Abstract
Aims: Restenosis after PCI and/or stent implantation is still one of the challenging problems in the field of interventional cardiology. Different approaches to prevent and to treat restenosis include the use of drug-eluting stents, which have shown to reduce restenosis. Another approach is the treatment with drug-coated balloons. This approach has been proven for different indications, e.g., in-stent restenosis and treatment of peripheral artery disease.
Methods and results: Patients from the PEPCAD III multicentre randomised trial in two study centres (Homburg and Hannover, Germany) were asked to participate in this intravascular ultrasound (IVUS) study at nine-month follow-up. At baseline (nine months before), patients were randomly assigned to receive either a paclitaxel-coated balloon (drug-coated balloon [DCB]) plus a premounted bare metal stent (DCB/BMS) or a sirolimus-eluting stent (drug-eluting stent [DES]) to treat de novo lesions. IVUS at follow-up was performed in order to analyse the restenosis for potential understanding of the mechanism leading to restenosis. IVUS data is available for 55 patients; 26 patients were treated with Cypher® DES (Cordis, Miami Lakes, FL, USA) and 29 patients with DCB/BMS. A focal malapposition of the stent was seen in six patients; four after DES and two after DCB/BMS. Stent expansion, calculated as symmetric expansion index, was equal for both groups (0.89 and 0.90). Mean stent area was also equal for both groups (6.25±1.7 vs. 5.65±1.5 mm², p=n.s.). The neointimal hyperplasia (calculated as stent area minus lumen area) was significantly different between both groups (0.69±0.49 [DES] vs. 1.08±0.53 mm² [DCB/BMS], p<0.01). This resulted in a significantly higher in-stent restenosis in the DCB/BMS group (19.7 vs. 11 %, p<0.01). There is no evidence of geographical mismatch.
Conclusions: First IVUS insights for the DCB/BMS showed a comparable, low incidence of malapposition for the combination of drug-coated balloon and premounted bare metal stent compared to the DES, and stent expansion was good and comparable to DES. However, at nine-month follow-up, the combination of drug-coated balloon and premounted bare metal stent showed higher in-stent restenosis compared to sirolimus DES. Geographical mismatch can be excluded as a reason for this result.
Introduction
Coronary in-stent restenosis is one of the challenging problems in the field of interventional cardiology. The incidence varies in different risk populations, e.g., diabetic patients, up to 35% after bare metal stent (BMS) implantation and is reduced, but still existent and problematic with clinical evidence, after implantation of drug-eluting stents (DES). Different therapeutic options have been developed and have been shown to reduce or treat in-stent restenosis, e.g., intracoronary brachytherapy, DES or, as a relatively new tool, the drug-coated balloon (DCB).
Stent-based local drug delivery (e.g., drug-eluting stents) provides sustained drug release using technologies like polymers. In experiments in pigs, intracoronary delivery of paclitaxel by contrast media1,2 or by drug-coated balloon3 was found to potentially lead to significant reduction in neointimal proliferation. In these animal studies, the most pronounced effect in reduction of neointimal proliferation was seen with paclitaxel-coated balloons4.
This interventional device has been proven in both preclinical and randomised clinical trials to be effective in the treatment of coronary in-stent restenosis and de novo and restenotic lesions in peripheral artery disease5-9. Further indications are tested in non-randomised trials with promising results that may lead to further possible indications like the treatment of de novo stenosis in small vessels10, treatment of bifurcation lesions11 or chronic total occlusions. Further indications are being tested in ongoing trials.
One problem with the treatment using the combination of drug-coated balloon and bare metal stent is the so-called “geographical mismatch”. Geographical mismatch implies that the BMS is in part deployed in vessel areas not treated with the drug-coated balloon, e.g., the stent edges. This phenomenon leads to higher rates of restenosis, especially focal restenosis in the area of geographical mismatch.
The aim of the PEPCAD III trial was to assess the safety and efficacy of a paclitaxel-coated balloon in combination with a premounted BMS for the treatment of de novo lesions in patients with stenosis in native coronary arteries. This device was compared to a sirolimus-eluting DES in a randomised fashion12. The aim of this study was to further investigate the implantation results in regard to restenosis and to understand the mechanism of restenosis (proliferation, stent mal-expansion, stent malapposition, geographical mismatch) with regard to this investigational device, and to do so, we performed an intravascular ultrasound (IVUS) study in two participating centres.
Methods
Patients with stable or unstable angina or documented ischaemia due to a significant lesion in a native coronary artery, with the intention to treat one lesion with one stent were included in this study. The lesion diameter had to be ≥2.5 mm and ≤3.5 mm with a lesion length <24 mm. Main exclusion criteria were treatment of in-stent restenosis, acute myocardial infarction or treatment of unprotected left main stenosis.
Patients were randomly assigned to receive treatment with either a paclitaxel-coated balloon (paclitaxel-iopromide coating) in combination with the Coroflex Blue® bare metal stent (B.Braun Melsungen AG., Melsungen, Germany) or with the sirolimus-eluting Cypher® stent (Cordis, Miami Lakes, FL, USA). Predilation before stent implantation was recommended. The aim was to treat one lesion with one stent, but in case of dissection or remaining significant stenosis proximal or distal to the stent, it was possible to implant a second stent (same product). During PCI all patients received unfractionated or low-molecular heparin. All patients were treated with 300-600 mg clopidogrel as loading dose, followed by clopidogrel 75 mg daily for up to 12 months, and daily aspirin (100-325 mg).
All patients were asked to come for follow-up angiography at nine months.
In two centres (Homburg/Saar and Hannover, Germany) all patients were also asked to participate in the IVUS study. They were asked to participate at the time-point when the informed consent for the nine-month follow-up catheterisation was obtained. Patients were also informed that the participation in the IVUS study was voluntary. This was at least one day before the procedure was performed. In all patients who gave informed consent, IVUS was performed at nine-month follow-up in order to analyse the stent. This study protocol was approved by the local ethics committees.
IVUS was performed after angiography using a commercially available IVUS imaging system (Boston Scientific, Fremont, CA, USA; or Volcano, Rancho Cordoba, CA, USA) with an automatic catheter pullback (speed 0.5 mm/sec). In Hannover, all patients were investigated using the Atlantis® SR Pro 40 MHz device (Boston Scientific), in Homburg all patients were investigated using the Eagle Eye® Gold device (Volcano). Before the IVUS catheter was inserted into the coronary artery, 100 to 200 µg of nitroglycerine was routinely inserted. The pullback was started at least 5 mm distal to the stent and performed for at least to 5 mm proximal to the stent. The analysis of IVUS images was performed offline in the corelab at Hannover Medical School, blinded to the treatment arm. The tapes were analysed according to the clinical expert consensus documentation standards for the acquisition, measurement, and reporting of IVUS of the American College of Cardiology by an experienced investigator13. Calibration was performed with grid marks encoded in the images. One image per second was analysed. Using computerised planimetry (Medical Imaging System [MIA], INDEC Systems Inc., Santa Clara, CA, USA), borders of the vessel lumen, the stent struts and of the external elastic membrane (EEM) were identified and cross-sectional area (CSA) measured. In-stent proliferation CSA was calculated by stent CSA minus lumen CSA for each image and as percentage of stent CSA. For each CSA, minimal and maximal diameter was calculated. Each in-stent region was manually reviewed for malapposition of stent struts. Therefore, data was analysed frame by frame for complete apposition over the entire length of the stent. To evaluate the evidence of geographical mismatch, we compared the burden of in-stent proliferation, calculated as a percentage of stent CSA for stent inflow and stent outflow with the mean in-stent proliferation for the entire stent. The symmetric expansion was calculated as a ratio between minimal and maximal stent diameter.
The primary endpoint of the main study was late lumen loss at nine-month follow-up assessed by angiography by an independent and blinded core lab. Secondary endpoints were procedural success, binary restenosis rate and MACE at nine months, one and three years.
The endpoints of this IVUS study were the expansion, apposition and amount of in-stent-proliferation and the presence of geographical mismatch at nine-month follow-up in a subgroup of all patients in two participating centres.
The statistical analysis was performed using GraphPad Prism® 5 (GraphPad Software, La Jolla, CA, USA). Continuous data are presented as mean+SD. The chi-square test or Fisher’s exact test was used for the comparison of categorical variables. The Student’s t-test was performed to compare continuous variables; a p-value of <0.05 was considered statistically significant.
Results
In this IVUS study on an experimental drug-coated balloon/stent system versus the sirolimus-eluting Cypher® stent, 55 patients were included. Of these patients, 26 patients were treated with the Cypher® drug-eluting stent (DES) and 29 patients with the paclitaxel-coated balloon in combination with the bare metal stent (DCB/BMS). Baseline characteristics as well as procedural data are outlined in detail in Table 1. Stent diameter and stent length were comparable in both groups, and also the number of diabetic patients were comparable in both groups. Stents were implanted in both groups with comparable inflation pressures (DCB/BMS 14.1±1.7 vs. DES 14.4±1.7 atm). As requested by protocol, the inflation time chosen for implantation was significant longer in the DCB/BMS group compared to the DES group (34.6±10.3 vs. 21.5±12 seconds, respectively). In a slightly higher, but statistically insignificant number of patients, a predilatation was performed in the DCB/BMS group (64 vs. 58 %). There were no complications related to the IVUS procedure at nine months. The mean procedural time for angiography and IVUS was 37 minutes for both groups.
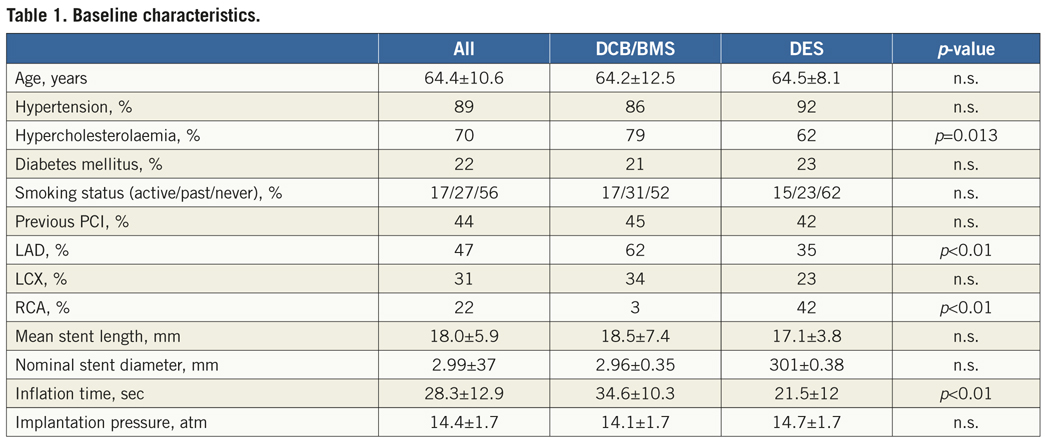
A focal malapposition of the stent was seen in six patients, four of whom were treated with DES and two with DCB/BMS (p=n.s., [Figure 1]). The stents were well expanded in both treatment arms: symmetric stent expansion, calculated as ratio between minimal and maximal stent diameter, was comparable in both groups (0.90 vs. 0.89, p=n.s.).
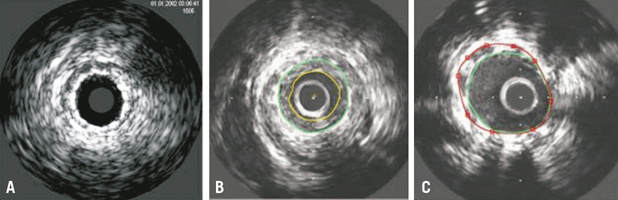
Figure 1. A) 3.0/16 mm DCB without intima proliferation, well-expanded stent; B) Example of in-stent stenosis in DCB (2.75/16 mm). Green circle indicates the stent, yellow circle indicates the lumen; C) Example of malapposition in DCB (3.0/19 mm). Green circle indicates the stent, red circle indicates the external elastic membrane (EEM). Gap between a portion of the stent and the vessel wall between 8 and 12 o’ clock.
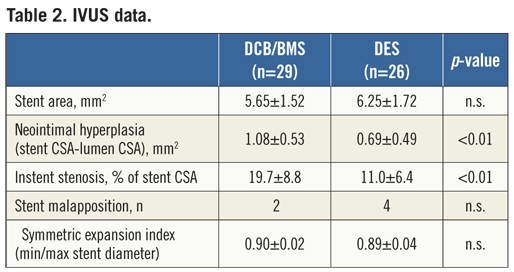
Mean stent CSA was equal in both groups (6.25±1.72 for DES vs. 5.65±1.52 mm² for DCB/BMS, p=0.18). The neointimal hyperplasia (calculated as stent CSA minus lumen CSA) was significant different between both groups in favour of DES (0.69±0.49 [DES] vs. 1.08±0.53 mm² [DCB/BMS], p<0.01). This resulted in a significantly higher rate of in-stent restenosis in the DCB/BMS group (19.7±8.8 vs. 11.0±6.4%, p<0.01) (Table 2).
We found no evidence of geographical mismatch when comparing the amount of in-stent proliferation for stent inflow (19.8±15.5%) and the stent outflow (15.8±10.4%) with the mean in-stent proliferation (19.7±8.8%) for the DCB/BMS group (p=0.33). One patient in this group showed significantly more proliferation in the stent inflow, but this did not result in a significant stenosis in angiography.
Discussion
In our study, IVUS was performed to analyse stent apposition and expansion as well as in-stent restenosis nine months after randomised treatment with the paclitaxel-coated balloon in combination with a bare metal stent or the sirolimus-coated Cypher® stent in patients with coronary artery disease. All patients were treated in stable clinical conditions due to the exclusion of acute myocardial infarction and all lesions were located in native coronary arteries. The main finding was that there is no significant difference in expansion (measured as symmetric stent expansion) and malapposition and there was no evidence for geographical mismatch. There was even a slightly higher rate of malapposition seen in those patients treated with DES, but this did not reach statistical relevance. However, this positive implantation data did not result in better outcomes in terms of restenosis. Neointimal hyperplasia was significantly higher in patients treated with the combination DCB/BMS. The reason for this finding is unexplained. Possible influencing variables could be the stent system used, the amount of drug, the stent and balloon lengths, and an interaction of the mounted stent with drug release from the coated balloon. Another surprising finding of this study was the result seen with the sirolimus DES, which was better than expected.
The phenomenon of geographical mismatch implicates that the bare metal stent is in part deployed in vessel areas not treated with the drug-coated balloon, e.g., the stent edges10. In this trial, the stent was already premounted on the drug-coated balloon. Consistently we found no evidence of geographic mismatch; IVUS showed not more in-stent proliferation in the part of stent inflow or outflow compared with the entire stent. However, the optimal ratio between stent and balloon length in the clinical application of DCB/BMS is unknown.
The interaction of the pre-mounted stent with the coated balloon may have influenced paclitaxel dose, drug distribution, and residency of the drug in the vessel wall. In the PERfECT Stent trial, an anti-CD34 antibody-coated stent was post dilated with the same paclitaxel-coated balloon catheter used in this trial.14 However, stent and coated balloon were two separate devices that did not influence drug application by the balloon. In this trial, the paclitaxel-coated balloon was about 4 mm longer than the stented segment in order to address neointimal proliferation in the segment proximal and distal to the stent. The device used in our trial had only small overlap of the balloon. Furthermore, predilatation with an uncoated balloon was mandatory in the PERfECT Stent trial, whereas direct stenting was used in almost half of the patients in our trial. Despite a reduced dual antiplatelet therapy of only three months in the PERfECT Stent study there were no cases of stent thrombosis14.
In the PEPCAD III study, the angiographic late lumen loss (LLL) in-segment for the DCB/BMS device used in our trial was 0.20±0.52 mm12, which is comparable to published data on different drug-eluting stents. In the PERfECT Stent trial in-segment LLL was 0.16±0.40 mm for the sequential use of an anti-CD34 antibody-coated stent and the paclitaxel-iopromide-coated balloon versus 0.61±0.47 mm without DCB (p<0.001), resulting in a significant reduction of major adverse cardiovascular events from 15.5% to 4.8%14. In an Asian diabetic population predilation with a DCB before BMS resulted in a similar angiographic and clinical outcome compared to a DES (LLL in-segment 0.37±0.59 mm vs. 0.35±0.63 mm, respectively)15. A recent meta-analysis revealed an in-segment late lumen loss from 11 randomised DES trials of between –0.05±0.30 and 0.35±0.47 mm16. For the bare metal stent arms in these studies, the late lumen loss was between 0.47±0.47 mm and 0.82±0.58 mm16.
Published data for the stent system mounted on the DCB is comparable to other bare metal stents. Bocksch et al reported the results of the real world Coroflex Blue Registry. In this registry in a non-randomised fashion without control group, the incidence of target vessel revascularisation (TLR) after six months was 5.5% and the cumulative six-month acute/subacute stent thrombosis rate was 1.6%17. The above-mentioned meta-analysis reported TLR rates for different bare metal stents of between 12.1 and 24.9%16.
The clinical efficacy of the iopromide-paclitaxel-coated balloon in stand-alone use has been demonstrated in different clinical trials and indications5-11,14,15. Furthermore, a pre-clinical study in pigs analysed neointimal formation after different inflation times using DES or DCB/BMS. In this study there was no difference between 10 sec, 60 sec or 120 sec balloon inflation time for a DCB/BMS system18. Therefore, the chosen inflation time of 30 sec may not have influenced the results. However this pre-clinical study was done with a higher paclitaxel dose of 5 µg/mm² in nonatherosclerotic vessels.
Conclusions
In summary, in this small cohort, to prevent restenosis it was safe to treat native coronary arteries with the paclitaxel-coated balloon in combination with a pre-mounted bare metal stent compared to a sirolimus DES without higher rates of malapposition. However, neointimal hyperplasia was higher than observed in the DES group. At this point, DCB with premounted BMS is not yet a replacement for DES. The potential benefit for DCB may be the reduced need for stents, but this concept needs further evaluation. A strategy of DCB angioplasty avoiding additional stent implantation (“DEB only” concept) may become an alternative in long and complex lesions, bifurcations or in patients with contraindications for DES (chronic anticoagulation, planned non-cardiac surgery, etc.)19. The concept of a BMS mounted on a DCB as a direct alternative to a DES will undergo further design evolution.
There are some limitations in this study. First, we did not perform IVUS at baseline, so some measurements regarding stent expansion could not be made. Second, the number of patients included in this IVUS study is limited. Third, quantitative coronary analysis (QCA) data for this cohort is not available.
Conflict of interest statement
D. Fischer receives speaking honoraria from AstraZeneca and Daiichi-Sankyo. He received a travel grant from B. Braun Melsungen. B. Scheller reports being named as co-inventors of a patent application for various methods of restenosis inhibition, including the technique employed in this trial, by Charité University Hospital, Berlin. B. Scheller and B. Cremers receive lecture fees from B.Braun. The other authors have no conflicts of interest to declare.

