Introduction
Peripheral arterial disease (PAD) is a common and devastating manifestation. Anatomically, approximately 30% of the arterial lesions are located in the iliac arteries, 70% in the femoropopliteal and tibial tract. Isolated lesions below the knee (BTK) are present in only 15% of the cases. Approximately 30% of the symptomatic PAD patients have diffuse arterial disease, and the majority of patients with critical limb ischaemia (CLI) have distal arterial disease with occlusions in the tibial arteries. CLI is typically characterised by diffuse multi-level infrainguinal and tibial arterial occlusive disease, primarily associated with diabetes, whereas femoropopliteal disease is often characterised by long, diffuse lesions and long occlusions. Surgical bypass has long been considered the gold standard treatment when symptoms could not be controlled by risk factor modification, exercise therapy or medication. However, with the introduction of new interventional techniques and devices, endovascular intervention has become the first-line therapy to treat PAD and even complex arterial diseases. Despite an initial technical success rate of more than 95% for percutaneous transluminal angioplasty (PTA) to recanalise the superficial femoral artery (SFA)1, in addition to vessel recoil and dissections recognised and treated during the procedure restenosis occurs in 40 to 60% of the treated segments after six to 12 months2-4. Percutaneous angioplasty of BTK arteries is an accepted therapy for patients with CLI. Limb salvage rates were reported in 80% to 90% after PTA5-7. However, after failed revascularisation attempt, 40% to 50% of the patients will lose their leg within six months, and the mortality rate will be about 20%7.
At the level of the pelvic arteries, balloon expandable stents or self-expanding nitinol stents are used with excellent acute and longer term revascularisation rates8. However, despite being more effective than balloon angioplasty for preventing coronary artery restenosis, a benefit of stenting in the vessels of the lower extremities remains to be confirmed9-12. Compared with PTA or stainless steel stents, newer generations of nitinol self-expanding stents appear to have a positive impact on primary patency rates in femoropopliteal arteries of patients with short, complex stenosis and occlusions13-15.
Stents stabilise dissection and prevent rapid elastic recoil and late negative vessel remodelling, but increase inflammation, thereby leading to more intimal hyperplasia resulting in in-stent restenosis. Drug-eluting stents (DES) have emerged as a successful strategy in the primary prevention of coronary restenosis as documented in preclinical and clinical trials16-18. Whereas in the coronaries the role of DES in the treatment of in-stent restenosis is well defined, very limited data exist about the use of DES in the treatment of in-stent restenosis in peripheral arteries. In a small number of trials, DES were investigated for their applicability and/or compared with bare metal stents in the treatment of femoropopliteal lesions19,20 as well as of infrapopliteal obstructions and critical limb ischaemia21-26. While DES failed to indicate restenosis inhibition in the superficial femoral artery, there are some data showing that BTK DES is safe and associated with low rates of in-stent restenosis or occlusion21-25.
DES are characterised by sustained drug delivery due to special features for slow release (mostly polymers) from the stent struts to the arterial wall. Late thrombotic complications27,28, delayed and incomplete endothelialisation of the stent struts29 due to high drug concentrations on the stents put the long-term safety of DES into question. On the other hand, incomplete suppression of neointimal hyperplasia at the stent margins or between the struts may limit the efficacy of DES18,30, especially of those stents used for SFA lesions due to larger stent strut distances.
New concepts to overcome the limitations of DES should avoid the need for sustained drug release from stent struts to allow for earlier endothelialisation and healing. Preclinical studies including cell culture experiments31,32 and the porcine coronary overstretch model32-35 showed that short exposure to paclitaxel can result in prolonged inhibition of cell proliferation and neointimal hyperplasia provided that the drug reaches the vessel wall in sufficient concentration. The concept of non-stent based local paclitaxel delivery was prompted by the surprising discovery that single-dose administration of the drug is sufficient, refuting the assumption that sustained release is necessary for long-lasting inhibition of restenosis. The finding of effective inhibition of neointimal formation using paclitaxel-coated balloons in the porcine model of coronary overstretch was confirmed in both first-in-man clinical trials (PACCOCATH-ISR-I and PACCOCATH-ISR-II), accessing the safety and efficacy of this device in coronary in-stent restenosis36,37. Paccocath® coated balloons are standard angioplasty balloons coated with paclitaxel at a dose of 3µg/mm2 of balloon surface in a specific matrix coating (paclitaxel is admixed to a small amount of the x-ray contrast medium Ultravist®). An additional preclinical study38 investigating different inflation times and increased doses due to overlapping coated balloons –two critical features of DEB application– indicates that neointimal proliferation was significantly reduced without obvious signs of toxicity (e.g., thrombotic occlusion or aneurysms). This was the case regardless of the doses tested. It was also shown that contact time was not a critical issue since the results with 10-second inflation time were almost identical to those obtained at inflation times of up to 120 seconds. These preclinical and clinical studies have shown that drug-coated balloons have a number of advantages over standard PTCA (as well as PTA) and stent technologies including:
(i) the potential for homogeneous drug delivery to the vessel wall, which is not accomplished using drug-eluting stents;
(ii) an immediate drug release without the use of a polymer which can induce chronic inflammation and late thrombosis as observed with some drug-eluting stents;
(iii) the option of using balloon catheters alone or in combination with a bare metal stent;
(iv) no foreign object such as drug-eluting stents left behind in the body;
(v) the potential of reducing antiplatelet therapy; and
(vi) lower restenosis rates in target coronary arteries compared to treatment with uncoated balloons.
Albrecht et al39 investigated whether the preclinical coronary data on the efficacy of paclitaxel-coated balloons could be translated to peripheral arteries. In the porcine model, stenosis in stented segments of the superficial femoral or popliteal arteries was significantly reduced by local short-term administration of paclitaxel delivered via balloon or in a contrast medium during PTA and stent implantation. Based on these data, and already before results of the first coronary ISR trial became available, two additional trials (Thunder and FemPac) were started enrolling patients with de novo stenosis and occlusions as well as restenosis in the superficial femoral or popliteal arteries40,41.
Tübingen/Rosenheim experience in the SFA-area
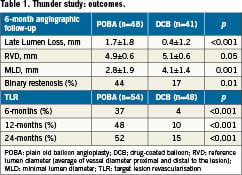
The Thunder trial40 was a German, randomised, blinded, multicentre study comparing Paccocath® paclitaxel-coated and conventional uncoated balloon catheters with respect to efficacy and tolerance in inhibiting restenosis in the peripheral arteries. In this trial, which included a two-year follow-up, a total of 154 patients with stenosis or occlusion of the superficial femoral or popliteal arteries were enrolled. Fifty-four patients were treated with uncoated balloons (=control group), 52 with a Paccocath® coated balloon and 48 with uncoated balloons and paclitaxel dissolved in the contrast medium (Ultravist®; 17.1 mg paclitaxel in 100 mL). The primary endpoint was late lumen loss of vessel segment at 6-months; the secondary outcomes measures included binary restenosis, target lesion revascularisation, and thrombosis. At 6-month follow-up, treatment of patients with Paccocath® balloons was found to be associated with significant reductions in late lumen loss compared to patients of the control group or patients treated with paclitaxel dissolved in the contrast medium (Table 1). Importantly, the rate of target lesion revascularisation at 6, 12 and 24 months after intervention remained significantly lower in the Paccocath group compared with both other groups. Only 4% of the patients in the Paccocath group received additional stents in target lesion versus 22% in the control group. No increase in thrombotic or embolic events was observed in the Paccocath group. The Thunder trial is still ongoing and awaits results from 5-year follow-up.
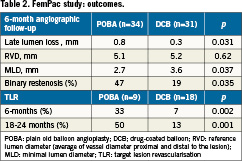
In the FemPac trial41, 87 patients with femoropopliteal artery occlusion or stenosis were randomly assigned to the treatment with either standard balloon angioplasty or the Paccocath® balloon. Forty-two patients were allocated to the control group (=uncoated balloon) and 45 patients to the Paccocath group. At 6-month follow-up, patients treated with the Paccocath® coated balloon had significantly reduced late lumen loss compared with the control subjects (Table 2). The difference between both treatment groups was maintained 18-24 months after intervention. Furthermore, patients in the coated balloon group also showed improvement in Rutherford class, but no difference in the improvement in ankle brachial index was found. Nine percent of the patients in the Paccocath group vs. 14% in the control group required additional stent implantation in target lesion. Compared to the Thunder trial, late lumen loss in the control group was lower in this study. Nevertheless, the FemPac trial confirmed the results of the Thunder trial, demonstrating that short-term exposure of injured peripheral arteries to paclitaxel may be sufficient to inhibit restenosis. Both studies create new hope to surrender the problem of restenosis development especially in the SFA area.
Despite the enthusiasm there are also limitations. The Thunder and FemPac trials were designed to compare the effect of the drug-coated balloons compared to non-coated balloons. In these studies, however, patients with very heavy calcification in the SFA, which might request stenting, were not enrolled. Highly calcified atherosclerotic plaque in SFA occurs in up to 15% of the patients. In such heavily calcified arteries DEBs might be less effective because of the low drug concentration reaching the vessel wall due to the calcium shielding. A possible approach to overcome this limitation is to combine atherectomy first with the use of DEBs. Directional atherectomy devices such as the SilverHawk® Plaque Excision System (ev3 Endovascular, Inc., Plymouth, MN, USA) are designed for the treatment of de novo and restenotic atherosclerotic lesions located in native peripheral arteries. Long, diffuse, even calcified lesions in the SFA could be treated successfully with the SilverHawk® atherectomy device42,43. Since in theory, plaque removal might work well together with local inhibition of excessive neointimal formation, an interesting approach would be the combination of local drug delivery with atherectomy devices. Thus, the concept of this combination in calcified femoropopliteal lesions will be investigated in the DEFINITIVE™ AR study. This European prospective, multicentre pilot study will randomise 100 patients with calcified SFA lesions either to receive a DEB only or atherectomy with the Silverhawk® or Turbohawk® catheter (Ev3) followed by local drug therapy using the Cotavance Paccocath® balloon (Medrad, Inc., Pittsburgh, PA, USA). An additional 25 patients with severe calcifications will be treated in a registry. The primary endpoint is target lesion percent stenosis at 1-year. A case of a diabetic patient with restenosis in the SFA, who underwent atherectomy followed by treatment with a DEB, is shown in Figure1. The procedure was safe and feasible, showing no side effects or any sign of restenosis at six months. The combination of atherectomy and DEB might be especially suitable for treating lesions of the popliteal artery, a highly mobile vessel not very amendable to stent placement. Furthermore, each method might overcome limitations of the other method. Despite the ability to remove calcified plaques atherectomy does not address excessive cell proliferation resulting in restenosis –on the other hand DEBs are designed to decrease neointimal proliferation but might have limitations in calcified arteries. Obviously, there is still much to learn about DEBs with respect to their benefits, limitations, side-effects, indications and contraindications. The same also applies to atherectomy. Atherectomy alone might not remain a niche technology, but it might help to overcome limitation of other technologies, e.g., by being applied in combination with DEB.
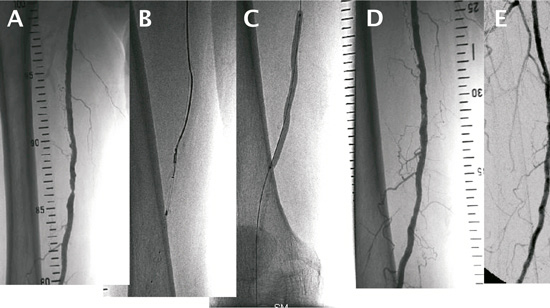
Figure 1. Angiograms of a diabetic patient with restenosis in the SFA. (A) depicts the initial angiogram of the SFA lesion which underwent atherectomy (B) followed by the treatment with a paclitaxel-coated balloon (C). (D) shows the post-procedural angiogram. At 6-months, no side effects and no restenosis in the treated SFA area were seen (E).
DEB: (The Bad Krozingen) experience below the knee
At the same time, tibial arteries are no more the forgotten region of PTA due to the emergence of innovative medical technologies making tibial procedures feasible, safe and durable. Endovascular stenting using balloon-expandable coronary stents has been shown to be of potential value to improve the results of infrapopliteal angioplasty44. DES were recently proposed treating infrapopliteal arteries. Data from single-centre studies reported significantly reduced restenosis rates in the infrapopliteal arteries21,22,45 at 6-month follow-up, and a sustained one-year benefit with a significant decrease of clinically driven reinterventions (i.e., target lesion revascularisation) in the Cypher™ (sirolimus-eluting stent) group compared with the bare metal stent group46. Recently published DES-registries reported primary one year patency rates exceeding 80% and excellent limb salvage rates47-49.
Very recently, the first randomised controlled YUKON-BTK trial reported significantly higher primary and secondary patency rates in infrapopliteal arteries at 12 months for the polymer-free sirolimus-eluting stent vs. bare metal stent (primary and secondary rates: 81% vs. 56%, p <0.004 and 92% vs. 71%, p <0.005, respectively)26. Despite these encouraging results, there are limitations of DES in infrapopliteal disease. In particular, CLI patients frequently suffer from long diffuse lesions up to 30cm in length. Those long lesions cannot sufficiently be treated with DES in their full length due to the lack of long DES and spot stenting had been shown to be associated with high restenosis rates in the lesion segments that are not covered with DES46. Moreover, lesions located at the level of the ankle or below are contraindications for balloon expandable stents due to external compression forces applied to the stent with the risk of stents compression and consecutive vessel occlusion.
Encouraged by the results with paclitaxel-coated balloons in SFA, two (INPACT-DEEP and PICCOLO) studies are currently investigating the impact of DEBs in BTK lesions in patients presenting with critical limb ischaemia (CLI). These trials are randomised, single blinded, two-arm, multicentre studies. The PICCOLO trial assesses the efficacy of Paccocath® coated vs. uncoated conventional balloons for prevention of restenosis in 114 patients with stenotic lesions in small arteries below the knee. Follow-up includes control angiography after six and 18 months and clinical follow-up examinations up to 18 months. The primary endpoint is late lumen loss at six months. Eighteen months follow-up of the trial had been recently completed, and the first results from this study are expected to be released in April 2011. A case of a patient with a long occlusion in the anterior tibial artery (ATA) treated with Paccocath® coated balloons in the PICCOLO study is presented in Figure 2. In the INPACT-DEEP trial, an estimated 357 patients with BTK critical limb ischaemia (Rutherford Class 4-6) are treated with either an IN.PACT™ Amphirion drug-coated balloon (Invatec S.p.A., Roncadelle, Italy) or a standard PTA balloon. The primary endpoints are late lumen loss at 12 months as assessed by quantitative vascular angiography in a sub-cohort of 168 patients with lesions up to 10 cm in length, and clinically driven target lesion revascularisation in the amputation-free surviving patients at 12 months. This study has a long-term follow-up schedule up to five years. Until October 2010, 104 patients had been enrolled in the trial.
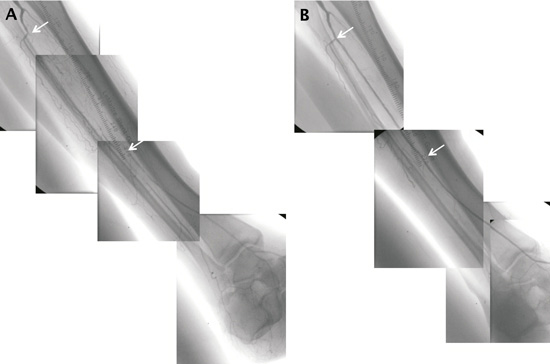
Figure 2. (A) The initial angiogram shows long total occlusion in the anterior tibial artery (ATA). (B) Completion angiogram after successful recanalisation of the ATA with two 12 cm long overlapping paclitaxel-coated balloons, showing restoration of blood flow.
The number of infrapopliteal cases treated with DEB in the Heart Center Bad Krozingen, Germany, has been significantly rising since dedicated low profile DEB with sufficient length became commercially available (7% and 24% of all infrapopliteal interventions in 2009 and 2010, respectively). Main indications for DEB application are recurrent stenosis, diffuse lesions longer than 10cm, single vessel run-off (in particular diabetics), distal lesion location (ankle, foot) and lesions in patients with complex wounds. The first Leipzig experience (Heart Center Leipzig; PI: Andrej Schmidt, MD) comparing two patients cohorts with BTK lesions with a mean length of 18 cm treated either with plain balloons or with IN.PACT™ Amphirion balloons showed a significantly lower restenosis rate in the DEB BTK group vs. uncoated group (angiographic restenosis >50% after three months: 27% vs. 69%, and restenosis of the whole treated segment: 11% vs. 56%). Although these first results are encouraging, there are some issues to be considered in using DEB in BTK lesions including:
(i) possibly significant drug loss on the way to target lesion which, however, might be prevented by predilatation of the vessel with a conventional balloon (should be shorter and smaller in diameter than DEB), and/or by the use of protected device introduction (e.g., guiding catheter, long sheath);
(ii) possible occurrence of early recoil and dissection in the target vessel after intervention;
(iii) possibly limited drug delivery from DEB to the vessel wall due to heavily calcified plaque; and
(iv) possibly incomplete lesion coverage with the drug.
The latter two limitations can be solved by performing plaque excision prior to drug delivery as an essential combination in BTK lesions as well as in SFA lesions. The rationale is firstly to mechanically remove the atherosclerotic plaque by atherectomy –to allow (i) DEB to cross the true lumen and (ii) homogeneous drug uptake by the vessel wall– and secondly to enhance prevention of neointima proliferation by drug delivery via DEB. This approach may also avoid early recoil and dissection, reduce the likelihood of bailout stenting and preserve the native vessel. However, the role of the combination of atherectomy with DEB in peripheral interventions has still to be investigated to rule out increased risk of pseudoaneurysm or aneurysm formation and to determine which debulking devices are appropriate for the combinational use with DEB.
The use of endovascular techniques including DEB application with or without upfront atherectomy or plaque modulation might become a strategy of the treatment of long diffuse SFA and BTK lesions. The future challenge is to improve the durability of the procedures. Debulking techniques and local drug delivery might play akey role in achieving this goal.
Conflict of interest statement
G. Tepe has received study support and is an advisor* for Abbott*, Bard, Cook, Ev3, Medtronic-Invatec* and Medrad. T.Zeller is an advisory member of Medrad, Medtronic-Invatec and gives study courses sponsored by Biotronik, EuroCor and Lutonix. S.Schmitmeier has no conflict of interest to declare.
References

