Abstract
Through continued innovation, percutaneous treatment of coronary and peripheral stenoses has evolved rapidly since balloon angioplasty was first introduced three decades ago. Significant advances were made with the introduction of bare metal stents and subsequently drug-eluting stents, which expanded the possibility of successful revascularisation in complicated lesions. Despite these advantages, efforts are still ongoing to improve patient outcomes further. In recent years, drug-coated balloons have emerged as an exciting technology developed to overcome the limitations faced by drug-eluting stents, such as stent thrombosis and dependency on prolonged dual antiplatelet therapy, and may prove efficacious in complex subsets such as small vessels and diffuse lesions where stent results are suboptimal. Several drug-coated balloons developed for coronary and peripheral applications were evaluated recently in preclinical and clinical studies with encouraging results. Drug-coated balloons have proven effective in treating in-stent restenosis; however, there is accumulating evidence on their utility in other clinical scenarios. We present a timely review of the mechanisms of action, key preclinical studies, emerging clinical indications, current clinical trial results, and future perspectives of this novel drug-coated balloon technology as it seeks to establish its role in percutaneous intervention.
Introduction
The introduction of balloon angioplasty in the 1970s transformed the treatment of coronary artery disease1. Since then, percutaneous coronary intervention (PCI) has made rapid progress by providing further innovations in revascularisation to combat restenosis. Bare metal stents (BMSs) were introduced to overcome vessel dissections, abrupt closure, and elastic recoil seen with balloon angioplasty, but their success was limited by in-stent restenosis (ISR)2. The introduction of drug-eluting stents (DESs) was a major breakthrough in further reducing restenosis rates by significantly inhibiting neointimal hyperplasia proliferation3,4. However, safety concerns surfaced with the new phenomena of late and very late stent thrombosis (ST) attributed to delayed endothelial healing, vessel wall inflammation, and impaired endothelial function5. Furthermore, dependency on prolonged dual antiplatelet therapy (DAPT) is associated with significant bleeding risk6. The long-term efficacy in preventing restenosis is also limited for DESs in difficult patient and lesion subsets, such as diabetics, complex coronary anatomy, including small vessels, diffuse disease, and bifurcation lesions7. This prompted the development of drug-coated balloon (DCB) technology as a potential therapeutic alternative. DCBs are semi-compliant angioplasty balloons covered with an antirestenotic drug that is rapidly released locally into the vessel wall during balloon contact. The objective was to achieve similar efficacy through local drug delivery without the need for prolonged DAPT. DCBs have demonstrated safety and efficacy in treating coronary ISR; currently, BMS ISR is the only approved indication for DCB use in the European guidelines. Endovascular interventions have replaced surgical bypass as the first-line treatment of symptomatic peripheral arterial disease and, with the limited long-term success seen with stents, DCBs offer a new and exciting therapeutic alternative. This review will discuss the mechanism of action of the DCB, preclinical data, emerging clinical indications, and results from clinical trials of this new and exciting technology.
Mechanism of action
(Online Appendix I, OnlineTable 1)
Preclinical studies
(Online Appendix II)
Clinical trials in coronary artery disease
IN-STENT RESTENOSIS (Table 1)
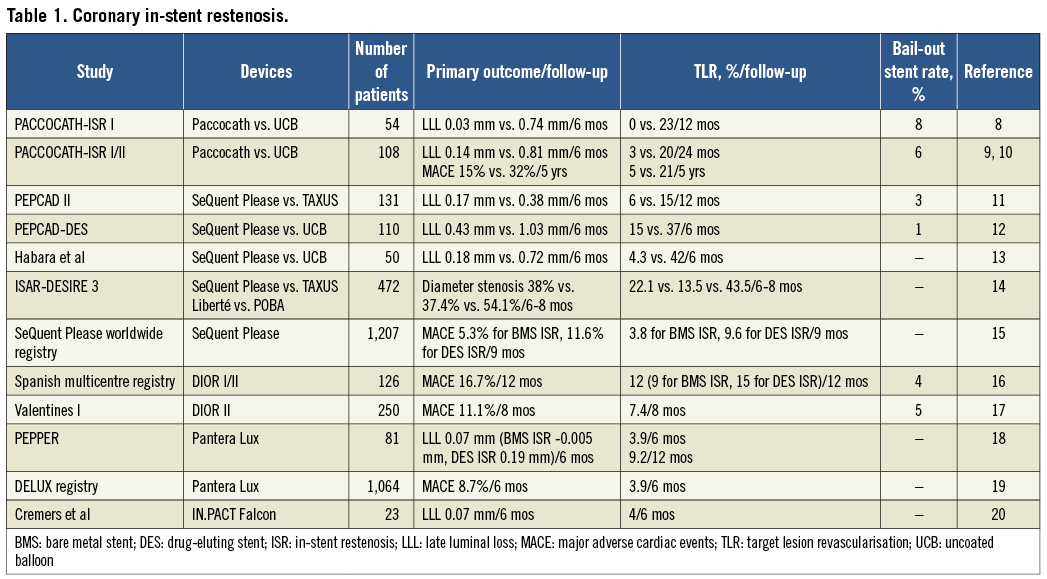
The majority of randomised trials conducted have been on treating native coronary artery ISR. DCBs were found to be superior to uncoated balloon angioplasty in treating both BMS and DES restenosis. Current European guidelines approve DCB use in BMS restenosis. The Treatment of In-Stent Restenosis by Paclitaxel-Coated Balloon Catheters (PACCOCATH-ISR) trial was a first-in-human DCB study8. In this study, patients with a single BMS ISR were randomised to Paccocath versus standard balloon angioplasty. There was a significant reduction in late luminal loss (LLL) (0.03 vs. 0.74 mm, p=0.002) and binary restenosis (5 vs. 43%, p=0.002) at six months with DCBs compared to standard angioplasty, translating to lower target lesion revascularisation (TLR) (0 vs. 23%, p=0.02) and major adverse cardiac event (MACE) rates (4 vs. 31%, p=0.01). A sustained clinical benefit was demonstrated for the combined group of patients from PACCOCATH-ISR I and II up to five years with no sign of late catch-up of TLR9,10. Of interest, there was no stent thrombosis up to five years despite only one month of DAPT.
Using the SeQuent Please, the Paclitaxel-Eluting PTCA Balloon Catheter in Coronary Artery Disease (PEPCAD) II study demonstrated non-inferiority to the TAXUS™ stent (Boston Scientific, Natick, MA, USA) in treating BMS ISR11. The primary endpoint of LLL at six months was lower with DCB, resulting in a lower binary restenosis rate (7 vs. 20%, p=0.06). Lower MACE occurred with DCB (9 vs. 22%, p=0.08), primarily driven by lower TLR.
The SeQuent® Please (B. Braun, Berlin, Germany) was shown to be superior to standard balloon angioplasty in PEPCAD-DES12, a randomised study of 110 patients with ISR of various DESs. Treatment with a DCB resulted in significantly lower six-month LLL (0.43 vs. 1.03 mm, p<0.001) and restenosis (17.2 vs. 58.1%, p<0.001), and significantly reduced MACE (16.7 vs. 50%, p<0.001), driven by a reduction in TLR (15.3 vs. 36.8%, p=0.005). Similar results were demonstrated in a smaller randomised study involving sirolimus-eluting stent restenosis patients13. SeQuent Please treatment resulted in superior angiographic and clinical outcomes than standard balloon angioplasty (MACE-free survival 96 vs. 60%, p=0.005). The recently presented Intracoronary Stenting and Angiographic Results: Drug Eluting Stents for In-Stent Restenosis (ISAR-DESIRE) 3 showed non-inferiority of SeQuent Please to TAXUS® Liberté™ (Boston Scientific) in patients with limus-DES restenosis: both paclitaxel platforms were superior to balloon angioplasty on intermediate-term angiographic follow-up14.
Various registry data support the safety and feasibility of DCB use in ISR15-20. A first-in-man study (Paclitaxel Releasing Balloon in Patients Presenting With In-Stent Restenosis, PEPPER) using the Pantera Lux balloon (Biotronik, Berlin, Germany) demonstrated impressive overall angiographic outcomes at six months: DCB treatment of BMS ISR resulted in superior LLL compared to DES ISR (–0.05 vs. 0.19 mm, p=0.001), translating to fewer revascularisations at one year (2.4 vs. 17.1%, p=0.001)18. Similarly, both the Spanish DIOR and the SeQuent Please registry highlighted better clinical outcomes with BMS ISR compared to DES ISR. However, outcomes of paclitaxel versus non-paclitaxel DES ISR treated with SeQuent Please did not differ (TLR: 8.3 vs. 10.8%, p=0.46). Perhaps the efficacy of DCBs in treating BMS ISR reflects the fact that a drug is introduced for the first time, whereas DCB is less efficacious in a “drug-resistant” vessel manifesting as DES ISR.
Besides comparable efficacy, a significant advantage in DCB treatment of ISR over DESs is the reduction of DAPT duration. There was no stent thrombosis up to five years despite just four weeks of DAPT in PACCOCATH-ISR, and <2% on midterm follow-up in other registries.
SMALL VESSELS (Table 2)
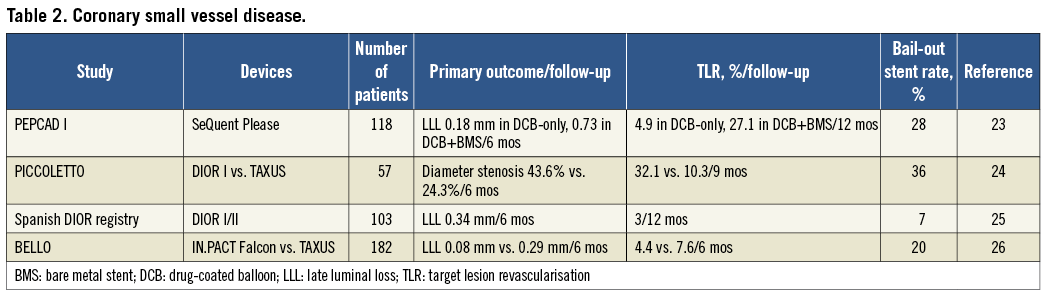
The main limitation of small-vessel stenting is restenosis21,22. The absence of polymer and metal with DCB reduces inflammation and abnormal vessel motion. The first study to explore DCB use in small vessels was the single-arm PEPCAD I using SeQuent Please23. Patients who received DCB-only treatment attained impressive six-month results. In contrast, patients who required bail-out BMS implantation had more restenosis and revascularisation. The majority of restenosis occurred at the stent edges, highlighting the potential pitfall of geographical mismatch, in which a longer BMS was placed beyond the shorter DCB-treated area. Vessel thrombosis occurred in 6.3% of the DCB+BMS treated group and none from the DCB-only group, despite a longer DAPT duration (three months vs. one month).
DIOR-I (Eurocor GmbH, Bonn, Germany) did not fare as well against TAXUS in Paclitaxel-coated balloon versus drug-eluting stent during PCI of small coronary vessels (PICCOLETTO), the first randomised trial in small vessels24. This study was prematurely terminated due to the clear superiority of DESs over DCBs, demonstrating less restenosis and a trend towards lower revascularisation. This negative result was attributed to lower tissue drug concentrations achieved from DIOR-I, as well as to procedural differences (lower predilatation rates and lower inflation pressures employed in the DCB group). Preliminary results from the Spanish registry (using DIOR-I and II in vessels <2 mm diameter) were more encouraging25. Although angiographic restenosis occurred in 1/5 of patients, only 3% required revascularisation, and one-year MACE was low at 5.8%.
A randomised study (Balloon Elution and Late Loss Optimization, BELLO) demonstrated clear superiority of IN.PACT Falcon (Medtronic, Minneapolis, MN, USA) to TAXUS26 in LLL at six months. However, >1/5 of DCB patients required bail-out BMS, and in this group the LLL (0.37 mm) was no different from that of DESs. Both DCB and TAXUS demonstrated similar MACE (10 vs. 16.3%, p=0.21).
With the exception of the Spanish registry, bail-out stenting seems common (occurring in 20-35% of cases). From current evidence, it appears that DCBs are effective in small vessels if no additional stent is implanted.
DE NOVO LESIONS (Table 3)
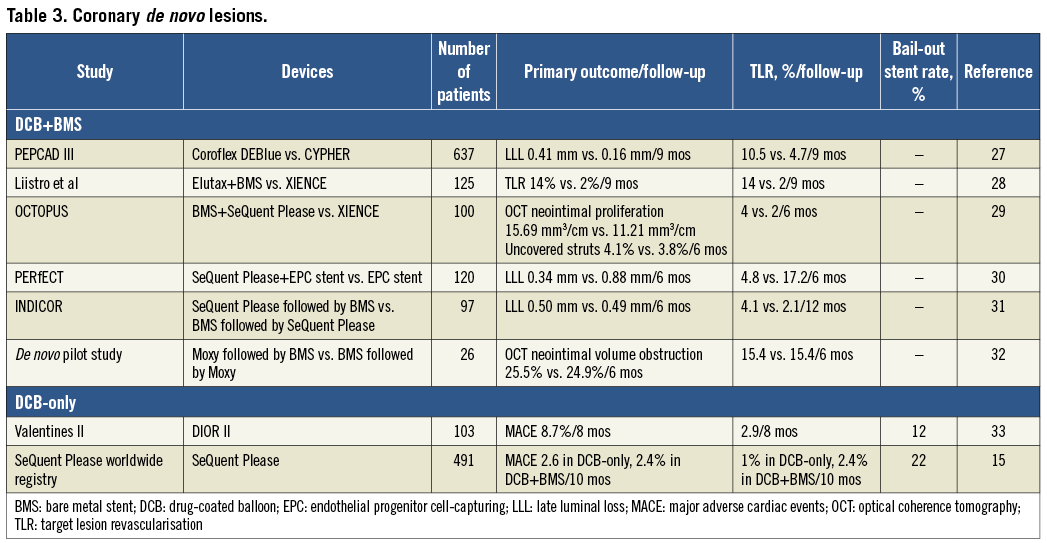
The evidence is less compelling supporting DCB in combination with routine BMS implantation. This combination aimed to provide homogeneous and rapid antirestenotic drug transfer, promote quick healing, overcome acute mechanical complications of balloon angioplasty, and potentially reduce DAPT duration in patients unsuited for DES.
The largest randomised trial comparing DCBs to DESs was PEPCAD III27, evaluating Coroflex® DEBlue (CoCr stent pre-mounted onto SeQuent Please; B. Braun) as an alternative to the CYPHER™ sirolimus-eluting stent (Cordis, Bridgewater, NJ, USA) in single de novo lesions. This DCB+BMS combination failed to meet non-inferiority to DES, with almost 3x higher LLL and double the revascularisations; however, the results were comparable against published paclitaxel-eluting stent efficacy data. Compared to current-generation DESs, predilatation with the Elutax® (Aachen Resonance, Aachen, Germany) followed by BMS was inferior to the everolimus-eluting XIENCE stent (Abbott Vascular, Santa Clara, CA, USA) with excessive TLR at midterm follow-up28. XIENCE V also inhibited neointimal proliferation more effectively than BMS+SeQuent Please post dilatation as assessed by optical coherence tomography (OCT) at six months29.
SeQuent Please was evaluated in conjunction with the endothelial progenitor cell (EPC) capturing stent (OrbusNeich Medical GmbH, Wiesbaden, Germany)30. Theoretically, adding paclitaxel enhances the restenotic efficacy while maintaining the EPC effect on endothelial healing. In this study, the EPC stent followed by DCB post-dilatation showed superior LLL (0.34 vs. 0.88 mm, p<0.001) and less restenosis (5.1 vs. 23.2%, p=0.006) compared to the EPC stent alone; no thrombosis occurred in either group with three months of DAPT. The positive results obtained may partly be attributed to the meticulous techniques employed: predilatation was mandatory and great care was taken to avoid geographical mismatch between DCB-treated and stented segments. Other studies evaluating the sequence of using DCBs for either predilatation or post-dilatation in conjunction with BMSs found no difference in terms of efficacy31,32.
ST risk is not negated when combining DCB+BMS. In PEPCAD III27, ST was higher with DCB+BMS compared to DES only (2.0 vs. 0.3% p<0.05), though DAPT duration was the same at six months. The Paclitaxel-Eluting PTCA-Balloon Catheter in Combination with a Cobalt-Chromium Stent (INDICOR) study reported ST rates of 3-6% in the first year31. The utility of this combination strategy is in doubt because of its inferior efficacy to DES and no apparent benefit in reducing DAPT duration. Moreover, emerging evidence suggests lower late loss when a stent is not implanted with a DCB. Thus, another strategy in treating de novo lesions is to use a DCB-only approach with provisional bail-out stenting.
The international Valentines II registry evaluated the feasibility of DIOR II as an adjunct to balloon angioplasty33. Follow-up at eight months indicated clinical efficacy and safety with 8.7% MACE, 6.9% target vessel revascularisation, and 2% cardiac death and myocardial infarction (MI); 11.3% required bail-out stenting. Angiographic follow-up of a subset of patients demonstrated LLL of 0.38 mm and a 14.3% binary restenosis rate, similar to results from the Spanish DIOR registry for small vessels. The SeQuent Please registry achieved similar favourable clinical outcomes using the DCB-only approach15, but had higher rates of bail-out stenting (22%). These patients, however, had similar outcomes to the DCB-only patients. These trials offer the DCB-only approach as a viable alternative in patients who may be unsuited to receive DESs.
COMPLEX CORONARY SUBSETS (Table 4)
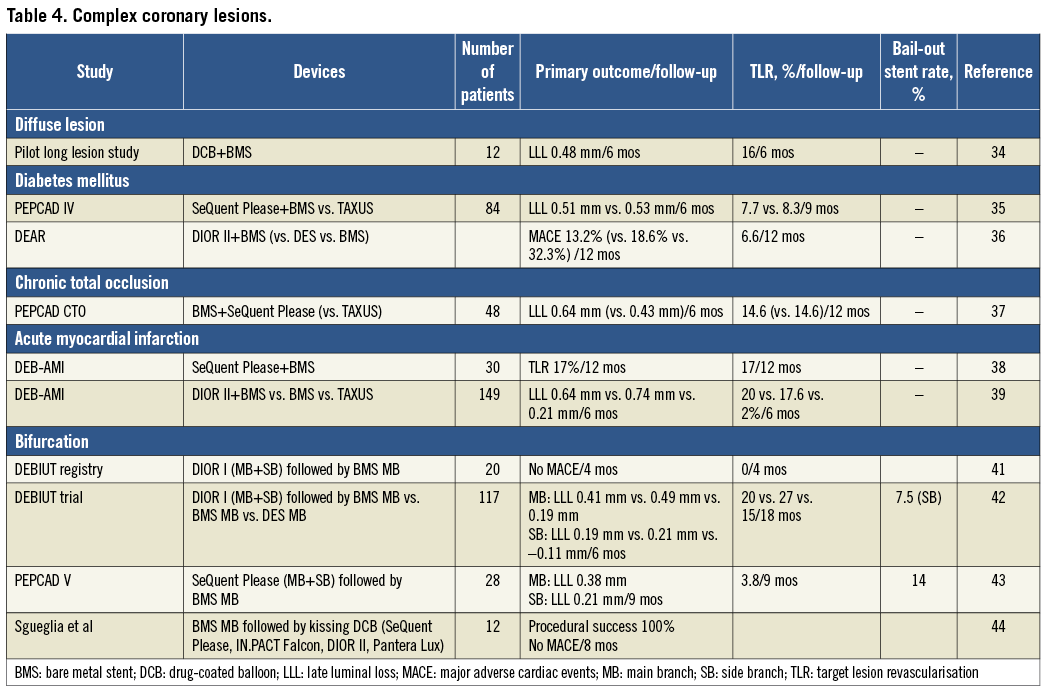
DIFFUSE DISEASE
The appeal of DCBs with spot stenting is in reducing the amount of metal in the vessels with its associated thrombotic and restenotic risk, while retaining the possibility of future bypass grafting. A small pilot study enrolled patients with mean lesion lengths of 74 mm34. Using this approach with optimal balloon angioplasty and intravascular ultrasound guidance, the six-month angiographic and clinical results were highly encouraging. Currently trials are being conducted testing this approach against DESs.
DIABETES MELLITUS
The homogenous drug delivery by DCBs may prove a valuable alternative to DESs in diabetic vessels, which are often diffusely diseased with high plaque burden. SeQuent Please+BMS achieved a similar, but no added advantage over TAXUS in angiographic and clinical outcomes in a randomised study of diabetic patients35. In the DiabEtic Argentina Registry (DEAR), DIOR II+BMS outcomes were comparable to historic diabetic cohorts using paclitaxel-eluting DESs, and superior to BMSs in target vessel revascularisation and MACE36.
CHRONIC TOTAL OCCLUSION
One of the most technically challenging lesion subsets in PCI is chronic total occlusion (CTO). DESs are superior to BMSs, but are limited in long-term efficacy and safety. The theoretical advantage of non-polymeric paclitaxel delivery over polymer-based DESs was tested in PEPCAD-CTO37. Following successful recanalisation and balloon dilatation of the native CTO, the entire lesion length was covered with a BMS followed by SeQuent Please treatment in the stented segments and beyond the stent edges. Angiographic and clinical restenosis achieved by BMS+DCB was no different from matched patients treated with the TAXUS stent. Moreover, no ST was reported up to one year with three months of DAPT. These findings highlight the exciting prospect of using DCBs in more complex lesions, especially in patients intolerant of prolonged DAPT.
ACUTE MYOCARDIAL INFARCTION
DCB treatment in acute myocardial infarction has led to disappointing results. A small pilot trial using SeQuent Please with BMS resulted in unacceptably high rates of revascularisation (17%) and vessel thrombosis (6%) at one year38. In another randomised trial in primary PCI39, using DIOR-II with BMSs conferred no advantage over BMS alone, and was inferior to TAXUS in angiographic and clinical outcomes. The pharmacokinetics of drug uptake in ruptured plaques and in the presence of thrombus remains unknown and requires further evaluation.
BIFURCATIONS
Side branch (SB) treatment remains a significant challenge in bifurcation PCI. Stenting often results in poor acute and long-term outcomes; currently, provisional SB stenting is the preferred approach40. Taking this into consideration, DCBs may prove advantageous when compared to regular balloon angioplasty in SB treatment, with the added potential of shortening DAPT duration. The Drug-Eluting Balloon in Bifurcation Utrecht registry evaluated the feasibility of DIOR-I to treat both the main vessel and the SB following adequate predilatation with regular balloons41. This was followed by provisional T-stenting with BMSs in the main vessel, and final kissing with regular post-dilatation balloons. None of the patients required additional SB stenting, and no MACE was reported at four months.
This was followed by the randomised Drug-eluting Balloon in Bifurcations Trial (DEBIUT)42, which employed the same provisional T-stenting technique in three arms: DIOR-I pretreatment+ BMS, BMS with uncoated balloon, and paclitaxel DESs with uncoated balloon. The DCB-pretreated arm failed to demonstrate superiority over BMSs and was inferior to DESs. This was attributed to unexpectedly good results with both BMS and DES, and also the inferior drug delivery attributes of DIOR-I. DCB use, however, proved feasible and safe with no vessel thrombosis with three months of DAPT.
A different technical approach was used in PEPCAD V. In this small, single-arm study, SeQuent Please was used to treat both the SB and the main vessel followed by provisional T-stenting similar to DEBIUT, except that there was no obligatory SB predilatation43. The procedure was successful in all patients. Angiographic follow-up at nine months demonstrated a DES-like effect in the SB. Additional SB stenting was performed in 14.3%, which resulted in higher LLL compared to DCB alone (0.66 vs. 0.12 mm). Of note, two patients (7.1%) suffered late ST beyond the prescribed three months of DAPT, attributed to stent underexpansion.
Another strategy is to avoid predilating the SB altogether, which may increase the risk of dissection and result in stenting. Instead, provisional stenting of the main vessel followed by final kissing DCB post-dilatation was suggested. A small study evaluated the feasibility of this approach using four different new-generation DCBs (SeQuent Please, IN.PACT Falcon, DIOR-II and Pantera Lux) in patients with anticipated low compliance to DAPT (prescribed for three months)44. The procedure was successful, and no MACE occurred up to eight months. A prospective registry of the kissing DCB technique is ongoing (KISSING DEBBIE study, NCT01009996).
BMSs were used for main vessel stenting in all of the above trials because there were no safety data for DCB use with DESs. A study is ongoing to evaluate SeQuent Please with paclitaxel DESs in the main vessel (Study of the Paclitaxel-Coated Balloon Catheter in Bifurcated Coronary Lesions, BABILON, NCT01278186). The possibility of using DCBs without stents should also be considered in future studies. While DCBs appear promising in SB treatment, the technical complexities of incorporating DCBs in bifurcation interventions still requires further evaluation.
PERIPHERAL ARTERY DISEASE (Table 5)
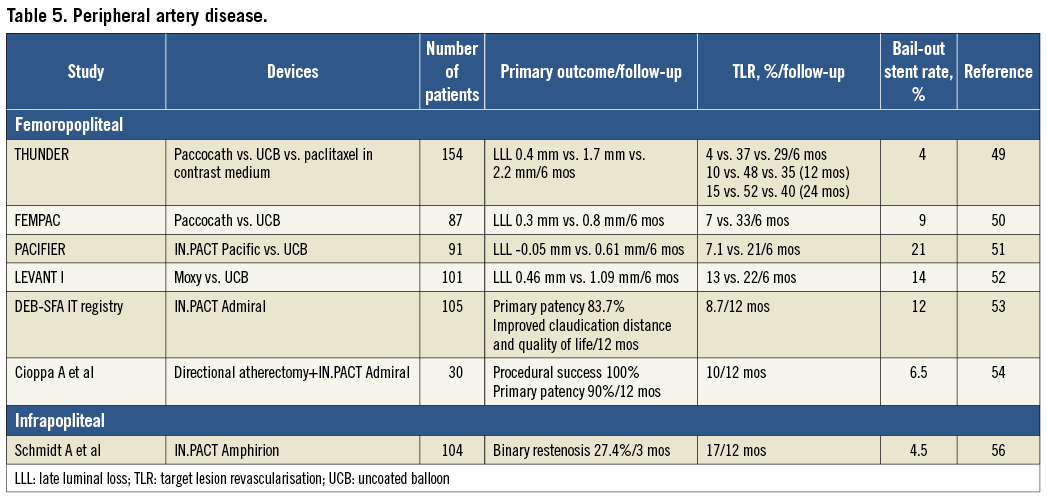
FEMOROPOPLITEAL ARTERIES
The superficial femoral artery (SFA) is subject to multidirectional mechanical forces. As such, although stenting is recommended as primary therapy45, restenosis rates remain high; DESs have, so far, failed to demonstrate efficacy over BMSs in long lesions46,47. Non-polymeric paclitaxel DESs may have a role in shorter femoropopliteal lesions where stent fracture risk is lower48. A DCB approach appears attractive in more diffuse lesions, and initial studies showed DCBs to be more effective than and as safe as standard angioplasty.
The Local Taxane with Short Exposure for Reduction of Restenosis in Distal Arteries (THUNDER) trial randomised patients with femoropopliteal disease to angioplasty with Paccocath, uncoated balloon, and uncoated balloon with paclitaxel dissolved in contrast medium49. Follow-up at six months demonstrated DCB superiority over the other groups, with TLR reduction sustained up to two years. Another randomised trial (Femoral Paclitaxel, FemPac)50, using Paccocath versus uncoated balloon angioplasty, demonstrated similar results. Despite showing biologic efficacy, these two trials were limited by small sample sizes, heterogeneous indications for enrolment (de novo, restenotic post-balloon angioplasty, ISR), varying disease severity (lesion lengths and total occlusions), inadequate blinding and follow-up. Luminal gain from positive remodelling was demonstrated with IN.PACT Pacific treatment in Paclitaxel-coated Balloons in Femoral Indication to Defeat Restenosis (PACIFIER)51, and at six months was superior to standard angioplasty across various lesion subsets. Another small randomised trial (Lutonix Paclitaxel-Coated Balloon for the Prevention of Femoropoliteal Restenosis, LEVANT 1) assessed the Moxy DCB against uncoated balloon angioplasty52, with or without the need for stenting. DCBs proved superior overall in reducing LLL and TLR at six months. The late loss did not differ in patients with or without stenting (0.49 vs. 0.45 mm). Following this, LEVANT 2 has just completed its enrolment of 476 patients in the first pivotal trial approved by the US Food and Drug Administration. The FREEWAY® balloon (Eurocor GmbH) is currently being evaluated against standard balloon angioplasty for ISR in a pilot study (Paclitaxel Balloon Versus Standard Balloon in In-stent Restenoses of the Superficial Femoral Artery, PACUBA, NCT01247402). Sustained improvements in absolute claudication distance and quality of life were demonstrated with IN.PACT Admiral and provisional stenting in an Italian registry53. The IN.PACT Admiral was also used successfully in combination with directional atherectomy in heavily calcified lesions with good midterm results54.
INFRAPOPLITEAL ARTERY
Treatment with DESs has shown encouraging results in various clinical trials and registries. However, DES use is limited in diffuse lesions due to the lack of long DESs. Spot stenting has been associated with high restenosis rates in segments not covered by DESs55. Stents below the ankle may also risk compression and occlusion, and DCBs may prove useful in this vascular territory. A registry explored this possibility56, and demonstrated lower restenosis with IN.PACT Amphirion compared to historical controls treated with standard balloon angioplasty (27 vs. 69% at three months). Further studies are ongoing assessing different DCBs in treating below-the-knee lesions causing critical limb ischaemia (Study of IN.PACT Amphirion Drug Eluting Balloon vs. Standard PTA for the Treatment of Below the Knee Critical Limb Ischemia, INPACT-DEEP, NCT00941733; Drug Coated Balloons for Prevention of Restenosis, PICCOLO, NCT00696956).
OTHER VASCULAR TERRITORIES
DCB treatment has recently expanded beyond coronary and leg arteries. Preliminary results have been encouraging in treating ISR of intracranial atherosclerotic disease with SeQuent Please or DIOR in conjunction with a self-expanding nitinol stent in 52 patients, achieving 81% procedural success and only one case (3%) of restenosis over one year57. A case report documented successful treatment of subclavian artery ISR using FREEWAY in conjunction with a cutting balloon58. Another small randomised trial demonstrated success of the IN.PACT DCBs in improving six-month primary patency of stenosed dialysis arteriovenous fistulas or grafts over standard balloon angioplasty (70 vs. 25%, p<0.001)59. These examples present exciting new directions for future DCB applications.
Clinical considerations
Bail-out stenting limits DCB efficacy and prolongs DAPT duration compared to DCB treatment alone. Acute recoil and dissections requiring bail-out stenting are more commonly encountered following balloon angioplasty in small coronary vessels and diffuse peripheral lesions than for in-stent restenosis. When using a stent with a DCB, good operator technique is essential to avoid geographical mismatch between the stented segment and the DCB-treated segment or there is a risk of suboptimal efficacy. It is also recommended to perform adequate balloon predilatation to create a stent-like angiographic result before deploying the DCB. Creating “micro-dissections” is thought to facilitate drug transfer and achieve adequate drug bioavailability in the tissues. The DCB should be sized longer than the area of balloon angioplasty to avoid geographical mismatch.
Technical issues relevant in peripheral intervention include minimising significant drug losses from long balloons during tracking and lesion crossing, which may be achieved by adequate lesion predilatation. Severe calcifications may limit the utility of DCBs unless they are used in conjunction with debulking devices for plaque modification; the early trials routinely excluded such lesions, although a small single-centre experience demonstrated feasibility with directional atherectomy54. This approach needs to be confirmed in larger randomised trials.
There is currently no consensus regarding the optimal DAPT duration with DCB use. Based on current evidence, one month of DAPT may be adequate in lesions treated with DCB alone. The risk of vessel thrombosis increases when coupled with stenting. Although initial studies with DCBs showed reasonable safety with just three months of DAPT, there were instances of ST occurring beyond six months on aspirin alone38,43. On OCT, however, good endothelial coverage is expected by six months29. A reasonable approach is to treat the DCB+BMS combination like DES with ≥6 months of DAPT. In complex lesion subsets, such as bifurcations, where the risk of vessel thrombosis may be higher, DAPT should perhaps be considered for ≥12 months, thus erasing the potential advantage that DCBs have over DESs in reducing DAPT duration. Future recommendations will depend on more robust clinical evidence.
Conclusions
While this new technology has established its efficacy in treating coronary ISR, especially BMS ISR, data for other indications are limited to a few small randomised trials and registries. Looking forward, larger randomised trials using clinical rather than surrogate angiographic outcomes are required. Also, different DCBs need to be compared head-to-head due to differences in manufacturing processes and drug-release kinetics. For now, this technology appears promising in treating small-vessel disease, de novo lesions, and femoropopliteal disease.
The immediate comparators to DCBs are DESs, which have high success and low restenosis rates in coronary artery disease treatment. Newer DESs have lowered the accrued risk of late ST even further. Besides a clear role in treating ISR, DCBs must establish equivalence to the newer-generation DESs across other clinical indications. As the interventional community awaits more robust clinical data, perhaps the greatest potential in DCB use is in patients not suited for DES implantation and intolerant of prolonged DAPT.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Appendix 1. Mechanism of action
RATIONALE
DCBs deliver an antirestenotic drug using a balloon catheter. Upon balloon inflation, the drug is rapidly released into the vessel wall on contact. Upon absorption by the vessel wall, the drug acts locally to prevent neointimal hyperplasia. The potential advantages of a non-stent-based local drug delivery include: rapid delivery with sustained antiproliferative effect limiting neointimal hyperplasia; homogenous drug delivery to the entire vessel wall; absence of polymer that may induce chronic inflammation; absence of metallic stent struts which preserves normal vasomotor function and retains the artery’s original anatomy (particularly important in bifurcations or small-calibre vessels); limited duration of long-term antiproliferative effect, thus promoting endothelial healing; and reduced dependence on prolonged DAPT.
DRUG
Paclitaxel is currently used in DCBs as it has been shown to be superior to other agents60,61. Paclitaxel inhibits cell replication in mitosis by suppressing microtubule disassembly, thus preventing arterial smooth muscle cell proliferation leading to neointimal hyperplasia62,63. Its lipophilic properties are ideal for drug transfer and absorption from balloon to the vessel wall64. Moreover, sustained drug release is not required for a prolonged antiproliferative effect because paclitaxel has the ability to remain in the vascular smooth muscle cells for up to a week63,65. Among rapamycin-based drugs tested, zotarolimus has lipophilic properties which appear promising in future DCB development66.
EXCIPIENT
Early preclinical findings show that solubility of paclitaxel is enhanced by the addition of an excipient. The excipient helps deliver paclitaxel by enhancing its penetrability into the arterial tissue67. Paclitaxel/iopromide formulation has been shown to inhibit neointimal formation effectively after stent implantation in porcine models, despite a short application time68,69. Iopromide as an excipient increases the contact area between paclitaxel molecules and the vessel wall, thus enhancing its bioavailability. This concept was used to develop DCB coating formulations. Paclitaxel admixed with a small amount of iopromide (Ultravist®; Bayer Healthcare, Wayne, NJ, USA) serves as an effective coating matrix and has been denoted as Paccocath® technology. Besides Paccocath, other DCBs use similar coating methods (paclitaxel+excipient) in a matrix prior to loading onto the delivery balloon70. Iopromide was the first excipient studied; others currently in use are urea, butyryl-tri-hexyl citrate, and shellac.
BALLOON (Online Table 1)
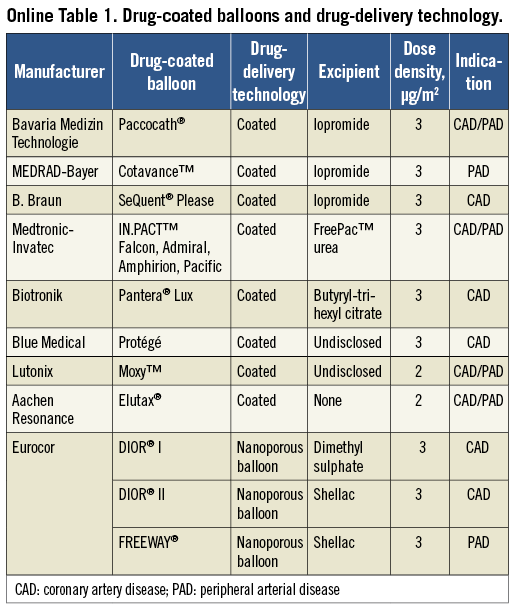
The DCB provides a means for more lesion-specific, rather than vessel-specific, drug delivery as with intracoronary drug administration. The Paccocath® balloon catheter (Bavaria Medizin Technologie, Oberpfaffenhofen, Germany) was the first DCB developed for clinical trials. The Cotavance® balloon catheter (MEDRAD-Bayer, Warrendale, PA, USA) was one of the first commercially available DCBs to use the Paccocath technology.
The SeQuent® Please (B. Braun, Berlin, Germany) balloon catheter uses the Paccocath formulation with a slightly modified coating and a different balloon platform. The IN.PACT (Medtronic-Invatec, Frauenfeld, Switzerland) series of DCBs for coronary and peripheral applications uses a proprietary coating called FreePac™. This hydrophilic urea coating was shown to be as effective as Paccocath in inhibiting neointimal formation in porcine models71. The Pantera® Lux (Biotronik, Berlin, Germany) uses butyryl-tri-hexyl citrate, while the Protégé (Blue Medical, Helmond, The Netherlands) and the Moxy™ (Lutonix, New Hope, MN, USA) DCBs use other undisclosed non-polymeric hydrophilic carriers.
The second-generation DIOR® DCB (Eurocor, Bonn, Germany) uses shellac, a natural resin coating that improves tissue drug concentration by up to 20x compared to the first-generation DIOR, which uses dimethyl sulphoxide as its excipient72,73, and is comparable to that achieved by SeQuent Please and Pantera Lux. As a result, the second-generation DIOR requires a shorter inflation time of 30-45 seconds instead of the previously recommended 60 seconds74. The FREEWAY® (Eurocor, Bonn, Germany) balloon catheter is similar to the DIOR II and is designed for peripheral intervention. The Elutax® balloon (Aachen Resonance, Aachen, Germany) uses a two-layer drug matrix (without excipient) that serves as a depot for homogeneous paclitaxel release, and uses a lower paclitaxel dose of 2 µm/mm2 compared with the other DCBs, which use 3 µm/mm2.
The DIOR balloon uses a shielding technique to prevent early drug wash-off during insertion and tracking. This method consists of a pre-folded balloon in its non-inflated state. The DIOR balloon is folded three times, while the FREEWAY balloon consists of four to six folds. Pantera Lux and Protégé use similar shielding techniques. In contrast, the SeQuent Please, which does not use shielding, releases ≈6% of paclitaxel detectable in the systemic circulation11. However, this systemic release is unlikely to result in harm as the doses are still much lower than those used for chemotherapy.
Several of the DCBs have pre-mounted cobalt-chromium (CoCr) stents. Some hybrid DCB+BMS systems include MAGICAL® (CoCr stent on DIOR II; Eurocor, Bonn, Germany), Pioneer (CoCr stent on Protégé; Blue Medical, Helmond, The Netherlands) and Coroflex® DEBlue (CoCr stent on SeQuent Please; B. Braun, Berlin, Germany).
Appendix 2. Preclinical studies
EFFICACY IN ANIMAL MODELS
The first proof-of-concept study tested the efficacy of DCBs in drug transfer and reduction in neointimal formation in a porcine coronary model. The study demonstrated a dose-dependent reduction in neointimal proliferation using an acetone-iopromide coating. Moreover, early endothelialisation of stent struts was observed in all samples on histomorphometry75. The same investigators also showed that, in addition to being as efficacious as DESs at four weeks, the drug delivered by the DCB on the vessel surface was more homogenously distributed76. A similar effect was demonstrated in the peripheral arteries of a porcine model using a 480-µg paclitaxel-coated balloon77. However, a more recent study by Nakamura et al demonstrated that DCB use as a distinct post-dilation inflation after stent implantation led to increased inflammation, stent strut malapposition, and reduced endothelial-dependent vasomotor function compared with uncoated balloons78. One limitation of this study was a shorter inflation time of 30 seconds compared to the 60 seconds commonly used in other studies.
INFLATION TIMES AND DOSE RESPONSES
A study by Cremers et al compared the effects of restenosis of different inflation times (10 sec, 60 sec, and 2×60 sec) and found no difference in efficacy between short and long inflations, suggesting that most of the drug was released on initial contact79. There was no further value in using two overlapping balloons to increase delivery dose, and there was also no added risk of thrombosis or aneurysm formation. Using two different paclitaxel matrix coating formulations (iopromide and urea), Kelsch et al demonstrated efficacy with a paclitaxel dose of 1 μg/mm2, with no increased efficacy beyond 3 μg/mm2. Intentionally excessive doses (9 μg/mm2) led to the occurrence of thrombotic events80.
EFFECT OF INFLATION PRESSURE
A study by Cremers et al demonstrated that employing low inflation pressure with 2 atmospheres was as effective as high pressure with 12 atmospheres in reducing late luminal loss (LLL) and intimal thickness. This has implications in DCB use in vessels that may not tolerate high inflation pressure81.
DRUG LOSS AND SYSTEMIC BIOAVAILABILITY
More than 10% of the initial drug from the balloon is lost during tracking through the guiding catheter to the lesion. Upon inflation, ≈80% of the drug dose is released and ≈20% of that will be taken up by the vessel wall, with the rest being washed off distally. At the end of the procedure, ≈10% of the initial dose remains on the balloon75,80. Peripheral interventions with long DCBs up to 10 cm demonstrated safe systemic levels and no untoward clinical effect of paclitaxel immediately post intervention, with levels declining rapidly thereafter. At two hours, more than half the samples reached undetectable levels and, by 24 hours, plasma levels were undetectable in all patients82.
EFFICACY AMONG DIFFERENT DCBs
A study by Joner et al compared different balloon platforms (uncoated, Pantera Lux, Elutax and SeQuent Please) for post-dilatation of BMSs and revealed significant heterogeneity of neointimal suppression among the devices tested83. Despite the study’s limitation of varying extent of vascular injury among groups, the results indicated that Pantera Lux had the most profound effect in suppressing neointimal hyperplasia with associated signs of delayed endothelial healing.

