be added. Following the aforementioned final statement from the FDA this year8, paclitaxel-coated DCBs or DES can be used without any concerns for safety issues. Sirolimus-coated DCBs still need to prove their efficacy.
Table 3. Summary of paclitaxel-coated balloon constituents for femoropopliteal indications.
| DCB marketing denomination | Company | Excipient | Paclitaxel concentration, µg/mm² | RCT reference(s) |
|---|---|---|---|---|
| IN.PACT | Medtronic | Urea | 3.5 | 28 |
| Ranger | Boston Scientific | TransPax – citrate ester | 2 | 29, 31 |
| Stellarex | Spectranetics/Philips | Unknown | 2 | 30 |
| LEGFLOW | Cardionovum | Shellolic acid | 3 | 34 |
| DCB: drug-coated balloon; RCT: randomised controlled trial | ||||
Table 4. Randomised controlled trials testing DCBs in patients undergoing femoropopliteal endovascular intervention.
| Study name | Year of publication | Exp/con arms | Primary endpoint | Number of patients enrolled | Clinical presentation: IC/CLTI, % | Mean length of treated lesions, mm | Primary endpoint met? |
|---|---|---|---|---|---|---|---|
| IN.PACT SFA28 | 2019 | Exp:IN.PACT DCBCon: POBA | Freedom from clinically driven target lesion revascularisation at 5 years | Exp: 220 Con: 111 |
Exp: 95/5 Con: 93.7/6.3 |
Exp: 89±49 Con: 88±51 |
Yes |
| COMPARE trial30 | 2022 | Exp: High-dose DCBCon: Low-dose DCB | Primary patency at 1 year | Exp: 207 Con: 207 |
Exp:93.7/6.3 Con: 95.2/4.8 |
Exp: 128.3±97.3 Con: 123.9±97.8 |
Yes |
| RAPID trial34 | 2019 | Exp: Supera+ DCBCon: Supera | Primary patency at 2 years | Exp: 80 Con: 80 |
Exp: NA Con: NA |
Exp: 158±74 Con: 158±76 |
Yes |
| CLTI: critical limb-threatening ischaemia; con: control; DCB: drug-coated balloon; exp: experimental; IC: intermittent claudication; NA: not available; POBA: plain old balloon angioplasty | |||||||
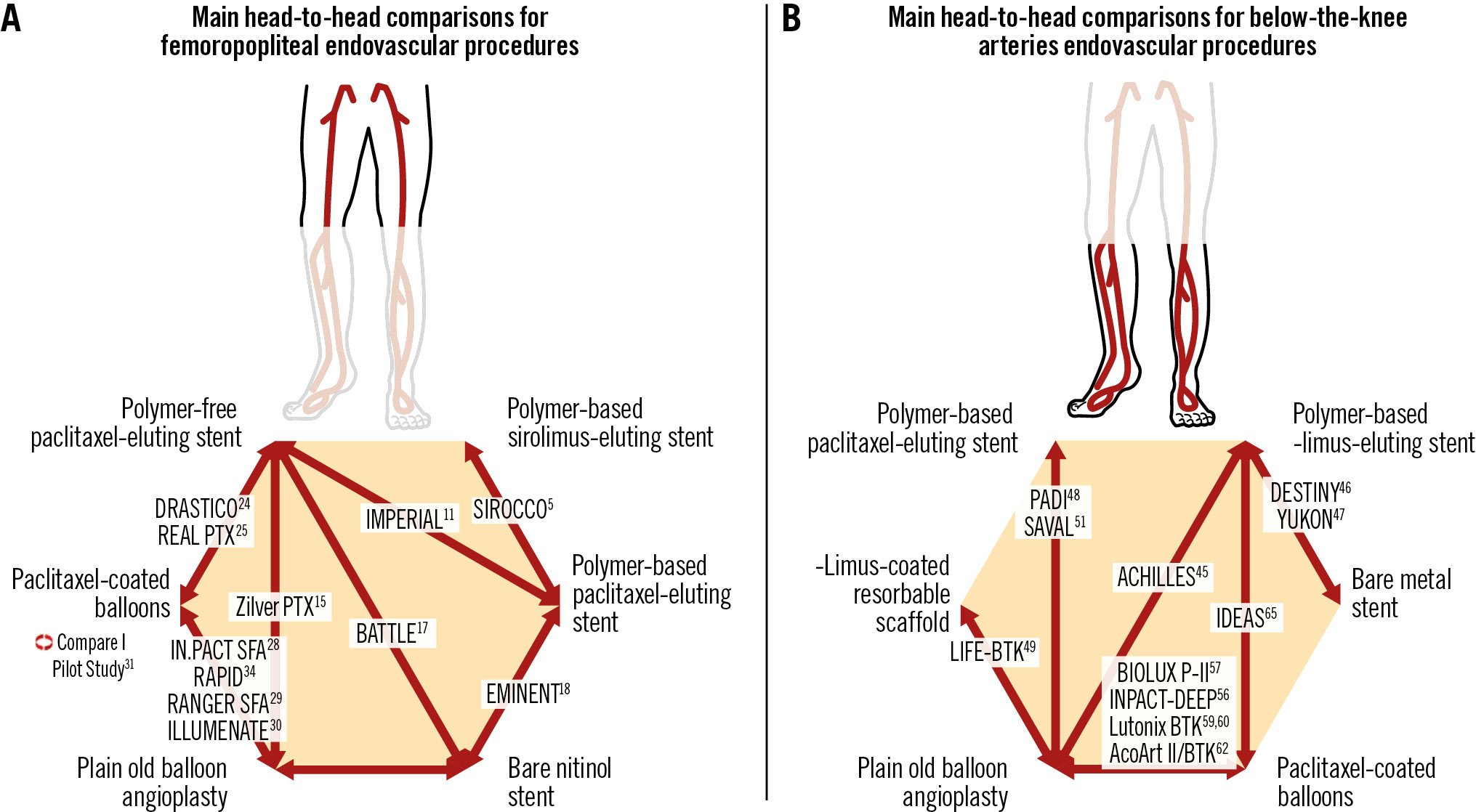
Central Illustration. Main head-to-head comparisons for femoropopliteal and below-the-knee artery endovascular procedures.
Endovascular drug elution below the knee
BTK atherosclerotic disease is a significant contributor to CLTI39. CLTI is considered to be responsible for approximately 90% of the major amputations performed worldwide and is a significant cause of morbidity and mortality40. Epidemiological and trial data have shown a 1-year mortality rate of 25% and a 10-year mortality of 75-80%3940. Plain old balloon angioplasty has become the first-line treatment strategy for BTK arterial disease43. However the midterm patency of POBA remains unfavourable because of several problems, including flow-limiting dissections, elastic recoil, residual stenosis and restenosis caused by intimal hyperplasia. The relatively disappointing 1-year primary patency rate of POBA varies between 40% and 70% in the literature44. Based on these shortcomings of POBA, several studies investigated the use of infrapopliteal drug-eluting technologies like DCB and DES (Table 5, Table 6). The main head-to-head comparisons for BTK endovascular procedures are shown in Central illustration B.
Table 5. Summary of drug-coated device constituents for below-the-knee revascularisation indications.
| Drug-coated device brand name | Company | Platform | Polymer | Drug | Drug concentration | RCT reference(s) |
|---|---|---|---|---|---|---|
| CYPHER SELECT | Cordis | Stainless steel316L cobalt-chromium | Poly n-butyl methacrylate +polyethylene-co-vinyl-acetate | Sirolimus | 1.4 μg/mm2 | 46 |
| XIENCE V | Abbott | L-605 cobalt-chromium | Non-bioresorbable acrylic-fluoropolymer | Everolimus | 1 μg/mm2 | 47 |
| Yukon-Choice stent | Translumina | Cobalt-chromium backbone | Polymer-free | Sirolimus | 1.4 μg/mm2 | 48 |
| TAXUS Liberté | Boston Scientific | Stainless steel | Styrene-isobutylene-styrene (SIBS) | Paclitaxel | 1 μg/mm2 | 49 |
| Esprit BTK | Abbott | Strutted scaffold of poly-L-lactic acid | Poly-L-lactide-coated backbone | Everolimus | 100 μg/mm2 | 50 |
| NA | STENTYS | Nitinol | Polysulfone | Paclitaxel | 0.8 μg/mm2 | 51 |
| NA | Boston Scientific | Nitinol | Polyvinylidene fluoride co-hexafluoropropylene | Paclitaxel | 0.24 µg/mm2 | 52 |
| Amphirion DCB | Medtronic | NA | Urea | Paclitaxel | 3 μg/mm2 | 57 |
| Passeo-18 Lux DCB | Biotronik AG | NA | Butyryl trihexyl citrate | Paclitaxel | 3 μg/mm2 | 58 |
| Lutonix 014 BTK DCB | BD | NA | Polysorbate and sorbitol | Paclitaxel | 2 μg/mm2 | 59, 60 |
| IN.PACT 014 DCB | Medtronic | NA | Urea | Paclitaxel | 3.5 μg/mm2 | 61 |
| Tulip & Litos DCB | Acotec | NA | Magnesium stearate | Paclitaxel | 3 µg/mm2 | 63 |
| DCB: drug-coated balloon; NA: not available; RCT: randomised controlled trial | ||||||
Table 6. Randomised controlled trials testing drug-coated devices in patients undergoing below-the-knee endovascular intervention.
| Study name | Year of publication | Exp/con arms | Primary efficacy endpoint | Number of patients enrolled | Clinical presentation: IC/CLTI, % | Mean length of treated lesions, mm | Primary endpoint met? |
|---|---|---|---|---|---|---|---|
| ACHILLES46 | 2012 | Everolimus-eluting stent/POBA | In-segment restenosis at 1 year | 200 | Exp: 0/100 Con: 100 |
Exp: 26.9±20.9 Con: 26.8±21.3 |
Yes |
| DESTINY47 | 2012 | Everolimus-eluting stent/BMS | Patency at 1 year | 140 | Exp: 0/100 Con: 100 |
Exp: 15.9±10.2 Con: 18.9±10.0 |
Yes |
| YUKON48 | 2011 | Sirolimus-eluting stent/BMS | Primary patency at 1 year | 161 | Exp: 48.8/51.2 Con: 58.2/41.8 |
Exp: 30±8 Con: 31±9 |
Yes |
| PADI49 | 2017 | Paclitaxel-eluting stent/BMS | Primary patency at 6 months | 137 | Exp: 100 Con: 100 |
Exp: Unknown Con: Unknown |
No |
| SAVAL52 | 2023 | Paclitaxel-eluting self-expanding stent/POBA | Patency at 1 year | 201 | Exp: 100 Con: 100 |
Exp: 68.1±35.2 Con: 68.7±49.2 |
No |
| INPACT-DEEP57 | 2024 | Sirolimus-coated balloon/ POBA | Clinically driven TLR and late lumen loss | 358 | Exp: 0/100 Con: 0.8/99.2 |
Exp: 102 Con: 129 |
No |
| BIOLUX P-II58 | 2015 | Paclitaxel-coated balloon/ POBA | 6-month target lesion primary patency without TLR | 72 | Exp: 0/100 Con: 100 |
Exp: 113.1±88.1 Con: 115.0±86.9 |
No |
| Lutonix BTK5960 | 2019 | Paclitaxel-coated balloon/ POBA | Primary patency and freedom from above-ankle amputation measured at 6 months | 442 | Exp 43.9/56.1 Con: 43.9/56.1 |
Exp: 111.8±92.6 Con: 94.7±85.4 |
No |
| AcoArt II/BTK63 | 2021 | Paclitaxel-coated balloon/ POBA | Primary patency at 6 months | 120 | Exp 2/98 Con: 100 |
Exp: 169.95±86.35 Con: 179.93±80.16 |
Yes |
| IN.PACT BTK61 | 2022 | DCB/POBA | Late lumen loss at 9 months post-procedure | 50 | Exp: 100 Con: 100 |
Exp: 215.41±83.81 Con: 218.19±80.43 |
No |
| IDEAS66 | 2014 | DCB/DES | Target lesion restenosis >50% at 6 months | 50 | Exp: Unknown Con: Unknown |
Exp: 127±46.5 Con: 148±56.7 |
Yes |
| LIFE-BTK50 | 2023 | Everolimus-coated resorbable scaffold/POBA | Freedom from the following events at 1 year: amputation above the ankle of the target limb, total (100%) occlusion of the target vessel, clinically driven TLR, and binary restenosis of the target lesion | 261 | Exp: 100Con: 100 | Exp: 43.8±31.8 Con: 44.8±29.1 |
Yes |
| BMS: bare metal stent; CLTI: critical limb-threatening ischaemia; con: control; DCB: drug-coated balloon; DES: drug-eluting stent; exp: experimental; IC: intermittent claudication; POBA: plain old balloon angioplasty; TLR: target lesion revascularisation | |||||||
1. DRUG-ELUTING STENTS FOR BELOW-THE-KNEE DISEASE
The similarity in diameter to coronary arteries led to the off-label use of coronary DES for relatively short BTK lesions. Two types of DES are described: the self-expanding cytotoxic paclitaxel-based DES and the balloon-expandable cytostatic -limus-based DES. As a result of a significant volume of high-level evidence supporting the safety and effectiveness of the latter in the literature, the TransAtlantic Inter-Society Consensus II update endorsed the use of DES in the treatment algorithm of CLTI for short focal lesions45. In the ACHILLES trial46, 200 patients with a mean total lesion length of 27±21 mm were randomised to receive the sirolimus-eluting DES (CYPHER SELECT [Cordis]) or POBA. The device success rate (achievement of a final residual diameter stenosis of 30%) was significantly higher for DES (95.5% vs 58.2%; p=0.001) compared to POBA, while DES at 12 months achieved significantly lower restenosis rates (22.4% vs 41.9%; p=0.019) and superior patency (75.0% vs 57.1%; p=0.025). The need for repeat revascularisation and amputation rates were similar for both arms. In the DESTINY trial44, 140 patients with BTK lesions up to 4 cm in length were randomised to receive either angioplasty with an everolimus-eluting DES (XIENCE V [Abbott]) or a BMS (Multi-Link Vision BMS [Abbott]). At 12 months, both primary patency (85% vs 54%; p=0.0001) and reintervention (85% vs 54%; p=0.0001) rates were significantly improved with DES use. Moreover, the XIENCE V DES significantly reduced both the mean in-stent stenosis (21±21% vs 47±27%; p<0.0001) and mean in-stent late lumen loss (LLL; 0.78±0.63 mm vs 1.41±0.89 mm; p=0.001). Similarly, in the YUKON trial48, 161 patients with lesions up to 4.5 cm in length were double-blind randomised to angioplasty treatment with either a polymer-free sirolimus-eluting DES (Yukon-S stent [Translumina]) or BMS (Yukon stent [Translumina]). The 12-month primary (80.6% vs 55.6%; p=0.004) and secondary (91.9% vs 71.4%; p=0.005) patency rates were significantly higher for the DES group, while changes in the Rutherford-Becker classification were also significantly superior in the DES group.
Besides the randomised data with -limus-eluting balloon-expandable stents, there is also one published RCT that tested paclitaxel-eluting balloon-expandable stainless steel coronary stents: the PADI trial49. In total, 74 limbs (73 patients) were treated with DES (TAXUS Liberté [Boston Scientific]) and 66 limbs (64 patients) with POBA and provisional BMS. The mean lesion length was 2.2 cm. The estimated 5-year major amputation rate was lower in the DES arm (19.3% vs 34.0% for POBA-BMS; p=0.091). The 5-year rates of amputation and event-free survival (survival free from major amputation or reintervention) were also significantly higher in the DES arm compared with POBA-BMS (31.8% vs 20.4%; p=0.043; and 26.2% vs 15.3%; p=0.041, respectively). Survival rates were overall comparable. The limited available morphological results showed higher preserved patency rates after DES than after POBA-BMS at 1, 3, and 4 years of follow-up.
Although the results of all the previously described drug-eluting balloon-expandable stents sound outstanding, the implementation of a permanent metallic scaffold in a small-calibre vessel could be considered an undesirable downside of the procedure. Recently the results of the LIFE-BTK Trial were released50. In this RCT, 261 patients with CLTI and BTK arterial disease were randomly assigned in a 2:1 ratio to receive treatment with a new everolimus-coated resorbable scaffold (Esprit BTK [Abbott]) or POBA for the treatment of infrapopliteal artery disease in patients with CLTI. The incidence of the primary efficacy composite endpoint (freedom from amputation above the ankle of the target limb, occlusion of the target vessel, CD-TLR, and binary restenosis of the target lesion) at 1 year was higher among patients who received an everolimus-eluting resorbable scaffold than among those who received angioplasty (Kaplan-Meier estimate 74% vs 44%, 95% confidence interval [CI]: 15-46; 1-sided p<0.001 for superiority). Interestingly, most patients presented with rest pain (Rutherford-Becker class 4 disease) in both groups with mean ankle brachial and toe brachial indices of the target limb of 0.88±0.32 and 0.49±0.29, respectively. Furthermore, the mean lesion length at the index procedure was 43.8±31.8 mm in the everolimus-eluting resorbable scaffold group and 44.8±29.1 mm in the angioplasty group.
Nevertheless, all these devices are characterised by a short available length, a higher associated cost (especially when using more than one device), and a higher risk of crushing deformities (certainly in the distal half of the tibial vessels and in specific anatomical areas such as the proximal anterior tibial artery where it perforates the interosseous membrane); these are significant issues that could impair the daily use of these devices in CLTI treatment.
Based on the success of paclitaxel-eluting stents in the femoropopliteal area, self-expanding paclitaxel-eluting devices were also introduced in the BTK area. Despite some initial success in a prospective, single-arm, multicentre trial studying a paclitaxel-eluting self-expanding coronary stent (PES-BTK [STENTYS])51, a subsequent RCT with this kind of device failed to show a positive outcome. The SAVAL trial was a prospective, multicentre trial randomising 201 patients with CLTI and infrapopliteal lesions, with a total lesion length ≤140 mm and stenosis ≥70%, in a 2:1 ratio to treatment with a self-expanding paclitaxel DES (SAVAL; n=130) or POBA (n=71)52. The target lesion length was 68.1±35.2 mm for the DES group and 68.7±49.2 mm for the POBA group. The 12-month primary patency rates were 68.0% for the DES group and 76.0% for the POBA group (p for superiority=0.8552). The major adverse event-free rates were 91.6% and 95.3%, respectively (p for non-inferiority=0.0433). No benefits related to effectiveness and safety with the nitinol DES compared with POBA could thus be demonstrated52. Besides the differences in antiproliferative agents, nitinol stents, such as those used in the SAVAL trial, have thicker strut profiles as well as other geometric differences compared with balloon-expandable metallic coronary stents, which may affect re-endothelialisation and thus provide an explanation for the observed lack of treatment benefits53. Figure 2 shows the rates of major amputation and freedom from TLR in RCTs testing different DES for endovascular interventions.
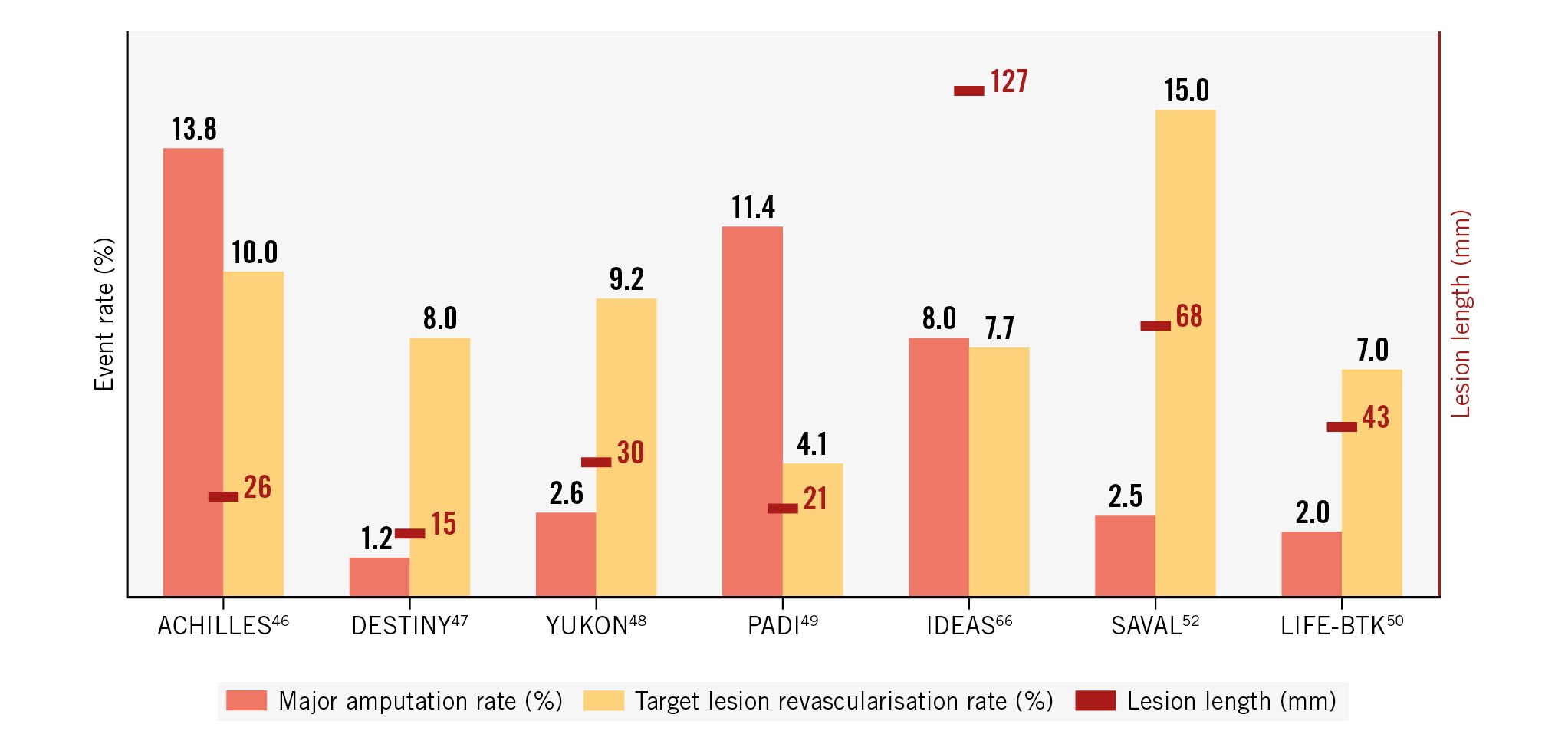
Figure 2. Major amputation and freedom from target lesion revascularisation rates in randomised controlled trials testing drug-eluting scaffolds for below-the-knee endovascular interventions.
2. DRUG-COATED BALLOONS FOR BELOW-THE-KNEE DISEASE
Success with DCBs in the femoropopliteal area has been extensively illustrated by numerous trials demonstrating their safety and efficacy54. The step towards their application in the BTK arteries was relatively small, the idea being to offer a potential valid solution for all the aforementioned DES caveats in tibial vessel treatments. Despite the initially promising results deriving from single-centre studies5556, 4 prospective multicentre RCT studies failed to prove any clinical superiority of DCBs over POBA.
The INPACT-DEEP study investigated 358 CLTI patients randomised in a 2:1 ratio to receive treatment with the IN.PACT Amphirion paclitaxel-coated balloon (Medtronic) or POBA57. No statistically significant differences were detected in the primary efficacy outcomes of CD-TLR (9.2% DCB vs 13.1% control; p=0.291) and LLL (0.61±0.78 mm for DCB vs 0.62±0.78 mm for control; p=0.950) at 1-year follow-up. The composite primary safety endpoint (6-month all-cause mortality, major amputation, and CD-TLR) was similar between the Amphirion group (17.7%) and the control group (15.8%). However, the major amputation rate at 12 months was more than twice as high in the DCB arm compared to the POBA arm (8.8% vs 3.6%; p=0.080).
In the BIOLUX P-II multicentre RCT study, 72 patients were randomised in a 1:1 ratio to receive either the Passeo-18 Lux DCB (Biotronik) or the uncoated Passeo-18 POBA (Biotronik)58. No significant differences were noted in terms of 6-month primary patency loss or 1-year major amputation in the DCB group versus POBA (17.1% vs 26.1%, and 3.3% vs 5.6%, respectively). The 30-day composite primary safety endpoint (all-cause mortality, target extremity major amputation, target lesion thrombosis, and TLR) was numerically lower in the DCB group while not reaching statistical significance (0% PCB vs 8.3% POBA; p=0.239).
The Lutonix BTK trial compared the performance of the Lutonix 014 BTK DCB (BD) to POBA in a 2:1 fashion in 442 patients with CLTI5960. The mean lesion length was slightly longer in the DCB group relative to the POBA group (11.18±9.26 cm vs 9.47±8.54 cm; p=0.03). The 30-day primary safety endpoint of freedom from major adverse limb events (which included significant reintervention, above-ankle amputation and perioperative death) did not differ between treatment arms (99.3% in the DCB group vs 99.4% in the POBA group). The composite efficacy endpoint of primary patency and limb salvage at 6 months was 74.7% versus 64.2% for DCB and POBA, respectively (p<0.001). This observed difference was, however, not sustained at 1 year (p=0.54). A similar observation was made concerning reintervention rates. At 6 months, there was a statistically significant difference in favour of the DCB arm (8.5% vs 17.5%; p=0.01), whereas no statistically significant difference could be identified at 1 year (17.8% vs 21.8%; p=0.39). Finally, the combined amputation-free survival endpoint did not differ between treatment arms throughout the 3 years of follow-up.
The IN.PACT BTK trial was the most recent prospective, multicentre study randomising 50 CLTI participants 1:1 to IN.PACT 014 DCB (Medtronic; n=23) or POBA (n=27), with a mean lesion length of 215.41±83.81 mm in the DCB group and 218.19±80.43 mm in the POBA group (p=0.806)61. The primary effectiveness endpoint, LLL at 9 months post-procedure, was 0.892±0.774 mm for the DCB group and 1.312±0.720 mm for the POBA group (p=0.07). The Kaplan-Meier estimated freedom from CD-TLR up to 9 months was 91.1% for DCB patients and 91.8% for POBA patients (log-rank p=0.942). Again, no significant differences in LLL, safety, nor efficacy between DCBs and POBA were detected.
Of note, the patency rates of POBA in all the aforementioned RCTs were unexpectedly high when considering the results from previous BTK POBA studies. The reason for this discrepancy remains unclear. Prolonged inflation times, better vessel preparation and correct sizing protocols within the controlled setting of RCTs could be partially responsible for these findings. Lack of luminal gain, recoil, residual stenosis, and flow-limiting dissections could be other important causes for the lack of clear DCB benefits over POBA in the BTK segment. Also, these observed results may be attributed to embolisation of the paclitaxel-coating components into the distal vascular bed and may potentially impair wound healing − a topic which needs to be further clarified in future studies62. These potential drawbacks of paclitaxel-coated balloons as a stand-alone treatment are opening the discussion about combination therapies with debulking tools and scaffolds or switching to cytostatic, and potentially less toxic, -limus-coated balloons or drug-eluting resorbable scaffolds. Ongoing research will hopefully offer more insights in this difficult treatment area. A first glimpse into this research has been offered by the investigators of the AcoArt II/BTK China trial. They randomly allocated 120 patients with CLTI and BTK lesions in a 1:1 ratio to either treatment with the Tulip & Litos DCB (Acotec; n=61) or POBA (n=59). Freedom from CD-TLR at 12 months was observed in 91.5% of patients in the DCB group and 76.8% in the control group63. The rates of major amputation and freedom from TLR in the aforementioned RCTs are illustrated in Figure 3.
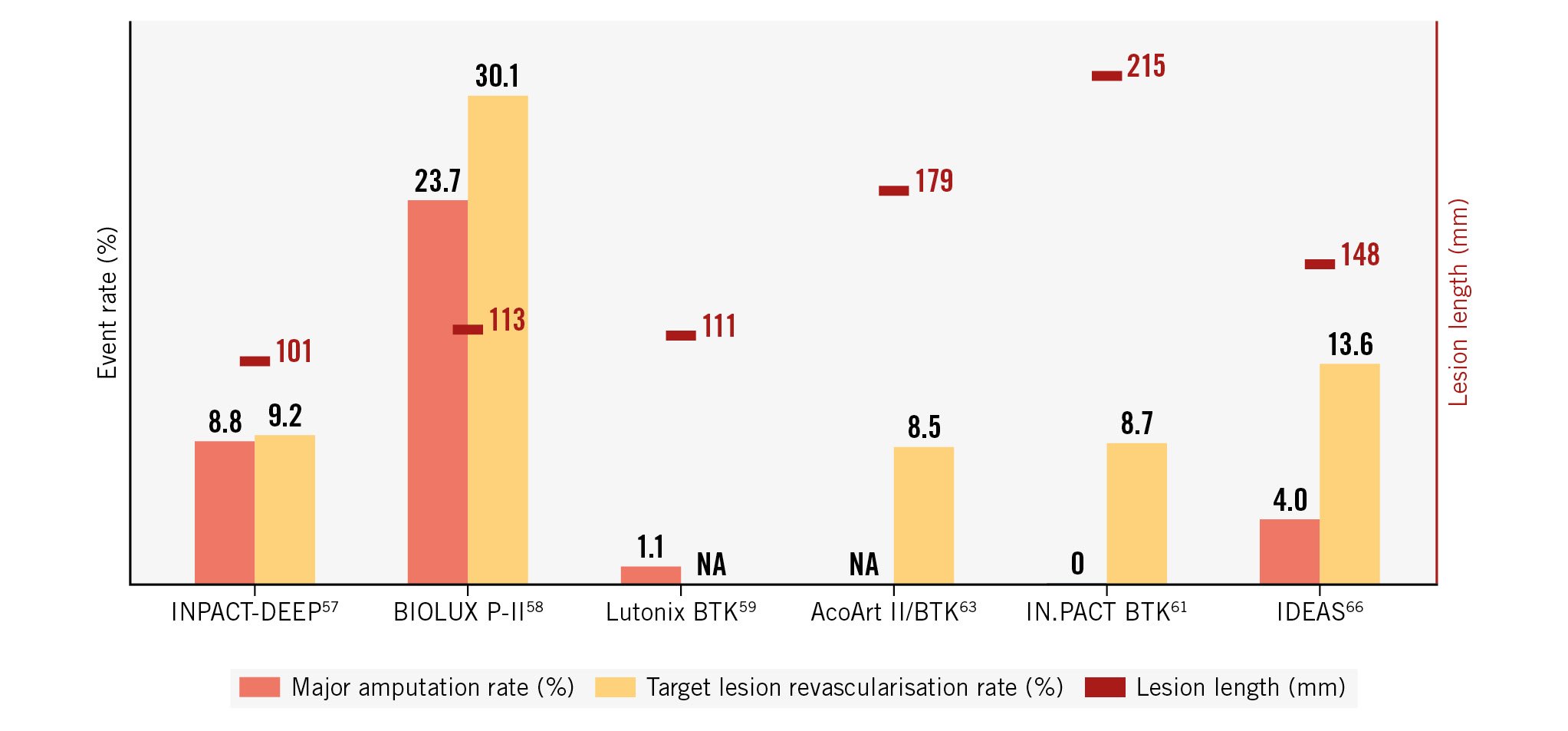
Figure 3. Major amputation and freedom from target lesion revascularisation rates in randomised controlled trials testing drug-eluting balloons for below-the-knee endovascular interventions. NA: not available
3. COMPARISONS BETWEEN THE TWO TREATMENT OPTIONS
In a meta-analysis of 16 RCTs (1,805 patients), Katsanos et al demonstrated that BTK DES treatment was associated with significantly lower rates of restenosis, TLR, and amputations as well as improved wound healing as compared to POBA and BMS61. Importantly, DES had significantly better results when compared with DCBs for the most important, strong clinical endpoint of amputation. The same conclusions were made by Zhang65 in his meta-analysis of 9 studies (707 and 606 patients in the DCB/DES group and standard percutaneous balloon angioplasty/BMS group, respectively). He suggested that compared with standard POBA/BMS, DES may decrease the risk of CD-TLR, restenosis and amputation rates without any impact on mortality. However, DCBs had no obvious advantage in the treatment of BTK disease. In the Infrapopliteal Drug-Eluting Angioplasty Versus Stenting (IDEAS-I) trial, Siablis et al66 directly compared both technologies in BTK disease treatment in a relatively small group of 50 patients (25 patients in the paclitaxel DCB group and 27 in the DES group). Immediate residual post-procedure stenosis was significantly lower in the DES treatment arm (9.6±2.2% vs 24.8±3.5% in PCB; p<0.0001), and at 6 months, the binary (>50%) angiographic restenosis rate was also significantly lower in the DES treatment arm (7 of 25 [28%] vs 11 of 19 [57.9%] in the DCB treatment arm; p=0.0457). There was no significant difference regarding TLR rates (2 of 26 [7.7%] in DES vs 3 of 22 [13.6%] in DCB; p=0.65).
Due to the fragility of the BTK patient population as well as the difficult arterial disease pattern (sometimes described as small arterial disease with media calcification [SAD-MAC] and big arterial disease with intra-arterial calcium [BAD-IAC])67, the lack of clear treatment strategies and the mainly small-diameter tibial vessels, the treatment of CLTI disease remains extremely challenging and still results in worse outcomes compared with treatment in other lower limb vessel segments5759. Although previous trials of -limus-eluting balloon-expandable coronary stents have been encouraging, the short lengths, the crushability, the lack of flexibility and permanent nature of DES along with the lack of hard clinical outcome improvements (mainly wound healing, ischaemic pain relief and major amputation rates) remain important drawbacks. There is currently insufficient evidence to recommend the widespread use of paclitaxel-eluting balloons below the knee. Despite being adequately adapted for long lesion treatment, the problems of acute recoil, lack of lumen gain, residual stenosis and flow-limiting dissections remain the main causes of disappointing trial results. Better sizing, optimising vessel preparation (potentially with dedicated BTK debulking devices), plaque modification (such as IVL), combination with appropriate (including bioresorbable) scaffolding, and a shift towards -limus-elution devices hold promise in the near future. Also, distal tibial and below-the ankle disease deserves our highest attention.
Safety profile of paclitaxel-coated balloons and stents in endovascular interventions for peripheral artery disease
The increasing popularity of DCBs and DES can be attributed to their ability to inhibit neointimal hyperplasia, thereby reducing the risk of restenosis following lower limb revascularisation166869. Numerous pivotal trials have consistently demonstrated the favourable impact of drug-coated devices on surrogate endpoints such as target lesion patency, binary restenosis, LLL, and TLR70. These positive outcomes have fostered their adoption in clinical practice and have resulted in regulatory approvals, leading to widespread implementation of these techniques across several countries
Paclitaxel, a hydrophobic natural diterpenoid initially derived from Taxus brevifolia, is the predominant drug used in drug-coated devices for PAD applications71. More recently, DCB and DES devices coated with mammalian target of rapamycin (mTOR) inhibitors like sirolimus and its derivatives have also emerged for PAD applications6769. While mTOR inhibitors act as cytostatic agents by interrupting the G1 phase of the cell cycle, paclitaxel exhibits cytotoxic properties and exerts its antiproliferative effects by impeding the M phase of the cell cycle through the interference of microtubule formation, ultimately inhibiting the formation of the mitotic spindle. When attached to balloons or stents, both paclitaxel and sirolimus are released locally, directly targeting the arterial wall, and effectively reduce neointimal tissue formation. As opposed to sirolimus and other -limus derivates, the hydrophobic nature of the core structure of the paclitaxel molecule makes it comparatively easier to reach a high local uptake in the vessel wall, particularly when coated on balloon catheters, which may be one reason why the PAD medical device industry have preferentially used paclitaxel over mTOR inhibitors as the main antiproliferative coating in peripheral arteries.
In December 2018, Katsanos et al conducted a study-level systematic review and meta-analysis of 28 RCTs comparing paclitaxel-coated devices (DCBs and DES) with non-drug-coated devices. Surprisingly, their investigation revealed an elevated incidence of late mortality associated with the use of paclitaxel-coated devices8. While all-cause mortality was comparable between the 2 groups at the 1-year post-intervention follow-up (risk ratio [RR] 1.08, 95% CI: 0.72-1.61), the use of paclitaxel-coated devices was associated with higher mortality rates at both the 2-year (RR 1.68, 95% CI: 1.15-2.47; results from 12 RCTs with 2,316 cases) and 5-year (RR 1.93, 95% CI: 1.27-2.93; results from 3 RCTs with 863 cases) follow-ups. The analysis specifically focused on patients treated for femoropopliteal lesions. In the meta-analysis, a total of 89% of the patients under analysis presented with intermittent claudication, in turn, reflecting the rather skewed PAD severity status in current device trials as compared to PAD patients undergoing lower limb revascularisation within routine clinical practice73. Notably, none of the included studies in the meta-analysis were specifically designed to investigate all-cause mortality nor any other hard endpoint (such as limb salvage rates and functional status improvement). Instead, safety assessments in the individual trials primarily relied on composite endpoints, which incorporated various adverse limb events including less robust endpoints such as target lesion revascularisation. However, a preliminary and rather crude estimate in the meta-analysis also indicated a dose-response relationship8. Subsequent studies with precise dosing information could not replicate this finding, challenging the notion of a causal connection between paclitaxel-coated device usage and mortality74. The initial dose-response estimation relied solely on the density of paclitaxel on the balloon or stent, treated lesion length, reference vessel diameter, and the duration of available follow-up. As a result, patients assigned to higher paclitaxel doses in the meta-analysis also demonstrated longer lesions, indicating a more complex lower limb PAD status, which is widely acknowledged as a significant risk factor for both mortality and severe limb events. Therefore, the initial dose-response claim might instead have reflected a non-surprising relation between lesion length and mortality. Moreover, the paclitaxel dosages utilised in endovascular procedures are, by several orders of magnitude, lower compared to those administered in systemic oncology therapies. For instance, a drug-coated balloon with a diameter of 6 mm and a length of 150 mm, delivering a high dosage of 3.5 μg/mm2, contains approximately 10 mg of paclitaxel, calculated as ([6 x π] x 150) x 0.0035. In contrast, the recommended initial systemic dosage for breast cancer treatment in an individual weighing 70 kg and measuring 170 cm in height is approximately 400 mg. Additionally, the net dose delivery from paclitaxel-coated devices to the body is probably even less given that the initial surface area dose product may, to some extent, be lost from the balloon or stent during preparation and handling before being introduced into the bloodstream75. The hypothesis suggesting potential embolisation of paclitaxel and/or coating carrier particles downstream from the target lesion during the intervention as a driver for all-cause mortality appeared improbable given the wealth of evidence indicating favourable effects on surrogate limb outcomes for these devices. Furthermore, it is pertinent to note that most patients in the meta-analysis had Rutherford category 1-3 disease (i.e., intermittent claudication) and that the overall number of reported amputations in the included trials was very low, in turn, opposing the theory of significant problems with distal embolisation. Hence, a plausible biological explanation for the identified mortality signal could not be proposed.
However, the observed mortality signal continued to be disquieting, as it was also subsequently corroborated by other researchers utilising data from the key RCTs that supported the US licensing of paclitaxel-coated products for PAD applications. This reanalysis comprised a mean follow-up period of 4 years, and through the post hoc acquisition of more comprehensive follow-up data on vital status, the strength of the mortality signal reduced, while the estimate precision improved (hazard ratio [HR] 1.27, 95% CI: 1.03-1.58)76. Both the FDA and the UK Medicines and Healthcare products Regulatory Agency (MHRA) conducted their own comprehensive reviews of available data and considered the observed mortality signal robust enough to issue safety communications cautioning against the continued widespread application of these devices in PAD patients undergoing lower limb revascularisations7778. However, in their communications, both regulatory authorities still affirmed the added effectiveness benefits of paclitaxel-coated devices in PAD treatment in terms of a variety of technical limb outcomes, especially in PAD patients at high risk for limb loss. Subsequently, Katsanos et al also repurposed their initial data into two additional, more focused meta-analyses on the subpopulations of patients explored in the first meta-analysis. In the first of these meta-analyses, paclitaxel-coated devices seemed to be associated with a lower chance of amputation-free survival in patients with CLTI (HR 1.52, 95% CI: 1.12-2.07; p=0.008)79, and in the second, it was even suggested that the use of drug-coated balloons resulted in a higher risk of major amputation (HR 1.66, 95% CI: 1.14-2.42; p=0.008)80.
Consequently, the long-term safety of drug-coated devices in PAD interventions came under scrutiny, in turn, evoking significant apprehension within the vascular community81828384. Three pragmatic ongoing investigator-initiated RCTs studying treatment with drug-coated devices from the perspective of more robust and patient-centred endpoints (BASIL-3 and SWEDEPAD 1 and 2) temporarily halted trial recruitment due to these safety concerns85.
Since then, a collaborative effort involving various stakeholders in the field − including clinicians, vascular researchers, and representatives from the medical device industry − has resulted in enhanced utilisation and repurposing of both observational data and trial data, with improved data quality and the addition of new or previously missing data, as well as extended follow-up periods within ongoing RCTs. These efforts have been amalgamated in new important datasets, both from individual trials as well as from novel meta-analyses, which have broadly investigated, from different angles, these tentative signs of harm in more refined analyses5765748687888990. Notably, a substantial body of large and ambitious observational studies have been conducted, employing sophisticated statistical techniques to account for potential confounding factors and efforts to explore causal relations. The most notable of these were the German BARMER Health Insurance studies919293, the US Veterans Health Administration study94, the Medicare SAFE-PAD study9596 and the study from the Vascular Implant Surveillance and Interventional Outcomes Network (VISION)94. None of these big data efforts have been able to replicate either a mortality signal associated with paclitaxel-coated devices or an excess risk for severe limb events associated with the use of paclitaxel-containing DCBs and DES. Moreover, previous apprehensions regarding a possible long-term increase in mortality related to the use of paclitaxel-coated devices were also refuted by an unplanned interim analysis from the largest ongoing RCT on this topic, SWEDEPAD (which, by the time of the interim analysis, had included 2,289 patients)98. Interestingly, when this pragmatic study was added to a new meta-analysis, its contributory weight exceeded 60% of the overall explanatory value, and the mortality signal could not be reproduced65. Additionally, several other updated meta-analyses have provided further evidence that ascertain the overall safety of paclitaxel-coated devices65889899. Most recently, a novel meta-analysis was published that focused on the comparative efficacy and safety of endovascular devices utilised in the treatment of intermittent claudication resulting from de novo atherosclerotic lesions (i.e., in a patient population that closely resembled the studied patient cohort in the initial meta-analysis). The risk estimates for all-cause mortality remained similar across short-, mid-, and long-term follow-ups for both treatment approaches88.
As additional data from many of the pivotal RCTs have become available, these have been further utilised in a collaboration between the FDA, device manufacturers, and external stakeholders. Championed by the FDA, device manufacturers participated in an updated meta-analysis that incorporated additional studies, more comprehensive vital status information, and longer-term follow-up compared to previous investigations. The patient follow-up in these studies ranged from 2 to 5 years, with data from most studies extending up to 5 years100. Following a thorough review of the study data, FDA clinicians and statisticians concluded that this updated meta-analysis of RCTs does not indicate any association between the use of paclitaxel-coated devices and a late mortality increase, which prompted a recent update from the FDA removing the previous advice to restrict use to high-risk populations only (Table 7)9.
However, RCTs are limited by the small number of patients and missing data. To complement the patient-level pooled analysis of long-term mortality, an exhaustive national database analysis is required. Recently, DETECT, a nationwide, exhaustive, retrospective cohort study was published. DETECT is based on medico-administrative data from the French national healthcare system, representing >99% of the population. DETECT showed that exposure to paclitaxel-coated devices was not associated with a higher risk of mortality in patients undergoing endovascular revascularisation for lower limb peripheral arterial disease. DETECT also showed a lower unadjusted risk in the paclitaxel-eluting stent and paclitaxel-coated balloon groups than in the non-drug-coated device group for major amputation101.
Thus, the overall risk-benefit profile remains favourable for paclitaxel-coated devices, and paclitaxel-coated devices therefore continue to play a valuable role in the treatment of PAD, albeit the ultimate effects of both DCB and DES devices on endpoints that are most important to PAD patients (limb salvage rates, functional outcomes, and health-related quality of life) remain to be fully confirmed. From a broader scientific viewpoint, the events mentioned above also teach us an important lesson. They illustrate the utmost importance of thoroughly assessing the overall safety profile of medical devices before introducing them widely into clinical practice, especially when the device involves a biologically active component. Had a more rigorous assessment been conducted initially for drug-coated balloons and drug-eluting stents intended for PAD application, it is highly likely that the aforementioned sequence of events could have been avoided.
Table 7. Summary of data on all-cause mortality from individual randomised controlled trials, observational studies and meta-analyses.
| Author, reference | Devices compared | No. of patients | Duration of follow-up | Mortality difference |
|---|---|---|---|---|
| Katsanos8 | DCD vs non-DCD | 4,663 | 5 years | 14.7% (DCD) vs 8.1% (non-DCD) RR 1.93, 95% CI: 1.27-2.93 |
| Schneider74 | DCB vs POBA | 1,980 | 5 years | 15.1% (DCB group) vs 11.2% (POBA group) (p=0.092) |
| Dake16 | Zilver PTX vs POBA | 474 | 5 years | 10.3% (DES group) vs 16.9% (POBA group) (p=0.03) |
| Zhang65 | Paclitaxel DCD vs non-DCD | 9,164 | 5 years | 18.2% (DCD) vs 15.2% (non-DCD) RR 1.18, 95% CI: 0.92-1.51 |
| Lyden86 | DCB vs POBA | 589 | 4 years | 14.0±1.7% (DCB) vs 14.4±2.8% (POBA) (p=0.864) |
| Teichgräber87 | Luminor DCB vs POBA | 171 | 5 years | 11% (DCB group) vs 16% (POBA group) (p=0.38) |
| Hess89 | DCD vs non-DCD | 4,316 | 3.5 years | 12.1% (DCD) vs 12.9% (non-DCD) HR 0.95, 95% CI: 0.83-1.09 |
| Zeller57 | DCB vs POBA | 358 | 60 months | 39.4% (DCB group) vs 44.9% (POBA group) (p=0.727) |
| Xu90 | DCB vs POBA | 180 | 5 years | 19.1% (DCB group) vs 26.4% (POBA group) (p=0.263) |
| Heidemann92 | DCD | 14,738 | 5 years | Paclitaxel-related reduction of 5-year mortality; HR 0.84, 95% CI: 0.78-0.91 |
| Freisinger93 | DCD | 64,771 | 11 years | DES not associated with increased long-term mortality for 11 years post-implantation (all p>0.057)DCB was associated with decreased long-term mortality in the first year post-implantation (HR 0.92; p<0.001) and indifferent in correlation in the years thereafter (all p>0.202) |
| Secemsky95 | DCD vs non-DCD | 168,553 | 2.7 years | 53.8% (DCD) and 55.1% (non-DCD) HR 0.95, 95% CI: 0.94-0.97; p <0 .001 |
| Mao97 | DCD vs non-DCD | 11,452 | 1 year | No mortality increased by using drug-coated devices, HR 0.88, 95% CI: 0.79-0.98 |
| Nordanstig98 | DCD vs non-DCD | 2,289 | 2.49 years | 10.2% (DCD) vs 9.9% (non-DCD) HR 1.06, 95% CI: 0.92-1.22 |
| Dinh99 | DCD vs non-DCD | 2,288 | 60 months | 15.0% (DCD) vs 13.5% (non-DCD) RR 1.07, 95% CI: 0.96-1.20; p=0.20 |
| Parikh100 | DCD vs non-DCD | 2,666 | 4.9 years | No significant increase in deaths was observed for patients treated with paclitaxel DCD; HR 1.14, 95% CI: 0.93-1.40 |
| CI: confidence interval; DCB: drug-coated balloon; DCD: drug-coated device; DES: drug-eluting stent; HR: hazard ratio; POBA: plain old balloon angioplasty; RR: risk ratio | ||||
Conclusions
Nowadays, paclitaxel-eluting devices have demonstrated their safety and efficacy in comparison to POBA and bare metal stents for the endovascular treatment of the femoropopliteal segment. Data are still lacking for indications of drug-eluting devices for the endovascular treatment of the below-the-knee arteries. Numerous new scaffoldings and drugs are currently being assessed to improve the outcomes of lower limb endovascular treatment, but improvements should also focus on new ways of evaluating patients and lesions, and how to carry out more thorough vessel preparation.
Acknowledgements
The authors thank N Lombilla and J Callaert for their contribution.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement
Y. Gouëffic reports research funding from PrediSurge, Biotronik, W. L. Gore & Associates, and GE HealthCare; and honoraria for consulting, medical advisory board, educational course, or speaking from Abbott, BD, Bentley, Biotronik, Boston Scientific, Cook, Eclevar Medtech, GE HealthCare, iVascular, Medtronic, Penumbra, Sensome, Shockwave Medical, and W. L. Gore & Associates. K. Deloose reports consulting fees from Terumo, BD, Biotronik, iVascular and honoraria and speaker fees from Boston Scientific, Abbott, Biotronik, Terumo, BD and iVascular. J. Nordanstig is an advisory board member for AstraZeneca and iThera Medical; he has previously received honoraria for educational activities arranged by Medtronic, BD, and Bayer Medical. The other authors have no conflicts of interest to declare.

