We read with interest the German Drug-Eluting Balloon Consensus Group recommendations on the use of drug-eluting balloons (DEB), recently published in EuroIntervention.1 When approaching coronary bifurcation lesions, the consensus group advocates to always predilate both the side branch (SB) and the main vessel (MV) with regular balloons. If the result is good, the sequence is repeated with DEB. Otherwise, stenting is favoured with either a drug-eluting stent (DES) alone or a bare metal stent (BMS) in association with DEB; dual antiplatelet therapy (DAPT) is recommended for 12 months in both cases.
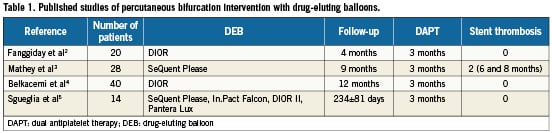
We believe that the most important feature of DEB technology is the ability to dilate stenoses combined with the effective transfer of a drug successfully inhibiting the vessel response to the dilation itself, without leaving anything behind (metal, polymer, eluting drug) that could trigger a delayed biological reaction. Accordingly, all DEB manufacturers recommend DAPT for only three months. This characteristic offers a new opportunity in percutaneous bifurcation intervention, namely the option to treat, with a result expectably similar to that obtained with a DES, patients that could not assume DAPT for 12 months as required after a DES has been implanted (e.g., patients with low compliance to drug therapy, high bleeding risk, important surgical indications). To date, published follow-up data after DEB treatment of coronary bifurcations are limited to 102 patients who assumed DAPT for three months (Table 1).2-5 Overall, stent thrombosis has been reported in only two patients enrolled in the PEPCAD V study and has been attributed to incomplete stent apposition.4 Therefore, we deem that the recommendation advocating 12 months duration of DAPT if a BMS is implanted after DEB treatment of a bifurcation is not supported by clinical evidence and weakens the advantages offered by DEB over DES, whose use in percutaneous bifurcation interventions is far more documented.
Moreover, to warranty effective DEB treatment, any effort has to be pursued to achieve total control of the bifurcation intervention. In that light, we consider that systematic SB predilation is questionable, especially if the vessel is free of disease. Indeed, primary predilation of the SB should be avoided because of the risk of dissection that can increases the need for potentially unnecessary stenting and the risk of rewiring the MV stent through a proximal cell.6 To the contrary, without SB predilation, it is possible to take advantage of the carina shift occurring during MV stenting to rewire the SB through the distal cell.6 Indeed, bench tests of provisional stenting have shown that rewiring through the cell closest to the carina provides far better scaffolding than proximal rewiring.7 Moreover, owing to the specific geometry of bifurcations favouring high atherosclerosis burden,8 success of percutaneous bifurcation interventions appears unlikely, unless adequate vessel scaffolding is provided.
To address all those issues, we have developed an approach consisting of stenting the MV with a BMS and then finalising the procedure with kissing inflation with DEB. We have shown that this approach is feasible and safe with all different DEBs.5 However, until a more robust demonstration of the validity of our strategy, we are currently restricting this approach exclusively to patients who could absolutely not be compliant to DAPT. Angiographic follow-up results accumulate slowly but are extremely encouraging, and our first findings with optical coherence tomography are especially reassuring against possible issues related to our strategy such as drug lost during DEB tracking (Figure 1).
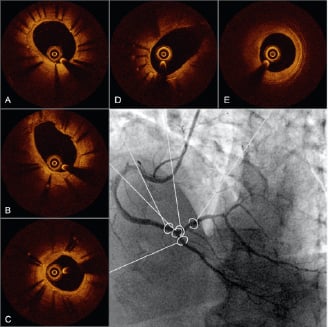
Figure 1. Optical coherence tomography (OCT) cross sections of the proximal main vessel (A), in-bifurcation segment (B), distal main vessel (C), side branch ostium (D) and para-ostial side branch of the distal right coronary artery bifurcation (E) obtained six months after percutaneous coronary intervention had been performed according to a strategy of provisional bare metal stenting completed by kissing inflation of drug-eluting balloons (DEB). Both angiographic and OCT images show absence of restenosis. Moreover, OCT images demonstrate lesser neointimal hyperplasia at the proximal main vessel than at the distal main vessel possibly related to higher dose of paclitaxel released during the index procedure because of the simultaneous inflation of two DEB.
Conflict of interest statement
The authors have no conflict of interest to declare.
References
Drug-eluting balloon offers a new opportunity in percutaneous bifurcation interventions
by Gregory A. Sgueglia, MD, PhD; Daniel Todaro, MD, PhD ; Edoardo Pucci, MD
We thank Drs. Sgueglia and Todaro for their interest in the German Consensus Group recommendations1. Their letter is related to the treatment of coronary bifurcations. As they correctly explained, the aim of the “DEB-only” concept is to avoid additional stent implantation when using drug-coated balloon catheters. The strategy of carefully preparing the lesion by predilatation to evaluate the risk for relevant dissections followed by local drug delivery with the drug-coated balloon seems to be a reasonable approach of translating the “DEB-only” concept into clinical practice.
The recommendations in the consensus paper are based on the results of randomised trials2-6, non-randomised studies7,8, registry data, and clinical experience with the SeQuent™ Please balloon. Drs. Sgueglia and Todaro include in their letter different types of drug-coated balloons suggesting a class effect. However, this assumption has not been proven. On the contrary, animal data show significant differences between different types of balloons9. The Dior balloon failed in randomised clinical studies10,11, whereas no randomised clinical data are available for In.Pact Falcon™12, Elutax™ and Pantera Lux™. The current European guidelines for revascularisation point to the fact that one cannot assume a class effect for drug-coated balloons13. Therefore, it seems to be very speculative to mix up results from this heterogeneous group of balloons.
The consensus group recommends only four weeks of dual antiplatelet therapy if no new stent is implanted, which is in contrast to the three months proposed in the letter of Drs. Sgueglia and Todaro. However, the minimal time for dual antiplatelet therapy when combining a drug-coated balloon with a new stent is unknown. Experimental data on paclitaxel-coated balloons with premounted stents indicates a persistence of paclitaxel in the vessel wall of at least six months14. In the clinical trials, cases of stent thrombosis occurred later than three months7,8,15. Therefore, the recommendation of the consensus group was to treat the combination of a bare-metal stent and a drug-coated balloon like a drug-eluting stent, e.g., 6-12 months of dual antiplatelet therapy.
Newer, randomised clinical data avoiding geographical mismatch and preferring post-dilatation of the bare-metal stent with the SeQuent™ Please balloon show no cases of stent thrombosis despite a dual antiplatelet therapy of only three months5,6. Therefore, a reduction of dual antiplatelet therapy down to three months in selected cases, e.g., when using only short stents (“spot stenting”) may be included in an upcoming update of the consensus group recommendations.
Coronary bifurcation disease is complex and heterogeneous. Therefore, we agree with Drs. Sgueglia and Todaro that there may be different ways to use drug-coated balloons in bifurcations. However, we doubt that a series of 14 patients using four different types of drug-coated balloons will be sufficient to prove that their approach is “feasible and safe with all different DEB”.
Well conducted randomised clinical trials for every type of drug-coated balloon are mandatory to clarify the future role of this new technology in interventional cardiology. A sufficient data basis from randomised studies should be the basis for evidence based recommendations in the official guidelines. A first step is the class IIa recommendation for the treatment of bare metal stent restenosis in the European revascularisation guidelines. However, this recommendation remains limited to the “specific devices with proven efficacy/safety profile, according to the respective lesion characteristics of the studies”13, e.g., paclitaxel-iopromide coated balloons.
Conflict of interest statement
B. Scheller reports receiving grant support from B.Braun; speaker honoraria from B.Braun and Invatec; major stock shareholder/equity in InnoRa GmbH; and is named as co-inventor of a patent application submitted by Charite University Hospital (Berlin, Germany). F.X. Kleber has a consulting agreement with B.Braun and has received lecture honoraria and free scientific research grants from B.Braun, Eurocor, Invatec-Medtronic and Biotronik. D.M. Mathey has received speaker fees from B. Braun. H. Rittger reports speaker honoraria from B.Braun.
References

