Abstract
Background: Numerous randomised controlled trials (RCTs) have demonstrated the superiority of paclitaxel drug-coated balloons (DCBs) over non-coated angioplasty balloons for treatment of femoropopliteal peripheral arterial disease (PAD). There is a paucity of clinical evidence in more complex patients who are often excluded from RCTs and long-term data up to 5 years are very limited in PAD revascularisation studies.
Aims: This is a report of the 5-year outcomes from the prospective, single-arm, international IN.PACT Global Study. The IN.PACT Admiral DCB was evaluated for femoropopliteal atherosclerotic disease treatment in a real-world patient population.
Methods: In total, 1,535 patients were enrolled at 64 international sites. The prespecified clinical cohort included 1,406 patients with claudication or rest pain. Patients were evaluated up to 5 years for the occurrence of adverse events and clinically driven target lesion revascularisations (CD-TLR).
Results: The mean lesion length was 12.1±9.5 cm in 1,774 lesions, 18.0% had in-stent restenosis, 35.5% were total occlusions and 68.7% were calcified. Per independent clinical events committee adjudication, the Kaplan-Meier estimate of freedom from CD-TLR up to 5 years was 69.4%, and the restricted mean survival time to first CD-TLR was 1,470.1 days. Outcomes were similar for males and females; freedom from CD-TLR was 69.1% in females and 69.6% in males (p=0.602). The cumulative incidence of major adverse events for the clinical cohort was 45.9% and freedom from all-cause mortality with the vital status update was 78.9% up to 5 years.
Conclusions: The IN.PACT Admiral DCB demonstrated safe and durable outcomes in real-world participants with complex femoropopliteal disease. ClinicalTrials.gov: NCT01609296
Introduction
The treatment of peripheral arterial disease (PAD) in patients with multiple comorbidities is challenging and remains a global health concern, impacting approximately 236 million people worldwide1. Drug-coated balloons (DCB) emerged as a novel endovascular treatment option a decade ago.
Numerous randomised controlled trials (RCTs)23456 and meta-analysis7 have demonstrated the superiority of DCB over uncoated percutaneous transluminal angioplasty (PTA) for the treatment of PAD in the femoropopliteal segment. Patients with more complex lesions, such as in-stent restenosis (ISR), lesions longer than 18 cm, and lesions with significant calcification, are usually excluded from RCTs, thus there are limited data on real-world patients. There are also few prospective studies on endovascular treatments for PAD with long-term (≥5 years) evidence2891011.
The IN.PACT Global Study was a large prospective study that assessed the IN.PACT Admiral DCB (Medtronic) for the treatment of atherosclerotic disease of the superficial femoral artery (SFA) and/or the popliteal artery in a real-world patient population. The study was designed with very broad selection criteria, allowing for the inclusion of a complex patient population. This article reports on the final 5-year outcomes of the full clinical cohort.
Methods
The IN.PACT Global Study was a prospective, international, single-arm study. It evaluated the safety and effectiveness of the IN.PACT Admiral DCB for the treatment of obstructive disease in femoropopliteal (including the entire popliteal artery) arteries caused by atherosclerosis. Details on the trial design and outcomes up to 3 years have been reported previously121314. Briefly, the inclusion criteria included Rutherford clinical category (RCC) 2-4 (claudication and/or ischaemic rest pain). De novo and restenotic lesions (including ISR) were permitted if they were severely stenosed or occluded and ≥2 cm in length. Relevant exclusion criteria included aneurysmatic and acute thrombotic lesions. The primary effectiveness endpoint was freedom from clinically driven target lesion revascularisation (CD-TLR) within 12 months. CD-TLR was defined as any reintervention of the target lesion(s) because of symptoms or a drop in the ankle-brachial index (ABI) of ≥20% or>0.15 when compared with the post-index procedure ABI. The primary safety composite endpoint was freedom from device- and procedure-related death up to 30 days, and freedom from major target limb amputation and clinically driven target vessel revascularisation (CD-TVR) within 12 months. A major adverse event (MAE) was defined as all-cause mortality, major target limb amputation, CD-TVR, or thrombosis at the target lesion site up to 5 years. Results from the 1-, 2-, and 3-year follow-up periods have been reported121314.
Dual antiplatelet therapy (DAPT) was required consistent with standard clinical practice, including aspirin (ASA) indefinitely and DAPT for 1 month (3 months for stented patients). Participants were followed up at discharge, 30 days, 6 months, 12 months and then annually up to 5 years. Follow-up evaluations, including medication compliance, were conducted via clinical visits up to 3 years. At years 4 and 5, follow-up was conducted over the phone to evaluate the incidence of adverse events, including revascularisations. To improve ascertainment of mortality, investigational sites were also asked to obtain vital status from study participants who withdrew or were lost to follow-up to verify safety information obtained as part of the original study design. Vital status results are specifically labelled as such when these additional data have been included.
An independent Clinical Events Committee (CEC; Syntactx) adjudicated all MAEs including death, major target limb amputation, CD-TVR, CD-TLR, and thrombosis at the target lesion site. The statistical methods were designed by the study sponsor and documented prospectively in the clinical trial protocol.
The study was conducted in accordance with Good Clinical Practice guidelines, the Declaration of Helsinki and all applicable country laws. The institutional review board or ethics committee at each participating site approved the study protocol, and informed consent was obtained from all participants prior to enrolment.
Statistics
Baseline characteristics and outcomes are summarised descriptively using percentages and frequencies for categorical variables and the mean, standard deviation, and number of observations for continuous variables. Time-to-event outcomes are summarised using the Kaplan-Meier (KM) method. Participants were censored at the time of withdrawal, loss to follow-up, or death. Confidence intervals (95% CI) were derived for time-to-event outcomes using the log-log transformation. Outcomes are also summarised using the restricted mean survival time (RMST) and 95% CI with a time horizon of 1,800 days. The RMST is the average time to an event within a fixed time period and corresponds to the area under the survival curve from the start of follow-up to the fixed time point. Baseline demographics, clinical characteristics, and outcomes are reported or analysed on a patient basis and lesion characteristics are reported on a lesion basis. Data were analysed according to the study protocol. A patient was included in the analysis if the study DCB was introduced into the sheath and after the guidewire had successfully passed through the target lesion. Annual cut-offs for the statistical analysis used 360 days per year (e.g., 1,800 days for the 5-year cut-off). The denominator for binary endpoints used the sum of those with an event and those with follow-up of at least 1,740 days. A prespecified subgroup analysis by gender is also presented.
An exploratory analysis of the association between baseline characteristics and the time to CD-TLR and MAE was computed using a Cox proportional hazards model for the full clinical cohort and each gender subgroup separately. Univariate models were computed first followed by a multivariable model that was derived using stepwise regression. The entry and stay criteria for the stepwise regression were 0.2 and 0.1, respectively, starting with the set of variables that had a p-value <0.2 in the univariate models. Single imputation stratified by gender was performed for missing data in the multivariable analysis for variables with less than 15% missingness, using the mean for continuous variables and the mode for categorical and binary variables. DAPT was included as a time-varying variable to allow for potential changes in use between discharge and 30 days.
No adjustment was made for multiple comparisons, and the level of statistical significance was set at 0.05. Statistical analyses were performed using SAS version 9.4 (SAS Institute).
Results
From May 2012 to March 2014, 1,535 patients were enrolled at 64 global centres. This report includes outcomes on 1,406 participants who were treated with the DCB. Patients from the 150 mm DCB cohort were enrolled non-consecutively and were excluded from the current analysis. The participant flow chart up to 5 years is shown in Figure 1. By the end of the 5-year follow-up window (1,885 days), 253 participants died, 186 withdrew or exited the study, 21 were lost to follow-up, and 28 did not have follow-up completed by the close of the study. Five-year data on 97.0% of the 946 eligible participants were obtained.
Baseline demographics and lesion characteristics have been previously reported121314 and are shown in Table 1. Medication compliance was collected up to 3 years; data are provided in Supplementary Table 1.
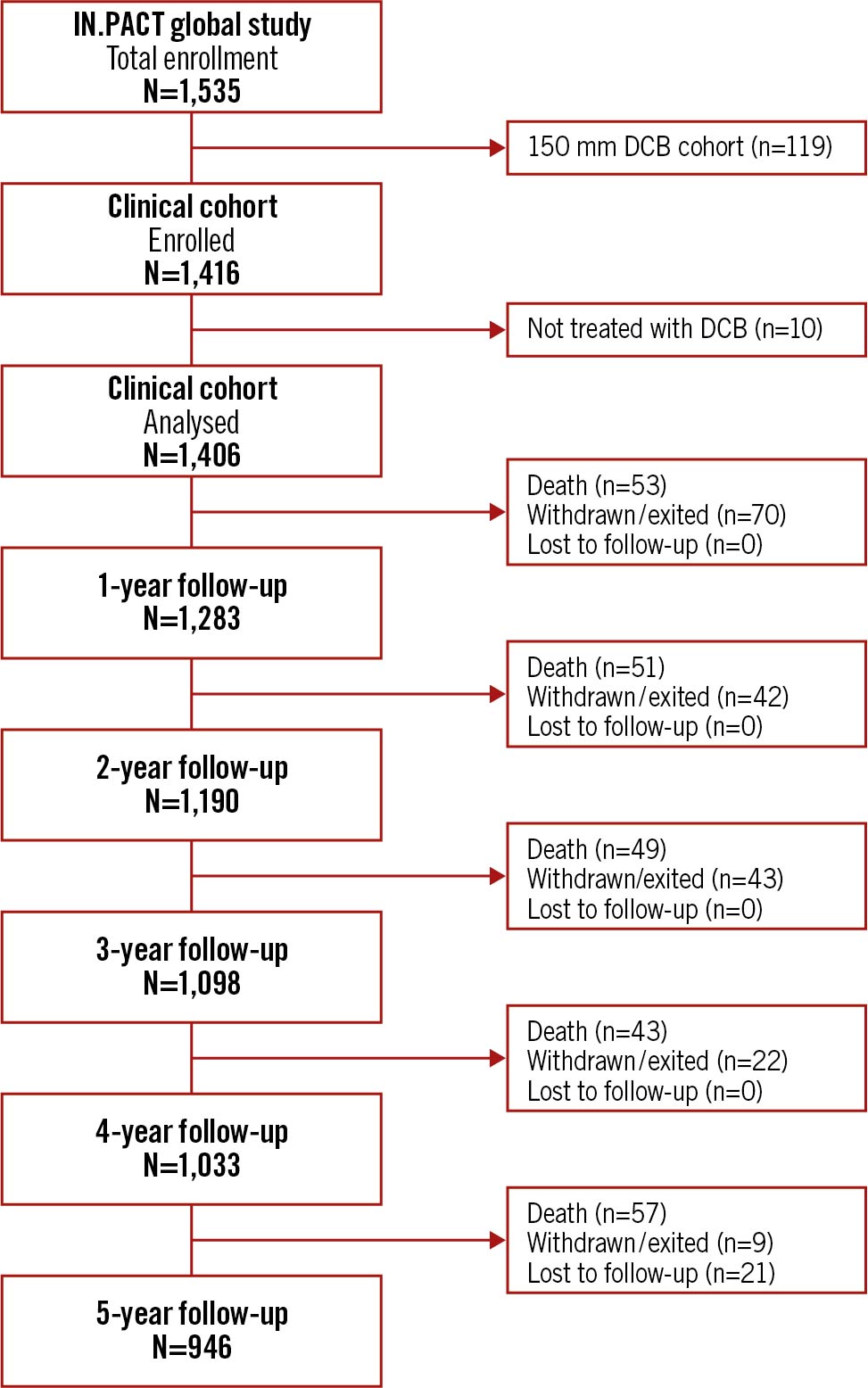
Figure 1. IN.PACT Global study patient flowchart up to 5 years. Follow-up windows were 365 days per year with windows of ±60 days. N represents the number of participants eligible for evaluation at each timepoint. DCB: drug-coated balloon
Table 1. Baseline and procedural characteristics.
| Patient characteristics | Clinical cohort(N=1,406 patients)(N=1,774 lesions) | |
|---|---|---|
| Age (years) | 68.6±10.1 | |
| Male | 67.8% (953/1,406) | |
| Obesity (BMI ≥30 kg/m²) | 20.5% (285/1,391) | |
| Hypertension | 83.4% (1,169/1,401) | |
| Hyperlipidaemia | 70.5% (960/1,362) | |
| Diabetes mellitus | 39.9% (560/1,402) | |
| Insulin-dependent diabetes mellitus | 17.8% (249/1,402) | |
| Coronary artery disease | 40.5% (540/1,332) | |
| Carotid artery disease | 20.2% (241/1,196) | |
| Current smoker | 31.8% (447/1,406) | |
| Renal insufficiency* | 11.2% (136/1,216) | |
| On dialysis | 2.7% (38/1,396) | |
| Previous peripheral revascularisation | 52.4% (737/1,406) | |
| Rutherford clinicalcategory | 1¶ | 0.1% (1/1,403) |
| 2 | 31.1% (436/1,403) | |
| 3 | 57.7% (810/1,403) | |
| 4 | 8.6% (120/1,403) | |
| 5¶ | 2.6% (36/1,403) | |
| ABI (mmHg ratio) | 0.7±0.2 | |
| Lesion characteristics | ||
| Lesion length (cm) | 12.1±9.5 | |
| Diameter stenosis (%) | 88.8±12.3 | |
| Occluded | 35.5% (630/1,774) | |
| Lesion type | De novo | 74.3% (1,318/1,774) |
| Restenotic (non-stented) | 7.7% (136/1,774) | |
| In-stent restenosis | 18.0% (320/1,774) | |
| Reference vessel diameter (mm) | 5.2±0.7 | |
| Calcification | 68.7% (1,217/1,772) | |
| Severe | 10.2% (181/1,772) | |
| Lesion location‡ | Superficial femoral artery | 87.6% (1,554/1,774) |
| Proximal popliteal artery | 27.3% (484/1,774) | |
| Procedural characteristics | ||
| Predilatation | 78.0% (1,097/1,406) | |
| Post-dilatation | 35.1% (491/1,397) | |
| Provisional stenting | 21.2% (373/1,762) | |
| Dissections | 0 | 56.7% (1,006/1,773) |
| A-C | 35.4% (628/1,773) | |
| D-F | 7.8% (139/1,773) | |
| Data are % (n/N) or mean±standard deviation. *Baseline serum creatinine ≥1.5 mg/dl. ¶Protocol deviations, patients enrolled and included in analyses. ‡Multiple lesion locations are reported in a single target limb, the total lesion locations could be more than the total number of target limbs. ABI: ankle-brachial index; BMI: body mass index | ||
Effectiveness and safety outcomes
The KM estimate of freedom from CD-TLR up to 5 years was 69.4% (95% CI: 66.7%-72.0%) (Central Illustration, Figure 2A). The RMST to first CD-TLR up to 5 years was 1,470.1 days (95% CI: 1,439.2-1,501.0 days).
The cumulative incidence of MAEs based on the KM estimate up to 5 years was 45.9% (95% CI: 43.2%-48.7%) including the following: arterial thrombosis 5.7% (95% CI: 4.6%-7.1%), major target limb amputation 1.7% (95% CI: 1.1%-2.6%), CD-TVR 31.9% (95% CI: 29.3%-34.6%) and all-cause death 19.5% (95% CI: 17.4%-21.8%).
In total, 19 participants underwent major target limb amputation. The mean time to amputation was 27.5±19.1 months. Details on the 12 amputations occurring within 3 years post-procedure have been previously reported13. Seven amputations occurred >3 years post-procedure; of these, all had 2 or more risk factors or concomitant atherosclerotic disease manifestations including diabetes mellitus (n=5), hypertension (n=5), hyperlipidaemia (n=6), coronary artery disease (n=4), renal insufficiency (n=2), currently smoking (n=2) and below-the-knee disease on the target limb (n=4). Four were classified as RCC 3 at baseline, 1 was RCC 4, and 2 were RCC 5. Five had a CD-TLR prior to amputation, and 5 died within the duration of the study, ranging from 8 days to 386 days post-amputation.
There were 253 deaths reported within the 5-year follow-up window (1,885 days). Deaths occurring within 3 years of the index procedure and the causes of those deaths have been previously reported13. Ninety-seven deaths were reported between 3 years and 5 years (1,800 days) post-procedure, and 9 deaths occurred after the cut-off of 1,800 days, but within the end of the 5-year follow-up window of 1,885 days. Per CEC adjudication, none of the deaths occurring within the 3-5 year window were procedure-related. One death was classified as device-related; the cause of death was right limb infection and sepsis, and the patient died 1,516 days post-procedure. CEC adjudicated outcomes are shown in Table 2.
After the vital status update, 96.4% (1,355/1,406) of the participants had vital status information, which resulted in 78.9% (95% CI: 76.7%-81.0%) freedom from all-cause mortality per the KM estimate (Figure 2B). Deaths found through the vital status data collection were not adjudicated by the CEC due to lack of adequate source documentation availability.
A summary of safety and effectiveness outcomes for the IN.PACT Global Study up to 5 years is reported in Table 3.
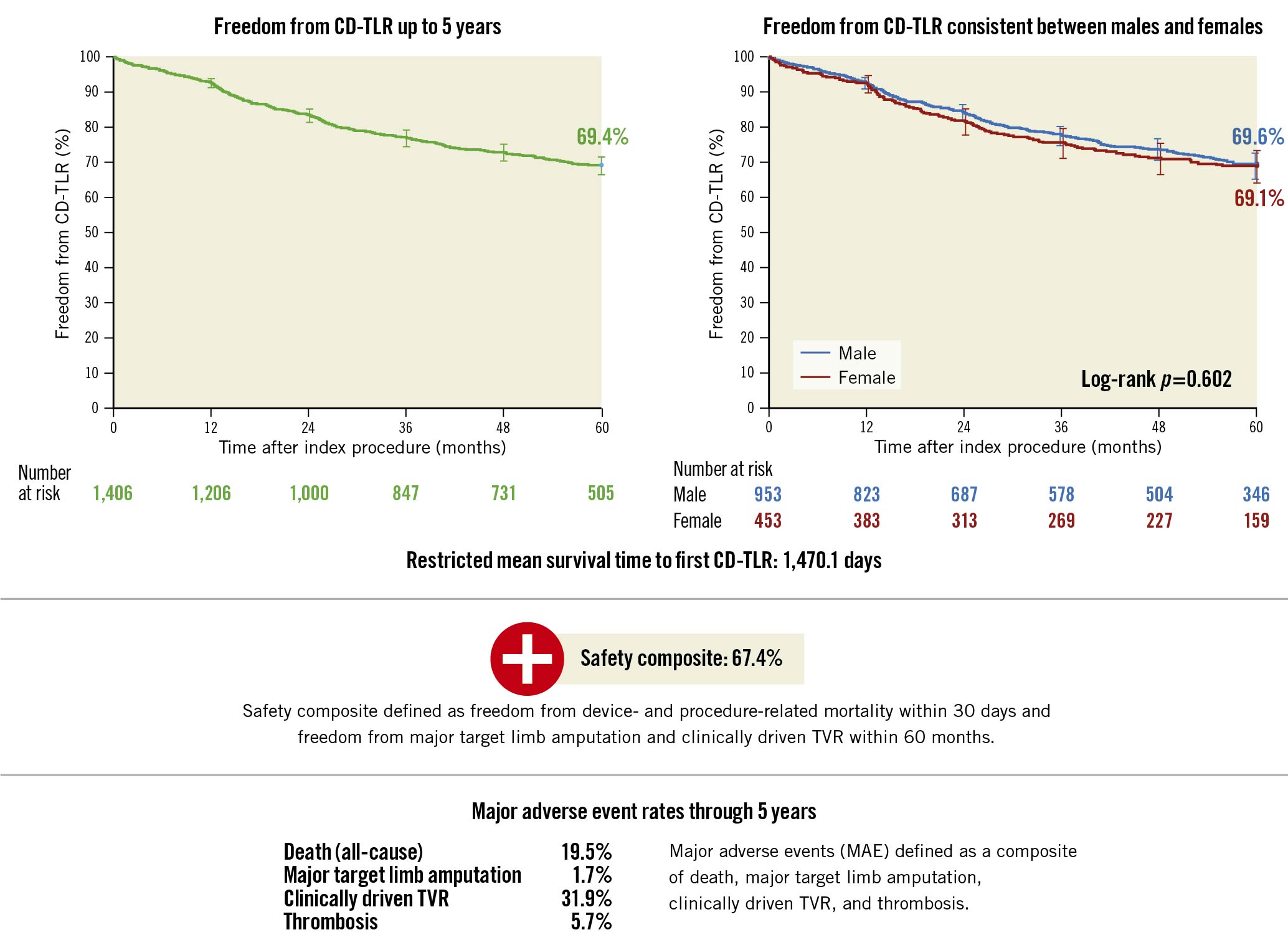
Central Illustration. Key outcomes at 5 years from the IN.PACT Global Study.
CD-TLR: clinically driven target lesion revascularisation; TVR: target vessel revascularisation
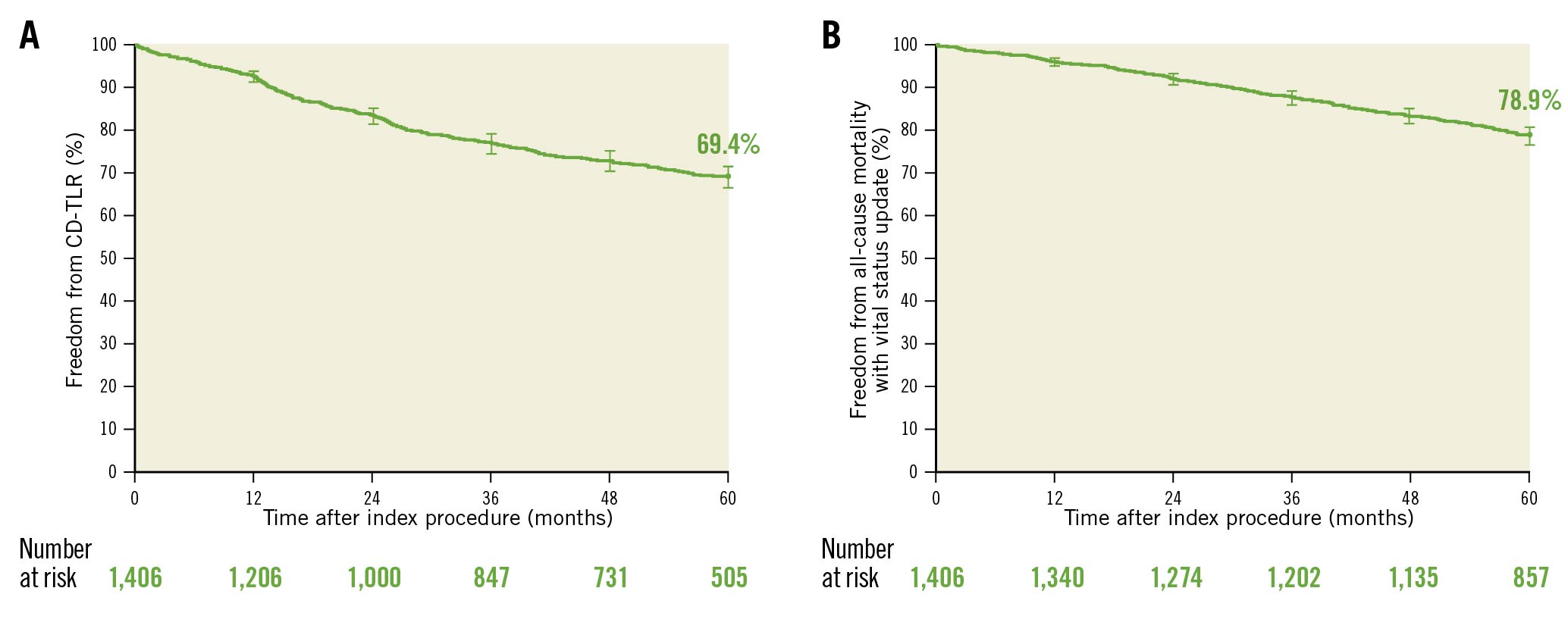
Figure 2. Freedom from CD-TLR and all-cause mortality: Kaplan-Meier curve up to 5 years. Bars represent 95% CI. A) Freedom from CD-TLR. B) All-cause mortality that includes additional vital status follow-up data. CD-TLR: clinically driven target lesion revascularisation; CI: confidence interval
Table 2. CEC adjudicated outcomes through 5 years*¶.
| Outcome | Rate |
|---|---|
| CD-TLR | 30.6% (366) |
| Any TLR | 31.3% (374) |
| Safety composite‡ | 67.4% (392) |
| Major adverse event composite | 45.9% (589) |
| Death (all-cause) | 19.5% (244) |
| CD-TVR | 31.9% (381) |
| Major target limb amputation | 1.7% (19) |
| Thrombosis | 5.7% (73) |
| *Up to 1,800 days post-procedure. ¶Per Kaplan-Meier estimate (number of patients with an event). ‡Defined as freedom from device- and procedure- related death through 30 days, major target limb amputation and CD-TVR within 5 years, per KM analysis. CD-TLR: clinically driven target lesion revascularisation; CD-TVR: clinically driven target vessel revascularisation; CEC: clinical events committee; TLR: target lesion revascularisation | |
Table 3. Summary of annual outcomes through 5 years.
| Key outcomes | |||||
|---|---|---|---|---|---|
| Kaplan-Meier estimates | 1 year | 2 years | 3 years | 4 years | 5 years |
| Freedom from all-cause death with vital status*¶ | 96.1% | 92.2% | 87.8% | 83.5% | 78.9% |
| Freedom from CD-TLR*‡ | 92.8% | 83.5% | 77.1% | 73.0% | 69.4% |
| Proportion rates, % (n/N)§ | 1 year [14] | 2 years [12]‡ | 3 years [13] | 4 years | 5 years |
| CD-TLR | 7.5% (98/1,311) | 16.8% (214/1,276) | 22.9% (289/1,262) | 26.1% (330/1,266) | 30.1% (366/1,215) |
| Major adverse events | 12.0% (157/1,311) | 25.5% (325/1,276) | 34.8% (439/1,262) | 40.7% (515/1,266) | 48.5% (589/1,215) |
| All-cause death | 3.5% (46/1,311) | 7.9% (101/1,276) | 11.6% (147/1,262) | 15.5% (196/1,266) | 20.1% (244/1,215) |
| CD-TVR | 8.1% (106/1,311) | 17.6% (224/1,276) | 23.7% (299/1,262) | 26.9% (341/1,266) | 31.4% (381/1,215) |
| Major target limb amputation | 0.2% (3/1,311) | 0.7% (9/1,276) | 1.0% (12/1,262) | 1.1% (14/1,266) | 1.6% (19/1,215) |
| Thrombosis | 2.9% (38/1,311) | 4.5% (57/1,276) | 5.6% (71/1,262) | 5.6% (71/1,266) | 6.0% (73/1,215) |
| *Kaplan-Meier estimates based on final locked database. ¶Vital status not CEC adjudicated. ‡Results updated since the original publication. §Data derived from different snapshots from the database; site monitoring and collection of additional data between follow-up timepoints impacted the sample size at each time point. CD-TLR: clinically driven target lesion revascularisation; CD-TVR: clinically driven target vessel revascularisation | |||||
Multivariable analyses
Exploratory multivariable Cox proportional hazards analyses (MVA) were employed to identify the predictors of CD-TLR, MAE, and all-cause mortality. Statistically significant key factors are listed below. All variables selected for the final model are shown in Supplementary Figure 1-Supplementary Figure 3. Univariate analysis outcomes are included in Supplementary Table 2-Supplementary Table 4. Variables associated with an increased risk of CD-TLR included ISR, moderate-to-severe target lesion calcification, longer lesion length, and higher post-procedure residual diameter restenosis. A larger reference vessel diameter (RVD) and having a target lesion confined to the SFA (vs extending into the popliteal) were associated with a reduced risk of CD-TLR. Variables associated with an increased risk of MAE included RCC ≥4, carotid artery disease, and coronary artery disease. Having a lesion confined to the SFA (vs extending into the popliteal) or a higher EQ-5D (EuroQol 5 dimensions) index score at baseline were associated with a reduced risk of MAE. Variables associated with an increased risk of all-cause mortality (with vital status update) included having previous target limb amputation, diabetes and older age.
Gender analysis
Prespecified analyses were performed to compare outcomes in males and females for CD-TLR and MAEs. Freedom from CD-TLR per KM estimate (Figure 3A) up to 5 years was not different between males and females (log-rank p=0.602). The RMST to CD-TLR up to 5 years was 1,480.9 (95% CI: 1,444.1-1,517.7) days in males and 1,446.9 (95% CI: 1,390.3-1,503.6) days in females (p=0.325).
Freedom from MAE per the KM estimate (Figure 3B) was not different between males and females (log-rank p=0.601). Females experienced a significantly greater number of thrombotic events (cumulative incidence based on KM of 7.5% vs 4.9%; log-rank p=0.045); other components of the endpoint were numerically similar and statistically non-significant.
In univariate analyses, gender was not significantly associated with CD-TLR (Supplementary Table 2) or MAE (Supplementary Table 3). A non-significant trend toward lower all-cause mortality including vital status data was observed in male patients versus female patients (HR 0.81, 95% CI: 0.63-1.03; p=0.08) (Supplementary Table 4) in the univariate analysis. However, gender was not selected in the final MVA model for all-cause mortality including vital status data. Exploratory MVAs show factors in the subgroup of males and females associated with risk of CD-TLR (Supplementary Figure 4) and MAEs (Supplementary Figure 5).
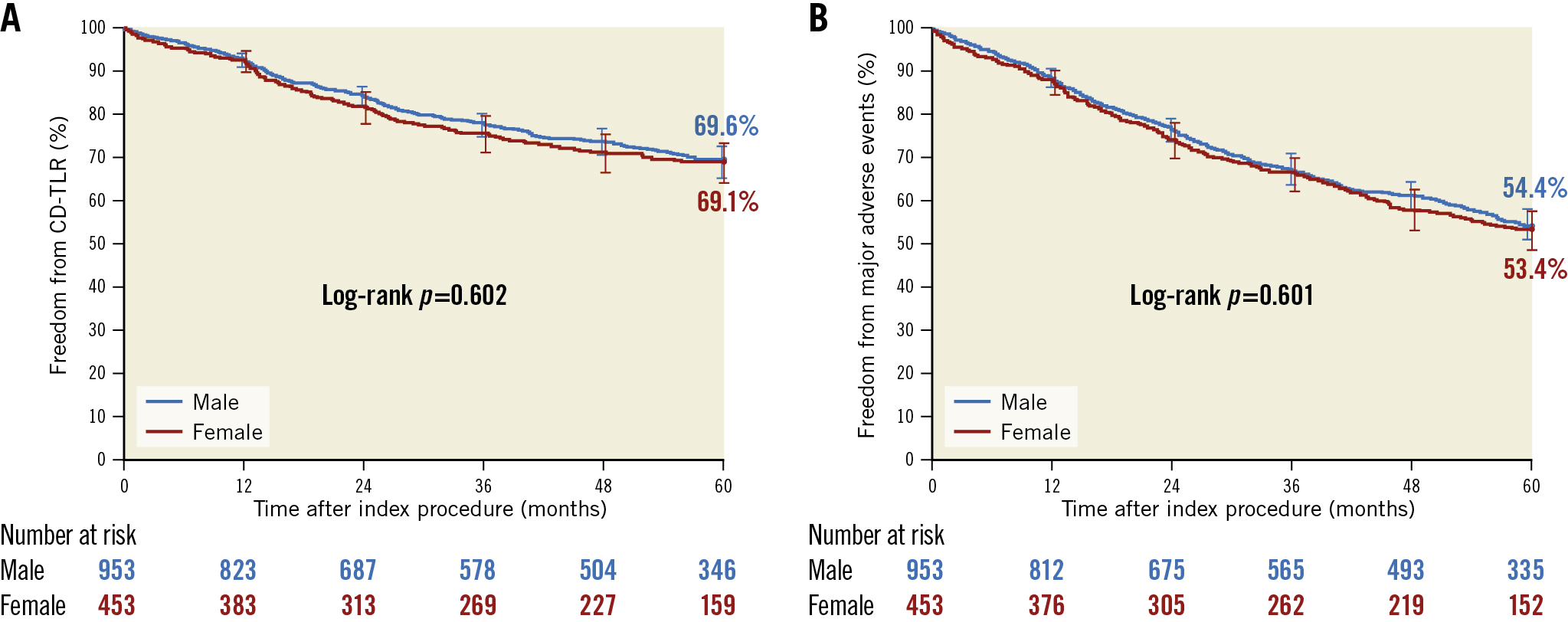
Figure 3. Subgroup analysis of freedom from CD-TLR and MAE up to 5 years. A) Freedom from CD-TLR in males and females not numerically or statistically different (log-rank p=0.602); B) Freedom from an MAE was not numerically or statistically different in males and females, (log-rank p=0.601). CD-TLR: clinically driven target lesion revascularisation
Discussion
The research and development of DCBs has been progressing steadily for the last 2 decades. Recently, DCBs achieved the highest level of recommendation (Class 1) per the Society for Cardiovascular Angiography and Interventions guidelines15. The data presented here add valuable evidence on the use of DCBs in a more complex patient population with 5-year follow-up and rigorous study conduct. The IN.PACT Global Study is the largest independently adjudicated real-world study of endovascular interventions to treat PAD to date. The uniquely broad selection criteria resulted in the inclusion of complex lesions and patients with known risk factors that contribute to disease progression. Additionally, follow-up up to 5 years is uncommon in this space, and high follow-up compliance was achieved through vigilant study management and oversight by each participating centre.
The 5-year freedom from CD-TLR rate observed in this study was 69.4%, which is lower than the 74.5% rate observed in the DCB arm and higher than the 65.3% rate observed in the PTA arm of the IN.PACT SFA randomised trial816. However, the randomised trial had tighter selection criteria and enrolled fewer complex lesions. As compared to the IN.PACT Global Study, the DCB arm of IN.PACT SFA had a shorter mean lesion length (8.9 cm vs 12.1 cm), fewer total occlusions (25.8% vs 35.5%), less calcification (59.3% vs 68.7%), and no ISR lesions as they were excluded, whereas 18.0% of lesions in IN.PACT Global Study were ISR16. The randomised THUNDER trial reported a 5-year cumulative TLR rate of 20.8% in the DCB cohort versus 55.6% in the PTA cohort, again in a patient cohort characterised by shorter lesion lengths (7.5 cm), fewer total occlusions (27%), and less calcification (50%) than observed in the IN.PACT Global Study1117.
As observed by an exploratory MVA in this study and others, ISR, calcification, a smaller RVD, and a longer lesion length are consistently associated with an increased risk for CD-TLR. The high prevalence of these challenging characteristics allowed for a critical assessment of this DCB’s efficacy beyond the initial tightly controlled randomised trials.
The use of adjunctive atherectomy or vessel preparation devices was not permitted in this study, in order to limit bias. However, vessel preparation may have further improved outcomes in lesions with a heavier plaque burden or with more calcification. It was hypothesised that the removal of plaque may increase the homogeneity of the drug application onto the vessel wall and increase diffusion into the vessel wall layers. The DEFINITIVE AR pilot study18, which also enrolled more complex lesions, tested this theory. A post hoc analysis showed a numerically higher 1-year patency rate observed in lesions where ≤30% residual stenosis was obtained following directional atherectomy prior to DCB treatment, as compared to lesions that had more than 30% stenosis remaining in the vessel before the DCB was delivered (84.2% vs 77.8%). Only 1 patient (5.3%) enrolled in the severe calcification arm required a stent, and no stents were needed in the randomised directional atherectomy plus DCB arm. The provisional stenting rate per lesion in the IN.PACT Global Study was 21.2%, suggesting directional atherectomy should be considered when stent avoidance is a priority18.
The efficacy of DCBs in women has not been consistently demonstrated, and very little long-term outcomes data have been reported. In the LEVANT 2 trial, women had lower 1-year patency rates in the DCB arm versus the control arm (56.4% DCB vs 61.4% PTA), but superior patency was observed in men treated with a DCB over PTA (70.6% DCB vs 48.4% PTA)19. In the DCB arm of the THUNDER trial, women had a higher rate of TLR at 5 years (38% vs 17% in men), but women treated with DCB had a lower cumulative rate of TLR as compared to women treated with PTA (38% vs 52%)11.
A recently published post hoc analysis of freedom from CD-TLR in the IN.PACT SFA trial showed women treated with DCBs had better outcomes than women in the PTA arm up to 5 years (67.4% for DCB, 52.9% for PTA; p=0.049)8. The gender subgroup analysis conducted in the IN.PACT Global Study showed no difference in freedom from CD-TLR between males and females (69.6% in men and 69.1% in women; log-rank p-value=0.602). This is consistent with the post hoc analysis on sex differences conducted in the IN.PACT SFA randomised trial up to 3 years20. Kohi et al reported freedom from CD-TLR in participants treated with the IN.PACT Admiral was 81.1% in women and 86.4% in men (p=0.285) at 3 years. The predictors in the MVAs were different in the subgroup analyses of males and females. Significant predictors of CD-TLR up to 3 years for women treated with DCBs were severe calcification, previous ipsilateral revascularisation, absence of hyperlipidaemia, and insulin-dependent diabetes. For men, predictors of increased CD-TLR consisted of a smaller RVD and a higher BMI20. In the current study, anticoagulant use at discharge was associated with an increased risk of CD-TLR and MAE in male patients. This association is not likely causal and may be related to underlying comorbidities.
The all-cause mortality rate observed in this study was 20.1%. A review paper that calculated 5-year mortality rates in the same manner reported similar rates in the more restrictively enrolled DCB arms of RCTs, including 15.8% in IN.PACT SFA, 20.3% in LEVANT 2, and 28.1% in the drug-eluting stent arm of the Zilver PTX trial21. Causes of death up to 3 years have been previously reported for IN.PACT Global Study but were not captured beyond 3 years13. Of note, as compared to the IN.PACT SFA randomised trial8, the IN.PACT Global Study enrolled patients with more comorbidities and more complex baseline lesion characteristics. Given the broader selection criteria and inclusion of critical limb ischaemia patients in the IN.PACT Global Study, putting the data into context with epidemiological studies is warranted. As expected, this population is associated with significant mortality, and individual trial rates will vary greatly depending on comorbidities and severity of PAD. Mueller et al reported the 5-year mortality rates of PAD patients ≥75 years old: 52% in those with diabetes and 38% in those without diabetes22. Heikkila et al reported a wide range of 5-year mortality rates when stratified by the type of intervention (open or endovascular) and severity level of PAD. The lowest rate was 24.7% in patients with claudication treated endovascularly and was highest, at 55.2%, in patients with severe limb ischaemia including tissue loss23.
Multivariable predictors of all-cause mortality up to 5 years observed in the IN.PACT Global Study included hyperlipidaemia as having a protective effect, but anticoagulation use was not significant. These results are counterintuitive and may be due to underlying comorbidities of patients prescribed anticoagulation medications. Likewise, having a hyperlipidaemia diagnosis would result in statin use, which has been shown to reduce the risk of mortality24.
Limitations
This was a single-arm study that did not include a control group. The results cannot be directly compared to other study or treatment outcomes. Assessments at years 4 and 5 were limited to CD-TLR, MAEs and mortality and did not include functional outcomes or medication compliance. While this study cohort represents a real-world patient population, the study follow-up requirements may have resulted in a higher medication compliance than might be seen in everyday clinical practice. Finally, the impact of provisional stenting on outcomes requires additional analyses that will be addressed in a future publication.
Conclusion
These long-term results from this large, international, real-world study add to the growing body of evidence that demonstrates the safety, effectiveness, consistency, and durability of the IN.PACT Admiral DCB for the treatment of femoropopliteal lesions.
Impact on daily practice
This study provides the first 5-year data on the use of the IN.PACT Admiral drug-coated balloon in real-world participants with complex femoropopliteal disease. Per independent CEC adjudication, the Kaplan-Meier estimate of freedom from CD-TLR up to 5 years was 69.4%. The data observed here validate the previously conducted RCT outcomes in a broader patient population. In-stent restenosis and severe calcification were identified as strong predictors of CD-TLR within 5 years; in these patients, vessel preparation may improve outcomes.
Acknowledgements
The authors would like to thank the following Medtronic employees: Stefanie Deckers and Eric Fernandez, MD for research support; Patrick Wang, MD, MPH and Kathleen Cahill, MS for technical review of the manuscript; and Meghan Schadow, MS for assistance in its preparation. Thank you to all the past and present principal investigators, subinvestigators and research staff for your ongoing dedication to this project for the last decade.
Funding
This study was sponsored by Medtronic. The authors received no specific funding for this work or preparation of the manuscript.
Conflict of interest statement
T. Zeller has received honoraria/consulting fees from Abbott Vascular, BIBA Medical, Shockwave, Biotronik, Cook Medical, Eformoral, Philips-Spectranetics, CSI, Intact Vascular, and Bayer; received grants or contracts from Bard, Biotronik, Veryan, Cook, Gore & Associates, Medtronic, Philips, Terumo, Trireme, Shockwave, MedAlliance, B. Braun, Intact Vascular, Boston Scientific, University of Jena, Pluristem, PQ Bypass, Reflow Medical, Ablative Solutions, and Surmodics; participated on a Data Safety Monitoring Board or Advisory Board for Medtronic, Boston Scientific, Gore, Veryan, Vesper Medical, and VentureMed. He also holds stock/stock options in QT Medical. M. Brodmann has received speaker honoraria from Bard Peripheral Vascular, Biotronik, Medtronic Spectranetics, and VIVA Physicians; and is a consultant for Bard Peripheral Vascular, Biotronik, Medtronic, and Spectranetics. G. Ansel received consulting fees from Medtronic, Boston Scientific, Veryan Medical, and Cook Medical; received royalties from Surmodics; honoraria from Cook Medical, and Medtronic; participated on a Data Safety Monitoring Board or Advisory Board for Syntactx, and Hart Clinical Consultants; and has stock/stock options in Reflow Medical. D. Scheinert has received consulting fees and/or speaker honoraria from Abbott, Acotec, Alvimedica, Bayer, Boston Scientific, Cook Medical, Cardionovum, CR Baird, IVascular, Gardia Medical/Allium, Medtronic, Philips, and Upstream Peripheral Technologies; and received payment for participation on a Data Safety Monitory Board or Advisory Board for Boston Scientific. G. Tepe has received study support from BARD, BSC, Gore, Medtronic, CSI, Philips, and Shockwave; grants or contracts from B Braun, Biotronic, Bayer, Verian, Gore, Shockwave and Philips; is on the Global Advisory Board of Medtronic, BSC, B Braun, and Philips; received payment or honoraria for presentations from BSC, Biotronic, B Braun, Verian, Gore and Shockwave; and has a leadership or fiduciary role in CX London. J. Menk is an employee of Medtronic and holds stock. A. Micari is on the advisory board for Medtronic; is a consultant for Boston Scientific, and Terumo; has received support for attending meetings from Medtronic, Boston and Terumo. The other author has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

