- drug-eluting balloon
- coronary artery disease
- valvuloplasty
- erectile dysfunction
- pudendal artery
- acute myocardial infarction
- peripheral vascular disease
Abstract
The drug-eluting balloon (DEB) is an exciting new technology that holds much promise. As an evolving technology undergoing intensive research, the device is being constantly refined and its numerous potential applications studied. Though initially created to fulfil specific needs in the coronary vasculature, there is great potential for its use in other vascular territories and structures including the management of valvular, congenital heart and neuro-interventional pathologies. In addition, the application of this device in conjunction with other existing technologies may enhance the clinical results.
Introduction
The field of interventional cardiology has experienced major disruptive technologies that revolutionised the management of patients whilst offering wider therapeutic options and improved outcomes.
Pioneering work by Dr. Andreas Gruentzig with the performance of the first coronary angioplasty on the 16th of September, 1977 marked the beginning of catheter-based interventions1,2. The first patient, Mr. Adolph Bachman, who was then 38 years old, received balloon angioplasty treatment to the proximal left anterior descending artery segment. The fact that a repeat angiography 23 years later, in 2000, showed excellent durable result at the treatment segment, bore testament to a therapy that worked3. Despite the excellent result in the first patient, plain old balloon angioplasty in general was beset with risks of acute vessel closure and high restenosis rates.
Then came the era of stents, with the first coronary stent implantation performed by Dr. Sigwart and Dr. Puel in Toulouse, France in 19864. It addressed the issues of vessel dissection, acute vessel closure and reduced restenosis by 30-40% resulting from negative remodelling of the external elastic lamina5,6. However, stents came with its new set of problem, i.e., restenosis from neointimal hyperplasia.
When drug-eluting stents (DES) became available, the amazing results –for example the first-in-man study (RAVEL trial7) and its durable five year follow-up data8– prompted many interventionalists to believe that they had found the panacea to restenosis. As with many new technologies, reality sets in a few years after the hype and hope faded. With time, there was a suggestion of a late “catch-up” phenomenon in the TLR rates. This observation, however, was not consistent and this TLR “creep” was not seen in other analyses9. More importantly were the disconcerting reports of late10 and very late stent thrombosis11. This was related to delayed endothelial healing12 which was seen in pathological specimens13 and angioscopy14 even many years after implantation15.
Hence, there is now a revival in the use of an old technology, the angioplasty balloon for local delivery of drugs. In essence, this “upgrade” allows the application of a drug to inhibit restenosis but without the issues associated with a stent.
Available DEB technologies
The drug-eluting balloon is very much an evolving technology. There is much to be learnt and discovered. The body of evidence needs to be built-up in order to realise its full potential. The results have been impressive as an option for the difficult problem of in-stent restenosis, and its result promising for small vessel disease. In other areas, the application of DEB is still experimental and speculative in many ways. Applications in different clinical scenarios and in conjunction with available interventional armamentarium are being continually explored and tested. Interest amongst the device companies in this technology is keen and fast growing. Many delivery systems are currently under development and some are in clinical use. Some available systems are listed in Table1.
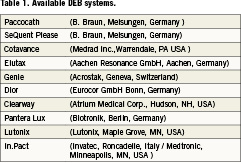
Beyond the coronaries, DEB may potentially be very useful for peripheral and neurovascular interventions, valvular heart disease as well as paediatric congenital solutions.
Aortic valve stenosis
Aortic valve stenosis may be prevalent in up to 2-6%16 of the unselected elderly population. Surgical valve replacement is the mainstay of treatment, especially if done early, as it potentially restores an almost normal life expectancy17,18. The recommendation for surgery is however impacted by the presence of multiple comorbidities, e.g., advanced age, neurological dysfunction, left ventricular dysfunction and, therefore, higher surgical risks. Owing to these reasons, this life-saving and symptom-improving surgery is not carried out in up to one-third of patients19-21.
The management of severe aortic valve stenosis has undergone rigorous scrutiny and research in recent years. This is largely due to the development of transcatheter aortic valve interventions (TAVI). The recently published multicentre randomised PARTNER trial concerning the placement of aortic transcatheter valves showed very favourable 1-year mortality and symptom-relief benefits of transcatheter aortic valve implantation in patients unsuitable for surgical valve replacement22.
Balloon aortic valvuloplasty was first described by Cribier in 198623. Prior to the development of TAVI, percutaneous aortic balloon valvuloplasty was frequently attempted but results were often disappointing. The acute success rate was moderate, the valves restenosed early, and the long-term survival rates remained dismal24,25.
Today balloon aortic valvuloplasty is reserved for the stabilisation of haemodynamically unstable patients, particularly as a bridge towards surgical valve replacement or TAVI.
Whilst there may be great interest to embark on a TAVI program, it is a very costly and resource intensive endeavour. Balloon aortic valvuloplasty (BAV) therefore is not a lost-cause and remains an attractive option to explore.
The pathology of a restenotic valve following balloon aortic valvuloplasty demonstrates active capillary growth, cellular proliferation with formation of granulation tissue, valve fibrosis and even ossification26. The insight from this histological picture suggests an inflammatory and proliferative response from the balloon intervention. Therefore, delivery of an antiproliferative agent to the valve may ameliorate some of the restenotic responses.
Utilising paclitaxel-eluting balloons in animal pre-clinical studies, Spargias et al were able to demonstrate significant delivery of this drug to the aortic root, aortic valve leaflets, as well as the left ventricular outflow tract after 2-4 inflations27.
The first-in-man paclitaxel-eluting balloon aortic valvuloplasty was performed on the 26th September, 2008 by Dr. Spargias at an interventional meeting. A press release from the company developing this balloon reported a reduction of the transaortic pressure gradient from 56 to 32mmHg after two inflations of a 20×40mm balloon.
There has been other interesting preclinical work (in press) announced in recent interventional meetings which showed a great rebound in the transvalvular pressure gradient soon after BAV with an uncoated balloon, but persistent reduction of pressure gradient after drug-eluting balloon valvuloplasty. Histological features of inflammation and cellular proliferation were also diminished after drug-eluting balloon valvuloplasty.
As the evidence-base for the application of this technology accumulates, we may see wider applications of drug-eluting balloon aortic valvuloplasty as an alternative to TAVI for patients who are not eligible for surgery. In fact, one may argue that even if DEB valvuloplasty results may not be as durable as TAVI (noting that TAVI’s long term results still require confirmation from longer follow-up data), DEB valvuloplasty is certainly attractive for a number of reasons.
This simpler procedure may be performed in most cardiac catheterisation laboratories without additional resources and is definitely more cost saving to organise and perform. Even if one were to project that the valves could restenose in approximately two to three years, the procedure is repeatable, and still at a fraction of the cost of a structured TAVI program.
Mitral valve intervention
The incidence of mitral stenosis, which is predominantly rheumatic in aetiology, has declined significantly in developed countries. However, it is still a prevalent problem in many of the poorer developing nations. Percutaneous mitral balloon valvuloplasty was first performed by Inoue in June, 198228 and it has since been the mainstay of treatment for this condition. Other variations of the balloon technique –e.g., the double-balloon technique was introduced but later fell out of favour with the introduction of the Inoue balloon technique which was simpler to perform– yielded similar results and was associated with lower complication rates.
Various restenosis rates have been reported, largely dependant on the definition adopted, final post-valvuloplasty mitral valve area obtained, patient age, follow-up period and valvular features, for instance, echo scores29-31. Event-free survival rates (from repeat percutaneous mitral balloon valvuloplasty, mitral valve replacement, cardiac death, high NYHA class) are generally good up to 10years32,33.
There are two main mechanisms for mitral valvular restenosis: commissural re-fusion and the progression of subvalvular thickening and/or degeneration. Turgeman et al34 reported that patients with mitral restenosis caused by symmetrical commissural re-fusion often responded well to repeat balloon commissurotomy procedures as compared to patients in whom the pathological mechanism of restenosis was mainly subvalvular and the commissures were not bilaterally fused but rather unilaterally or bilaterally split.
Similar to the drug-eluting balloon aortic valvuloplasty concept, it is logical to theorise that combining the Inoue balloon, which splits the commissure with an anti-proliferative drug coating, the long-term success of mitral valvuloplasty could be further enhanced.
Coronary interventions
Acute myocardial infarction
Primary percutaneous coronary intervention is a preferred reperfusion strategy in many ST-elevation myocardial infarction situations. The implantation of stents improved the acute results, but there is always a concern with risk of stent thrombosis in these acute coronary syndrome scenarios35,36. DES implantation in acute STEMI setting has been associated with an increased risk of late stent thrombosis37.
There are mechanistic reasons to suspect why this may be so. Angiographic stent thrombosis risk appears to increase when there is a larger thrombus burden at the time of primary PCI DES implant38. The presence of thrombus apposed on the stent surface causes highly variable and unpredictable antiproliferative drug delivery to the arterial wall39. It is suspected that such variable drug effect on the arterial wall may predispose to areas of uncovered struts and late stent malapposition.
In contrast, implanting a bare metal stent following a paclitaxel-eluting balloon dilatation in a native vessel resulted in better stent endothelialisation as seen on optical coherence tomography (OCT).
Early reports (unpublished) of primary PCI showed bare metal stent implantation after treatment with paclitaxel-eluting balloon resulted in superior stent endothelial coverage as seen on optical coherence tomography when compared to Cypher (a sirolimus eluting stent). The ongoing “Drug-Eluting Balloon in Acute Myocardial Infarction (DEB-AMI)” (Clinical Trials.gov Identifier: NCT00856765) study is testing this hypothesis. The OCT follow-up of its first patient showed excellent strut endothelial coverage40.
However, these concerns may not be translated to clinical significance as meta-analysis of DES randomised controlled trials in STEMI41 only showed a non-significant trend towards stent thrombosis, but no mortality concern, and significant benefits in reducing target lesion revascularisation. Hence, whether DEB will find its role in AMI percutaneous coronary intervention is left to be seen.
Paediatric intervention
For many years, paediatric interventional cardiologists have been performing balloon angioplasties for congenital aortic and pulmonary valve stenoses, vascular stenoses, e.g., pulmonary artery, aortic coarctation, pulmonary vein stenoses, surgical conduits (aorto-pulmonary shunts and other extra-cardiac conduits). In some cases, stent implants are necessary. Conduit stenoses tend to restenose easily after balloon angioplasty alone. Stent implantation offers better durability. These are either balloon-expandable stents or self-expandable ones.
Paediatric patients present a different challenge to interventionalists. The basic problem lies in the fact that the child continues to grow. Therefore, stent to vessel size mismatch is an issue. Balloon expandable stents allow some degree of further expansion with sequential larger balloon dilation as the child grows. It has good radial strength, allows accurate positioning with less foreshortening, and has a long history of use and experience. They are however stiffer and requires a relatively larger delivery system. The self-expandable stents are usually made of nitinol or cobalt-chromium alloy. They are of smaller profile and are more flexible. It is conformable to the vascular architecture and resists stent crush. However, it has lower radial strength and has limited potential for further expansion beyond its pre-set diameter. Stent restenosis, stent fracture, limitation in future surgical conduit replacement, significant regurgitation in a valved conduit and coronary artery compression are other potential stent-related complications42.
Realising these limitations, developments are in place for the possibility of bioabsorbable stents, or better still, avoiding stents altogether.
The possibility of using drug-eluting balloons for these indications is certainly attractive. It may offer durable benefits compared to balloon angioplasty alone and it certainly does away with problems related to stent implantation.
This potential has not escaped the attention of paediatric cardiologists and paclitaxel-eluting balloon treatment of congenital pulmonary vein restenosis has been described43.
Neuro-interventions
Catheter-based interventions for some intracranial vascular lesions sometimes require balloon angioplasty and also stent placement. Intracranial stenosis in particular is highly prevalent in Asians, Hispanics and African-Americans.
Symptomatic stenosis –i.e., hospitalisation for cerebral events– varies from 1% in non-Hispanic whites, to as high as 50% in Asian populations44,45.
Balloon angioplasty and stent implantation in the tortuous intracranial neurovascular anatomy is a challenge. Even with the use of coronary stents, its rigidity limits access. Procedural complication rates may be between 0-36%. There are dedicated intracranial stent devices, e.g., the Wingspan Device (Boston Scientific, Natick, MA, USA). However, they also present with a high restenosis rate (31%).
Some of these technical issues may be overcome with the use of drug-eluting balloon.
In fact, the first-in-man intracranial paclitaxel-eluting balloon (Elutax® from Aachen Resonance) neuro-intervention was performed in the basilar artery of a man in April, 2009. Follow-up angiography at five months revealed good vessel patency.
Other vascular interventional applications
The ability to perform therapeutic dilatation followed by delivering a drug locally to prevent restenosis has generated interests in applying the drug-eluting balloon technology to other parts of the vasculature.
One of the potential applications is in central vein stenosis angioplasty. Previous experiences with regular balloon dilatation or stent implant showed poor primary patency rates (less than 30%) at one year. Repeat angioplasties provided reasonable assisted primary patency rates and is the norm regardless of whether there was a stent implanted46. Aachen Resonance has initiated the first-in-man application of this technology for recurrent subclavian vein stenosis for a young gentleman on haemodialysis.
Stenosed and dysfunctional dialysis AV-fistula or grafts may receive angioplasty with the hope of prolonging the patency of the vascular access. The primary patency rates are variable and range from 43-77% at six months and are worse for angioplasty of an arterial-venous graft rather than fistula47-49. In addition to the choice of the cutting balloon to improve the procedural success and especially for durability, DEB may be an attractive option for dialysis access intervention.
Pudendal artery intervention for erectile dysfunction (ED)
Male sexual function has always been a topic of intense interest for many. Penile erection is a complex activity that involves neural pathways that modulate vasodilating parasympathetic discharges and also gonadal androgens that enhances local nitrous oxide (NO) release. The resultant increased arterial inflow encourages penile tumescence.
Whilst there may be many psychogenic drugs, as well as hypo-androgenic factors that interfere with the erectile function, vascular insufficiency is another contributing factor50.
It is clear that erectile dysfunction is a close correlate of coronary artery disease, sharing many of the same risk factors51 and has common co-existence52-55. Up to 70% of men with coronary artery disease have erectile dysfunction.
There are many effective therapeutic options available for ED today and the introduction of phosphodiesterase-5(PDE-5) inhibitors have revolutionised its management. However, there remain a significant number of patients who do not respond favourably to these modern treatments. This may be due to the unaddressed problem of vascular insufficiency. In ED patients with concomitant leg and hip claudication, stenosis of the common or internal iliac arteries may be the responsible pathology. This may be addressed easily via endovascular intervention techniques with good durable results. In other patients, the culprit lesions may be stenoses in the more distal pudendal arteries and its branches.
Reported at the TCT 2009 meeting, the “Pelvic Angiography in Non-Responders to Phosphodiesterase-5 Inhibitors (PANPI)” (Clinical study identifier: NCT00574184), unpublished study of 10 patients with coronary artery disease and ED which was unresponsive to PDE-5 inhibitors, 100% correlation was found between presence of angiographic CAD and pudendal artery disease. The pattern of disease was similar in both arterial territories, suggesting feasibility of treatment with stent implantation.
Angioplasty treatment of these distal vessels for arteriogenic impotence had been described as early as 198256,57. Long-term patency of the pudendal artery and its branches had been consistently poor.
At the same meeting, Medtronic reported the launch of the ZEN Study (Zotarolimus-Eluting Peripheral Stent System for the Treatment of Erectile Dysfunction in Males with Sub-Optimal Response to PDE5 Inhibitors). The results are eagerly awaited and it was reported that enrolment of the planned 50 patients from nine participating US centres had been enthusiastic.
However, experience from the coronary vasculature warns of potential problems with stents, especially in the penis.
Think about stent crush? It is not inconceivable to consider the traumatic penile injuries that may occur during sports, accidents or even vigorous sexual activity. The consequences of stent crush may be ugly. DES itself is associated with higher stent thrombosis risk. Risk of penile ischaemia, gangrene and even amputation is real.
Consider then, the use of DEB for pudendal artery stenosis. The durability of balloon angioplasty results could be improved, but, more importantly, complications associated with stent implant could be avoided. Hence, I believe that DEB application for ED should be seriously explored ahead of stent implantation.
Bioabsorbable stents
It is every interventionalist’s dream to one day be able to use a stent that does its role of opening and scaffolding the vessel and then later disappears; with this, we could do away with problems of stent thrombosis and restenosis.
Being able to incorporate an antiproliferative drug within this bioabsorbable stent would enhance its longer-term results58,59.
The furthest along in development is the the everolimus-eluting poly-L-lactide polymer based stent from Abbot Vascular (Abbott Vascular, Redwood City, CA, USA) which is being studied in the ABSORB trial. However, many issues still need to be optimised. The stent’s late-loss and higher-than-expected restenosis rates tells us that much work needs to be done. The ideal bioabsorption kinetics still need to be sorted out. At two years, imaging studies showed that at least one-third of the stent has been absorbed.
At present, these stents are still far from being in routine clinical use. There is a lot more that we need to learn about the best bioabsorbable material (that would provide the radial strength and scaffold), stent design and profile (that would determine deliverability), biocompatibility, radio-opacity as well as ideal absorption kinetics. To combine the need to improve on these issues with the requirement to elute an antiproliferative drug adds complexity to the development and may delay its progress.
One may suggest that perhaps, whilst we concentrate on the physical development of the bioabsorbable stents, adjunctive use of a DEB to deliver the antiproliferative drug to the vessel wall seems logical. Any takers?
Peripheral vascular intervention
This manuscript does not intend to discuss this topic in-depth as it is addressed in other submissions. However, there is no question that the next big “explosion” in the use of DEB would be its application for peripheral vascular intervention.
There are extensive trial programs with various DEB platforms for the management of peripheral arterial disease. Preliminary results demonstrating consistent improvements in late loss and restenosis rates when compared to uncoated balloon angioplasty with different balloon designs, herald its wide-spread use for lower limb intervention60,61.
Conclusion
The drug-eluting balloon is a resurrection of an old technology, i.e., the angioplasty balloon which returns to fulfil a niche that stents could not satisfy62.
The early promises of restenosis prevention with drug-eluting stents was offset by concerns of stent thrombosis, prolonged dual anti-platelet therapy and possible late TLR-creep.
With an antiproliferative drug coating of the angioplasty balloon, this “upgrade” allows the delivery of the drug without the problems related to stent implantation.
Evidence for DEB benefits is very favourable for the management of in-stent restenosis. Small vessel intervention is a possible indication.
DEB is still an evolving technology that is undergoing refinement. It is a technology that is here to stay and will complement the various percutaneous intervention strategies available.
There is great excitement on its potential applications for various coronary, cardiac and extra-cardiac interventions. Active research interests abound and we hope that in the future some of the DEB promises suggested in this discussion will bear fruit.
Conflict of interest statement
The author has no conflict of interest to declare.
References

