
The risk of restenosis after implantation of current-generation drug-eluting stents (DES) is considered to be very low1. However, the number of complex coronary interventions is increasing, often involving a larger number and total length of implanted stents. As a result, about 10% of all coronary interventions represent treatment of in-stent restenosis (ISR) (USA NCDR database). The causes of ISR are complex and multifactorial. The primary mechanism of excessive neointimal proliferation due to the stimulus of the permanent implant is often reinforced by mechanical causes such as insufficient expansion of the stent or fractures of the stent struts. Therefore, ISR therapy typically involves local drug delivery to reduce the risk of neointimal proliferation and mechanical measures to regain luminal area2.
Strategies for treating ISR are numerous and range from repeat balloon angioplasty to surgical revascularisation. However, based on the available evidence, only two procedures could be considered effective, namely the implantation of another DES or the use of a drug-coated balloon (DCB)3. Only these two device types have received a corresponding recommendation in the guidelines1. Both procedures have in common that in the restenotic area of the stent a renewed local drug delivery is applied to address the mechanism of excessive neointimal proliferation. Overall, the frequency of repeat target lesion reintervention (TLR) seems to be slightly lower for the stent-in-stent approach4. The reason for this difference may be that, while both procedures address the growth of neointima, the mechanical component of ISR is primarily better served by the additional radial force of the second stent. However, this does not lead to a reduction of hard endpoints such as death or myocardial infarction4. A recent meta-analysis of 4,590 patients comparing DCB and alternative treatments such as DES for coronary ISR and de novo lesions reported a significantly lower all-cause and cardiac mortality after three years when using DCB5. The proposed pathomechanism for this surprising finding is the avoidance of a permanent implant when treating patients with DCB. For DES, a device-related annual event rate of up to 2% has been reported6. For this reason, several authors suggest using DCB as the primary strategy for treating ISR7, even if the reduction in the risk of recurrence of TLR is somewhat lower4 (Figure 1).
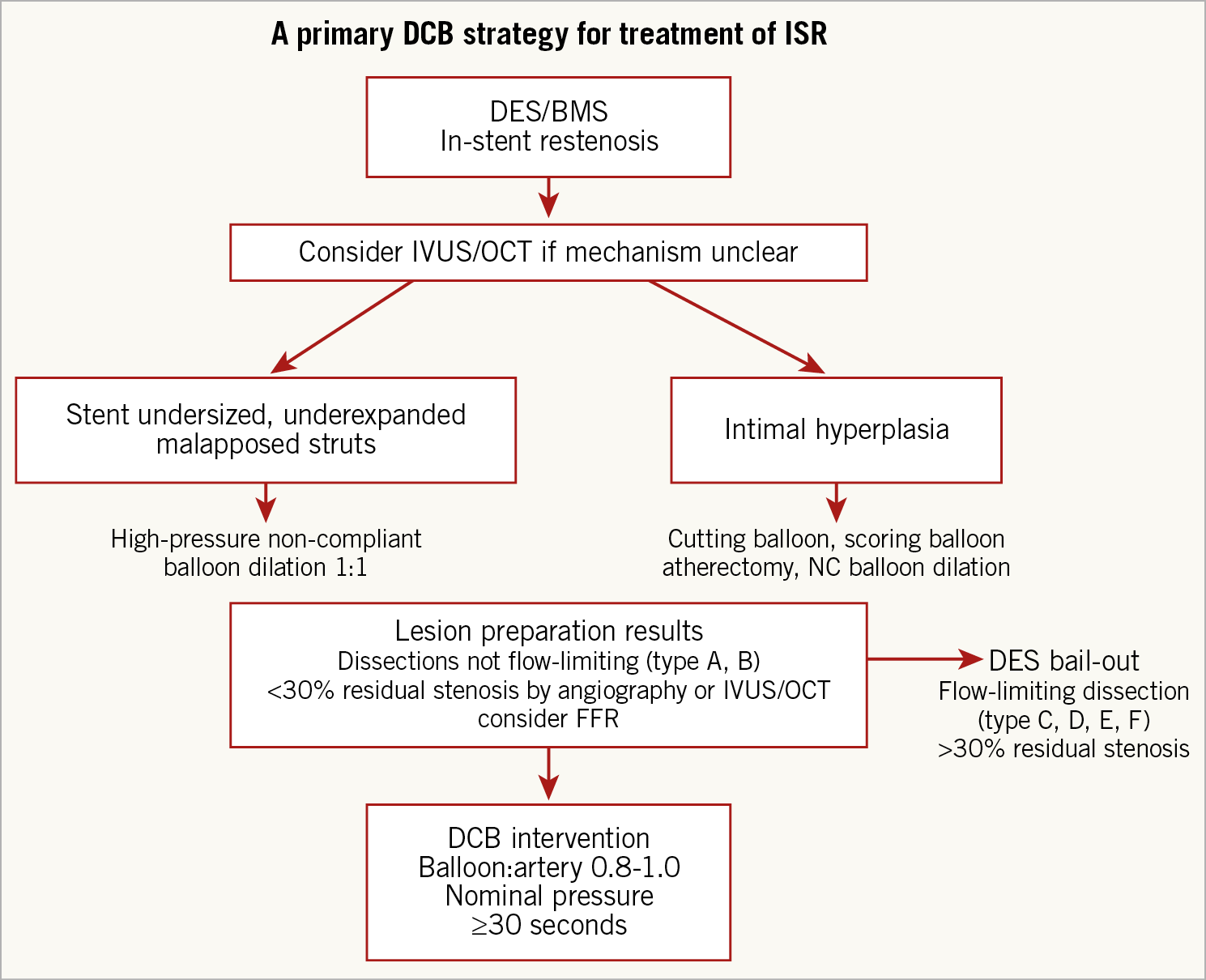
Figure 1. Proposed algorithm for the treatment of in-stent restenosis with a primary drug-coated balloon strategy. Adapted from Lansky et al7 with permission from the European Heart Journal.
The majority of clinical evidence on coronary DCB therapy from randomised trials has been generated using the paclitaxel iopromide coating which was originally investigated in the Paccocath ISR trial5,8. For this coating it could be shown that a balloon-based local application of paclitaxel in combination with an excipient leads to a long-term inhibition of neointimal growth9. The present study by Hamm et al is a mechanistic comparison of two different DCB10.
One hundred and twenty-five patients with ISR were randomly assigned to the iopromide DCB with a paclitaxel concentration of 3 µg/mm2 (n=60) or an acetyl tri-butyl citrate DCB with a paclitaxel concentration of 2 µg/mm2 (n=65). The latter coating was non-inferior to the paclitaxel iopromide DCB in the primary endpoint late lumen loss after six months. There were no significant differences in the baseline data. However, when looking in more detail, the paclitaxel iopromide group included slightly longer lesions (13.3 vs 11.7 mm; ns), a smaller reference diameter (2.48 vs 2.60 mm; ns), more lesions that actually required treatment according to angiographic criteria (96.6% vs 87.1%; ns), more calcified lesions (78% vs 60%; p=0.07), and a lower proportion of bare metal stent ISR (18% vs 25%; ns)10.
Lesion preparation followed by local drug delivery represents the fundamental principle of any DCB therapy11. It has been shown that the clinical outcome after ISR therapy with DCB depends on whether the quality criteria of the DCB consensus group are met or not12,13. The primary goal of the preparation of the lesion is to avoid flow-limiting dissections and to reduce the degree of residual stenosis to less than 30%12. From the perspective of an experienced DCB operator, one would expect more information on the quantity and technique of the required lesion preparation in the present trial. In this non-blinded study, numerical differences in post-procedural parameters such as minimal lumen diameter (1.77 vs 1.93 mm; ns) and the degree of residual stenosis (29.0% vs 25.7%; p=0.09) can be observed. Remarkably, the degree of residual stenosis in the paclitaxel iopromide group was 29.0±16.0%10, which means that almost half of the treated lesions did not meet the DCB consensus quality criterion of a maximum residual stenosis of 30%12. With regard to the calculation of the sample size, it should be critically noted that a standard deviation of 0.3 mm of the primary endpoint was assumed. In the studies cited, however, it ranged between 0.42 and 0.44 mm8,14,15,16, and in the present study even between 0.43 mm and 0.54 mm10. With these values, a significantly higher number of patients would have had to be included in order to prove non-inferiority of the primary endpoint statistically.
Nevertheless, the question that was investigated in the present study seems relevant. When we started with the first experiments on coated balloon catheters about 20 years ago, the amount of paclitaxel on the balloon was only just reproducible at about 3 µg/mm29. Due to the favourable clinical results in the first-in-man studies in terms of safety and efficacy, this dose was maintained and no further dose finding was done8,17,18. Preclinical studies indicate that, in the non-atherosclerotic animal model, lower concentrations of paclitaxel may also have antirestenotic effects19,20. The choice of the excipient seems to play a role21, but also very specific measures of the coating process itself. However, it is unclear whether higher drug doses are required in the atherosclerotic vessels of humans. From a scientific point of view, a clinical comparison of different dosages would be particularly useful if as few other parameters as possible are modified. Furthermore, the accepted clinical standard of use should be observed12, the comparability of the groups should be ensured, and sample size calculation should be based on realistic assumptions.
The underlying question for the interventional therapy of coronary heart disease is whether there is actually a need for solutions beyond the current generation of metallic DES. Modern stents enable us to treat complex coronary anatomies. The primary results are usually quite nice and the event rates in the first years low. However, the past enthusiasm for bioabsorbable scaffolds indicates that the necessity of avoiding permanent implants is perceived. In contrast to primary stent treatment, DCB therapy means a fundamental change in mindset and requires appropriate training. The most important part of the intervention is no longer the dropping of the stent but the preparation of the lesion. The coming years will reveal whether the current stent technology will remain the final solution or whether technologies such as DCB represent the next step in the evolution of percutaneous coronary intervention. The question of the best drug on the balloon will play much less of a role22. The decisive factor will be whether it will be possible to convince the majority of interventional cardiologists to implant fewer or no stents at all.
Conflict of interest statement
B. Scheller is a shareholder of InnoRa GmbH, Berlin, and was named as co-inventor on patent applications submitted by Charité University Hospital, Berlin, Germany.
Supplementary data
To read the full content of this article, please download the PDF.

