
Abstract
Aims: Although paclitaxel drug-coated balloon (DCB) angioplasty is an established endovascular treatment for peripheral artery disease, restenosis remains a major concern. Thus, we compared a novel paclitaxel-coated DCB with nano-coating technology with uncoated plain old balloon angioplasty (POBA).
Methods and results: This multicentre trial randomly assigned 171 patients with stenotic and occlusive lesions of the femoropopliteal artery to angioplasty with a novel DCB or uncoated POBA. The primary endpoint, late lumen loss at six months, was 0.92 mm lower in the DCB group (95% CI: −1.36 to −0.49 mm, p<0.001). Patients showed improved walking after DCB treatment at six months (p=0.021). In the DCB group, 44.6% and 50% of the patients improved by three Rutherford-Becker classification stages after six to 12 months, respectively (POBA: 27.8% and 36.8%, respectively). Only one patient needed TLR (1.3%) in the DCB group, compared to 14 patients (18.7%) in the POBA group after 12 months (relative risk [RR]=0.08, 95% CI: 0.01-0.53, p<0.001). Primary patency was 90.3% (DCB group) versus 65.3% (POBA group) after 12 months (RR=1.38, 95% CI: 1.14-1.67, p<0.001).
Conclusions: The novel DCB was effective and safe for inhibiting restenosis. Moreover, it demonstrated a better improvement in walking than POBA and showed no mortality concerns due to paclitaxel application after 12 months. Clinical Trials Identifier: NCT02540018
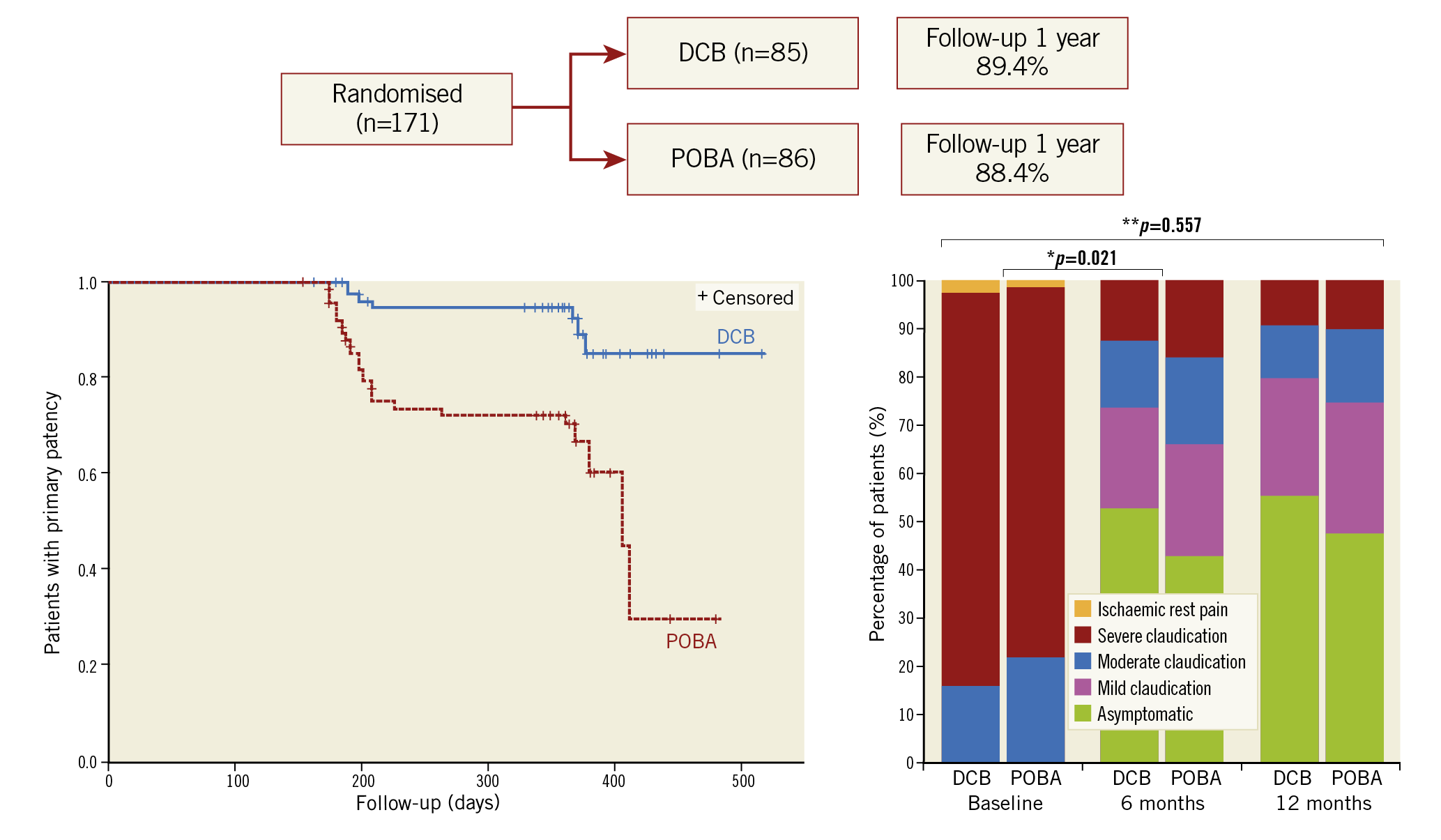
Visual summary. Patient flow, Kaplan-Meier survival curves for primary patency, and change in Rutherford-Becker category over 12 months.
Introduction
Intermittent claudication in the lower extremities is the most common symptom of peripheral artery disease and is often caused by stenosis or occlusion of the femoropopliteal artery segment1. Treatment in intermittent claudication aims to improve the pain-free walking distance and, ultimately, quality of life2. Uncoated plain old balloon angioplasty (POBA) followed by paclitaxel drug-coated balloons (DCBs) is increasingly considered as the treatment of choice for revascularisation in shorter lesions3,4. Early onset of neointimal proliferation is an important limitation that often leads to restenosis. Local drug delivery with paclitaxel DCBs is a promising method for inhibiting neointimal proliferation5,6. Different DCBs have already been tested; however, there is considerable heterogeneity (regarding efficacy) among such studies and a high risk of performance bias existed in earlier studies7,8. The current report outlines the 12-month outcomes of the EffPac trial, which compared a novel paclitaxel-coated DCB with nano-coating technology to uncoated POBA with regard to clinical benefit and safety.
Methods
STUDY CONCEPT
This investigator-initiated multicentre randomised controlled parallel-group trial was performed at 11 vascular centres across Germany. The trial was approved by the independent ethics review board at each of the participating institutions and all patients provided written informed consent. An independent clinical research organisation was appointed for the trial monitoring activities and a blinded independent core laboratory reviewed the primary endpoint measurements and duplex ultrasound measurements. The study protocol was published in the journal Trials9. The trial was reported according to the CONSORT statement10.
STUDY POPULATION AND ELIGIBILITY CRITERIA
Patients with symptomatic peripheral artery disease, with moderate to severe intermittent claudication or ischaemic rest pain (Rutherford-Becker classes 2-4), were eligible for enrolment. De novo stenotic or non-stented restenotic or occlusive lesions with a lesion length ≤15 cm were considered. Only lesions in the superficial femoral artery (SFA) and the proximal popliteal artery up to the P1 segment were included. Bail-out stenting in flow-limiting dissection was also considered. The inclusion and exclusion criteria are outlined in Supplementary Appendix 1.
INVESTIGATIONAL PRODUCT
In the experimental arm, patients were treated with the paclitaxel-coated Luminor® DCB (iVascular S.L.U., Life Vascular Devices Biotech, Barcelona, Spain). A description of the investigational product with its TransferTech® nano-technology coating is provided in Supplementary Appendix 2.
RANDOMISATION AND THE INDEX PROCEDURE
Patients were randomly assigned after predilatation in a 1:1 allocation ratio using a computer-generated randomisation list with random block sizes and stratification by vascular centre (stratified block randomisation). For non-flow-limiting or flow-limiting dissections, prolonged percutaneous transluminal angioplasty (PTA) with the same PTA balloon was performed. For persistent flow-limiting dissections, bail-out stenting with a bare metal stent was permitted (Figure 1). In case two or multiple PTA balloon catheters were used, a minimised overlap of 5 to 10 mm was required. A total of 93 drug-eluting Luminor 35 balloons were used.
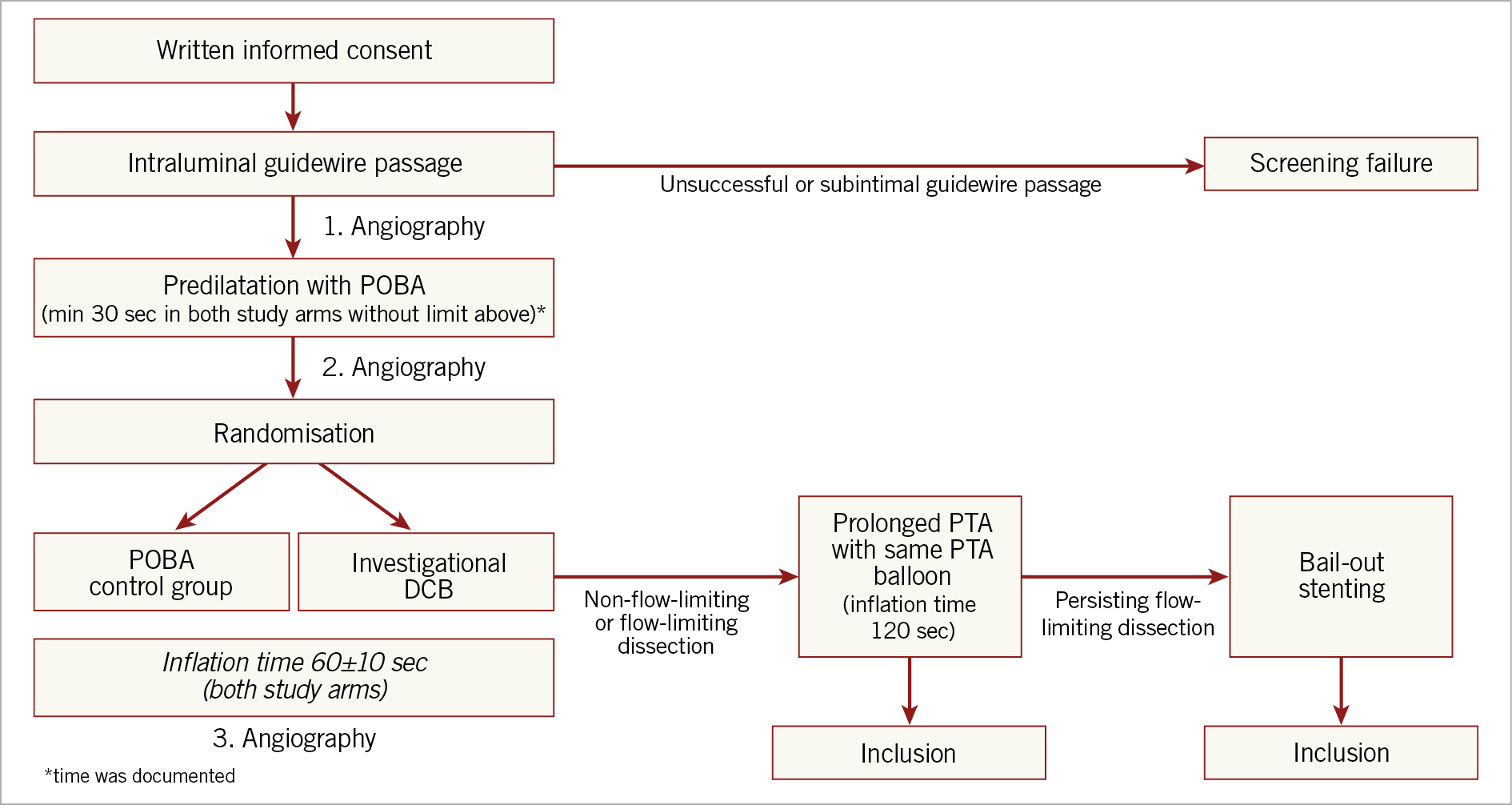
Figure 1. Flow chart of the index procedure.
ENDPOINTS AND FOLLOW-UP
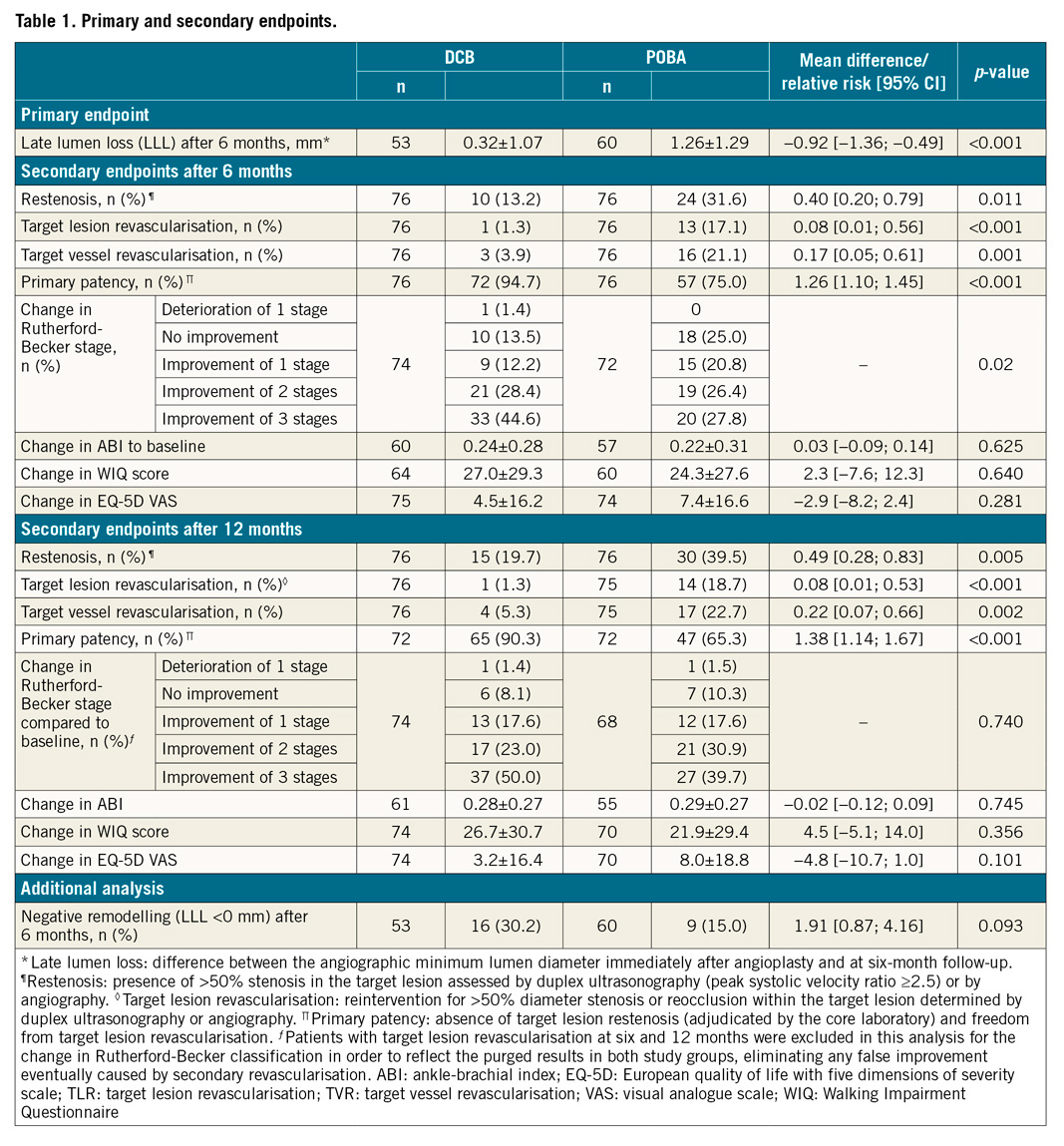
The primary efficacy endpoint of our study was late lumen loss (LLL) after six months (defined as the difference between the angiographic minimum lumen diameter immediately after PTA and that at follow-up). Safety endpoints included freedom from target lesion revascularisation (TLR), investigation- and procedure-related serious adverse events (SAE)/AE, all-cause mortality, and minor and major target limb amputations. The secondary outcomes were primary patency, regarded as TLR + freedom from binary restenosis assessed by duplex ultrasound peak systolic velocity ratio <2.5, or angiography (core laboratory adjudicated); freedom from target vessel revascularisation (TVR); change in walking impairment assessed by the Walking Impairment Questionnaire (WIQ) and Rutherford-Becker classification (RBC) at follow-up; change in ankle-brachial index (ABI) after the intervention and at follow-up; change in “quality of life” as assessed by European quality of life with five dimensions of severity (EQ-5D) scale at follow-up; number of bail-out stents.
STATISTICAL ANALYSIS
The sample size calculation was based on the results of a previous trial11. All analyses were performed according to the intention-to-treat principle. Multiple imputation of missing values was conducted for the primary endpoint using the fully conditional specification method to evaluate the robustness of the conclusions. Continuous data are presented as means and standard deviations or medians and interquartile ranges according to the data distribution. Absolute and relative frequencies are given for categorical data. Data were analysed with SAS 9.4 (SAS Institute, Cary, NC, USA). A two-sided p-value of <0.05 was considered to indicate statistical significance. The statistical analyses for each endpoint are described in Supplementary Appendix 3.
Results
STUDY POPULATION AND TREATMENT
A total of 171 patients were enrolled between September 2015 and December 2016. Only one drop-out due to small-vessel diameter occurred in the DCB group after randomisation. Eighty-six patients were treated with POBA and 85 with the investigational DCB. The patient flow diagram according to CONSORT 2010 is shown in Supplementary Figure 1. The groups were well matched in baseline demographics and comorbidities (Supplementary Table 1); 38.6% (66/171) of the patients were diabetics and 41.8% (71/171) were current smokers. Regarding the DCB versus POBA groups, the mean lesion length was 59±43 mm versus 56±39 mm, the total treated length was 89.8±48.6 mm versus 84.9±45.1 mm, and total occlusions comprised 20.2% (17/84) versus 25.6% (22/86) of the total lesions.
Predilation was performed in all but one POBA patient (DCB: 100% [84/84]; POBA: 98.8% [85/86]). The rate of dissections (DCB: 37.6% [32/85]; POBA: 40.7% [35/86]) and the bail-out stenting rate (DCB: 15.3% [13/85]; POBA: 18.8% [16/85]) were similar in both groups. Moreover, no significant differences existed in the other angiographic parameters at baseline (Supplementary Table 2). Periprocedural distal thrombotic embolisation was not recorded.
PRIMARY EFFICACY AND SAFETY OUTCOMES
Regarding the DCB versus POBA groups, 62.4% (53/85) versus 73.3% (63/86) of the patients underwent angiography after six months. LLL at six months was 0.14 mm (95% CI: −0.38 to 0.67) for DCB versus 1.06 mm (95% CI: 0.54-1.59) for POBA. The difference between the groups was −0.92 mm (95% CI: −1.36 to −0.49, p<0.001). We found no evidence that the results of the primary endpoint were biased due to dropouts. The TLR rate was 1.3% (1/76) and 17.1% (13/76) after six months in the DCB and POBA groups, respectively (p<0.001). The relative risk reduction for TLR was 91.8% after six months according to the Cochran-Mantel-Haenszel estimation, and the number needed to treat (NNT) to prevent one additional TLR after six months was seven (Table 1). After 12 months, the TLR rate was still significantly lower in the DCB group (1.3%, 1/76), with an NNT of six, than in the POBA group (18.7%, 14/75) (p<0.001). The Kaplan-Meier estimates for freedom from TLR are shown in Supplementary Figure 2.
Other safety endpoints did not differ significantly between the groups. There was one minor amputation (1.2%) and there were two deaths (2.3%) in the POBA group after 12 months versus one death in the DCB group (1.2%). All deaths were considered unrelated to the device, procedure, or index limb.
SECONDARY OUTCOMES
Primary patency was 94.7% (72/76) and 75.0% (57/76) after six months in the DCB and POBA groups, respectively, (p<0.001). After 12 months, primary patency remained significantly higher in the DCB group (90.3%, 65/72 vs 65.3%, 47/72; p<0.001). The additional analysis for negative remodelling is shown in Table 1. The Kaplan-Meier estimates for patency are reported in Supplementary Figure 3. Significantly more patients showed an improved RBC at six months after DCB angioplasty than after POBA (p=0.021). An improvement of three stages was noted in 44.6% (33/74) and 27.8% (20/72) of patients for DCB and POBA, respectively (Figure 2). The DCB group also showed better RBC improvement after 12 months: 50% of the patients (37/74) in the DCB group showed an improvement of three stages of RBC compared to only 39.7% in the POBA group (27/68), although the difference was non-significant (p=0.740). Further, compared to the POBA group values, the average WIQ score in the DCB group was 2.6 points (95% CI: −6.9 to 12.0) higher after six months and 5.3 points (95% CI: −4.6 to 15.2) higher after 12 months. Further results are shown in Table 1.
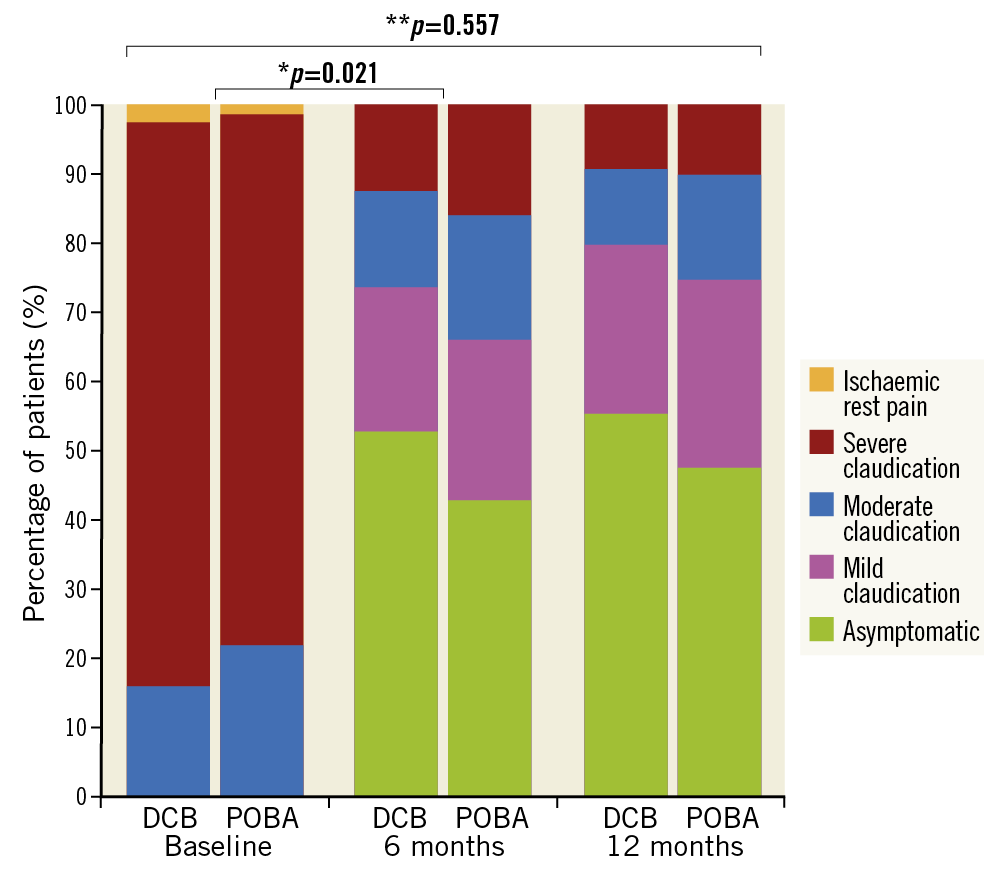
Figure 2. Percentage of patients with different Rutherford-Becker classifications at baseline, 6 months, and after 12 months
Discussion
Angioplasty with paclitaxel DCBs can effectively reduce neointimal proliferation12. Decisive factors for the effectiveness of DCB catheters are the loss of the coating layer during catheter transfer and incomplete drug delivery to the vessel wall. The DCB catheter in our trial is based on a new proprietary nano-coating technology, with very low drug loss during catheter insertion and advancement, as well as a high paclitaxel delivery to the vessel wall during inflation.
In the DCB group, LLL was lower than in previous DCB trials (e.g., PACIFIER, FemPac, and THUNDER trials), with a similar surface dosage11,13,14. “Negative remodelling” (negative LLL defined as lumen gain during follow-up) occurred in 30.2% of the DCB patients, i.e., twice as frequently as in POBA patients. Similar observations were shown in recent DCB trials with low TLR rates15,16. Negative remodelling can additionally indicate high DCB effectiveness. However, ectatic vessel changes were occasionally documented six months after DCB treatment17. In our trial, no such inadvertent aneurysmal dilatations of the target lesion were observed. Only one revascularisation was necessary after six and 12 months in the DCB group (TLR rate of 1.3%). Good TLR rates of 2-6% after one year have also been noted with other DCBs18,19.
Furthermore, EffPac showed a comparably low TLR rate in the control group, with an NNT of seven (i.e., with every seventh DCB treatment, one additional reintervention is prevented). Unlike in earlier trials, which partly suggested lower NNTs, EffPac did not show any significant treatment differences between the study groups (especially regarding predilatation and stenting rates), except for the applied catheter. This allows a more realistic assessment of the treatment effect and is consistent with newer trials that also performed predilatation before randomisation20,21.
Along with a lower reintervention rate, EffPac showed a higher primary patency rate after DCB treatment and thus fewer restenoses that did not require treatment (≥50%) defined by peak systolic velocity ratio by Doppler ultrasound ≥2.5. The NNT was six after six months and four after 12 months. This also suggests high antirestenotic ability and is comparable to the performance of other DCBs (e.g., in the AcoArt I, IN.PACT SFA, and ILLUMENATE EU trials)16,18,19. RBC is an easily applicable yet established clinical staging system for peripheral arterial disease and seems reliable for indicating the necessity of a possible reintervention22. Our trial demonstrates a significant improvement in RBC after DCB treatment at six-month follow-up. This is the most important outcome compared to the other endpoints, which may be considered surrogates. Also, after 12 months an improvement is notable, even though it loses its statistical significance. As a matter of fact, the results at 12-month follow-up are biased to walking improvement by the fact that 12 patients were revascularised in the POBA group and only one in the DCB group. Therefore, those patients obviously improved their walking capacity after secondary revascularisation. This represents a performance bias, which leads to the loss of statistical significance at 12 months.
A significant clinical improvement was also reported by the AcoArt I trial, but EffPac additionally demonstrated, for the first time, the clinical improvement for blinded follow-up visits under the same treatment conditions in both groups16. Of note, the randomisation was performed after predilatation; therefore, both study groups were pre-treated in the exact same way, minimising performance bias early in the study design (Figure 1). An improvement in walking capacity was also affirmed by the patient-blinded WIQ results. Although not significant, in all subdomains of the questionnaire, higher mean scores were noted in the DCB group after six and 12 months as compared to POBA. The change of ABI compared to baseline was not significant between the study groups. Possible reasons could be either the lack of sufficient statistical power, the impairment of run-off vessels below the knee or microangiopathy, especially in patients with diabetes.
In the two-year and five-year long-term follow-ups, we will investigate whether these clinical benefits will be preserved. According to the three-year data of the IN.PACT SFA trial and the five-year data of the THUNDER trial, the occurrence of a late catch-up seems unlikely23,24.
Limitations
Several limitations of this study need to be discussed. Although the Data Safety and Monitoring Board and core laboratory personnel were blinded to treatment, physicians performing the index procedure were not blinded because of the visible coating on the DCB catheter.
The risk of performance bias was minimised by the predefined treatment process (e.g., randomisation after predilatation, stent implantation only after persistent flow-limiting dissection). No significant differences in the key parameters of treatment were found.
Another limitation might be the short lesion lengths (approximatively 5.7 cm) compared to some other recent trials (AcoArt I, CONSEQUENT trial)16,25. However, shorter lesions are more suitable for a balloon-only approach (“leaving nothing behind”) and reflect clinical practice. Comparable trials that also focused on TASC II A and B lesions also investigated short lesions from 4.0-9.8 cm11,18,19,26,27,28. In longer lesions with more occlusions, the need for adjunctive treatment, e.g., atherectomy and stent implantation, increases.
When our trial was initiated in 2015, POBA was still the standard as the comparative device to drug-eluting balloon catheters and LLL was imperative as the primary endpoint to demonstrate technical efficacy. This was the only way to show that our investigated DCB was effective on the one hand, and safe compared with a non-paclitaxel-coated balloon catheter on the other hand. The safety of a DCB is best shown in an RCT with POBA as control group. At this time point, we are only able to prove that there are no mortality concerns due to paclitaxel application after 12 months in our EffPac trial. In a meta-analysis comparing all kinds of paclitaxel-coated devices for the SFA, Katsanos et al have recently shown an increased risk of death following the application of paclitaxel-coated balloons and stents after two years and five years29. The EffPac trial is a proof-of-principle study. Regarding the promising results of LLL as technical outcome, and TLR, patency and walking improvement as clinical outcomes after one year, we amended EffPac for additional 24-, 42- and 60-month follow-ups. Also, a head-to-head trial will be the next step allowing direct comparison to other DCB catheters. Finally, patients were recruited on the basis of strict inclusion and exclusion criteria; therefore, the generalisability and the clinical relevance of the data to real-world cases may be limited.
Conclusions
The EffPac findings further validate the superiority of DCBs, showing a notable LLL, and TLR and patency rates as technical and clinical outcomes, respectively. What defines the new-generation paclitaxel DCB is its significant improvement of walking capacity, which is the most relevant clinical endpoint in patients with intermittent claudication. This is an important contribution to clinical practice.
|
Impact on daily practice EffPac showed, for the first time, a statistically significant improvement assessing walking capacity as a “real” clinical endpoint and is therefore crucial for patients with peripheral artery disease. Our study uncovers the important role of additional measurement tools such as Rutherford-Becker classification and walking impairment tests. |
Appendix. Study collaborators
Peter von Flotow, MD; Department of Vascular Medicine, Westpfalz-Hospital, Kusel, Germany. Britta Heilmeier, MD; Center Internal Medicine, Ruprecht-Karls-University Heidelberg, Germany. Christian Erbel, MD; Center Internal Medicine, Ruprecht-Karls-University Heidelberg, Germany. Michael Werk, MD; Martin-Luther-Hospital, Berlin, Germany. Vicenç Riambau, MD; Hospital Clínic de Barcelona, Barcelona, Spain. Andreas Wienke, MD; Medical Epidemiology, Martin-Luther-University Halle, Halle, Germany.
Acknowledgements
We thank the Center of Clinical Trials Jena (ZKS), especially Isabella Schiller, Nicole Brillinger, and Tabitha Heller. The authors also thank Laura Graziani, our research coordinator, for her support.
Funding
This work was financially supported by the manufacturer of the investigational product, iVascular S.L.U., Life Vascular Devices Biotech, Barcelona, Spain, as well as by endoscout GmbH, Freiburg, Germany.
Conflict of interest statement
U. Teichgräber is a consultant for iVascular and endoscout GmbH. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

