
Abstract
Aims: The aim of this study was to investigate the use of a drug-coated balloon (DCB) in daily clinical practice and provide further evidence on the safety and efficacy of paclitaxel-coated balloon treatment using urea as an inert excipient.
Methods and results: Between December 2013 and December 2015, 757 patients treated for coronary lesions with the IN.PACT Falcon balloon were enrolled in this prospective real-world all-comers registry. The primary outcome was the clinically driven target lesion revascularisation (TLR) rate at 12 months. The secondary outcome was major adverse cardiac events (MACE) defined as cardiac death, myocardial infarction, TLR and target vessel revascularisation (TVR). Out of 805 lesions, 43.1% were de novo, and 53.2% drug-eluting stent (DES) or bare metal stent (BMS) in-stent restenosis (ISR). TLR at 12 months was 6.2% and TVR 8.3%. MACE occurred in 9.7% of patients with a composite of cardiac death in 0.8% and myocardial infarction in 2.7% plus TLR/TVR. Subgroup analysis confirmed a TLR rate of 7.5% for ISR (2.1% BMS and 9.5% DES) and 4.9% for de novo lesions.
Conclusions: The IN.PACT Falcon urea-based paclitaxel-coated balloon is safe and efficient in de novo and ISR lesions with low rates of TLR/TVR. The high proportion of treatment of de novo lesions indicates that a DCB-only strategy is nowadays common.
Introduction
Percutaneous coronary interventional revascularisation has improved markedly since the first balloon angioplasty revolutionised coronary revascularisation1. Elastic recoil with abrupt vessel closure, subintimal dissection, recoil and restenosis caused by cellular proliferation are major drawbacks of plain angioplasty. The use of intracoronary stents solved the problem of dissection and recoil, though the clinical outcome with bare metal stents (BMS) was limited by neointimal hyperplasia, leading to in-stent restenosis (ISR). The development of drug-eluting stents (DES) significantly attenuated the need for repeat revascularisation, although complex coronary territories such as bifurcations, small vessels, long lesions, saphenous vein grafts and diabetic disease have worse outcomes with DES than simpler lesions. Even with modern DES, late stent thrombosis and continued risk of restenosis remain a severe problem in selected patients2. Bioresorbable vascular scaffolds were designed to provide transient support against dissection and acute recoil with the capability of preventing neointimal proliferation by eluting immunosuppressive drugs. However, there are still major problems to overcome with this new technology, especially in the treatment of smaller vessels, and late scaffold thrombosis is of concern3. Drug-coated balloons (DCB) represent a potential alternative therapeutic option for ISR treatment and de novo stenosis, especially in small vessels or side branches in complex bifurcation stenoses, without implanting a permanent foreign object into the vessel lumen. The mitotic inhibitor paclitaxel was identified as the primary drug for DCB due to its rapid uptake and prolonged retention. According to European clinical practice guidelines, DCB therapy is recommended for the treatment of ISR4. This recommendation is based on preclinical and randomised clinical trials which demonstrated the safety and efficacy of DCB technology for patients with ISR with iopromide excipient-based paclitaxel-coated balloons5,6,7,8,9,10. However, no recommendation was given for the use of DCB in broader indications, e.g., in de novo lesions in small vessels, side branches in bifurcation stenosis and the increasing use for complex coronary territories or when stent implantation is not an option, as only limited data are available.
While DCB therapy with the urea-based paclitaxel-coated balloon IN.PACT™ Falcon (Medtronic, Minneapolis, MN, USA) for small vessels was superior in terms of late lumen loss and MACE as compared to treatment with the TAXUS™ DES (Boston Scientific, Marlborough, MA, USA)11, data for real-life situations in which the urea-based paclitaxel-coated balloon IN.PACT Falcon is used are only limited12. We therefore conducted the FALCON all-comers DCB Registry to provide further evidence for the safety and efficacy of paclitaxel-coated balloon treatment using urea as an inert excipient and to gain insight into the use of DCB therapy in daily clinical practice in a real-world setting.
Methods
The FALCON Registry was a European-wide, prospective multicentre, observational clinical registry to assess the safety and efficacy of the urea-based paclitaxel-coated balloon IN.PACT Falcon in a real-world setting. Further, the investigators intended to gain more insight into the indications for which DCB is commonly used nowadays. The study procedures were performed in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. The local ethics committee of the Hannover Medical School (as site of the principal investigators), as well as independent ethics committees at each site as per local regulations, approved the study protocol and the patient data release form. Key inclusion criteria were written informed consent, de novo lesions, restenosis or ISR in coronaries with a lumen >2.0 and <4.0 mm. As this was a real-world all-comers registry, sites were encouraged to include all patients treated with an IN.PACT Falcon DCB for coronary lesions in their centre during the recruiting period. Key exclusion criteria were patient incompliance, indication for bypass surgery, coronary lesions not treatable by PCI, cardiogenic shock, life expectancy <1 year.
The primary study endpoint was clinically driven target lesion revascularisation (TLR) at 12 months. Key secondary endpoints were major adverse cardiovascular events (MACE), a composite of cardiac death, myocardial infarction and clinically driven TLR or target vessel revascularisation (TVR) at 12 months. Details are shown in Supplementary Appendix 1.
STATISTICAL ANALYSIS
Continuous variables are presented as mean±SD. Discrete variables are expressed as counts and percent. Baseline differences between groups were analysed for significance using the Mann-Whitney U test for continuous data and the chi-squared test (or Fisher’s exact test where the expected cell value was less than 5) for categorical variables. SPSS statistical software, Version 24 (IBM Corp., Armonk, NY, USA) was used for all statistical calculations.
Results
Between December 2013 and December 2015, 757 patients with a total of 805 lesions treated for all indicated coronary lesions with the IN.PACT Falcon balloon were enrolled at 26 sites in eight countries in this prospective real-world all-comers registry (Supplementary Table 1). Patients had an overall mean age of 66.2±11.2 years and were predominantly male (78.9%). Five hundred and thirty-five (535) patients (70.5%) had hypercholesterolaemia and 567 (74.9%) hypertension; diabetes mellitus was present in 260 (34.3%) and 394 (52.0%) had a history of smoking. Three hundred and ten (310) patients (40.9%) presented with an acute coronary syndrome, of whom 30 had an ST-elevation myocardial infarction, 159 non-ST-elevation myocardial infarction and 121 unstable angina. A high proportion of patients (326; 43.1%) was treated for de novo lesions by a DCB-only strategy, 405 (53.5%) presented with ISR, 102 (13.5%) with BMS-ISR and 223 (29.5%) with DES-ISR. In 81 patients the stent type treated for ISR was unknown. Twenty-two (22) subjects could not be assigned to one of the lesion groups as either numerous different lesion characteristics were detected or the information was unknown (Table 1).
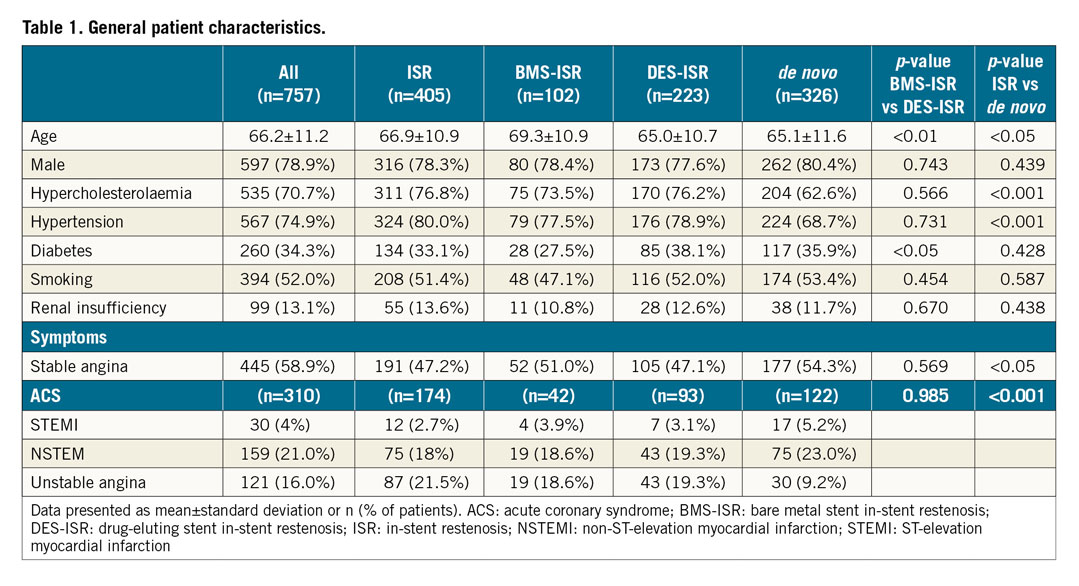
Most lesions presented in the left anterior descending, followed by the left circumflex and right coronary artery. A small number of lesions in bypass grafts were treated, and 16.5% bifurcation stenoses were present. Bifurcation stenoses were significantly more often found in the de novo lesion group than in the ISR group. Mean lesion length was 17.9±10.7 mm and reference vessel diameter 2.8±0.5 mm, which was significantly lower in de novo lesions (2.5±0.5 mm); 24.9% of lesions were >2.75 mm, while 75.1% were <2.75 mm (Figure 1). Forty-eight (48) patients (6%) were treated for multiple lesions (Supplementary Table 2).
Figure 1. Indications for drug-coated balloon treatment. In 3.6% indications could not be assigned to one of the groups. BMS: bare metal stent; DES: drug-eluting stent; ISR: in-stent restenosis
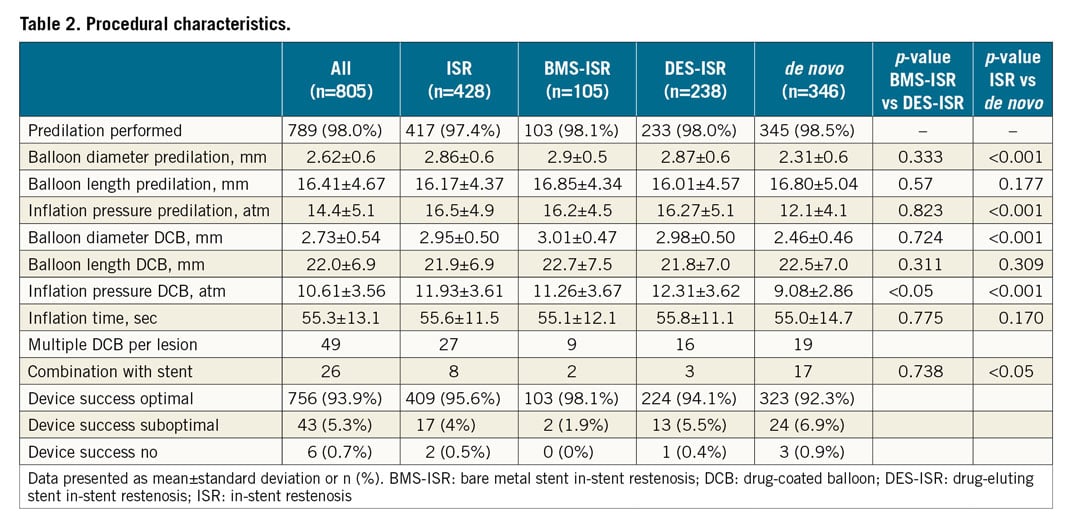
Predilation was performed in nearly all lesions (98%) with a mean inflation pressure of 14.4±5.1 atm. Mean DCB balloon diameter used was 2.73±0.54 mm with a length of 22.0±6.9 mm. Mean inflation pressure was 10.61±3.56 atm. Predilation balloon diameter, inflation pressure and DCB diameter were significantly lower in de novo lesions compared to ISR. Procedural device success was high (optimal result 93.9%, suboptimal 5.3%), and in only six lesions (0.7%) was the DCB treatment classified as not successful. In 26 lesions an additional stent implantation was performed, which was significantly more often carried out in the treatment of de novo lesions (Table 2).
Follow-up data were available for 745 (98.9%) of the 757 patients at 12 months. Overall clinical TLR was 6.2% in all patients, with 9.5% in patients with DES-ISR, while, as expected, it was lower at 2.1% in BMS-ISR. In de novo lesions TLR was 4.9%. Overall MACE was observed in 9.7% of all patients, while MACE was higher in DES-ISR (12.3%) vs BMS-ISR (8.2%) as well as in de novo lesions (8.0%). Cardiac death at 0.3% and myocardial infarction at 0.6% were low in patients with de novo lesions, and the MACE rate was primarily driven by TLR and TVR (7.4%) (Figure 2).
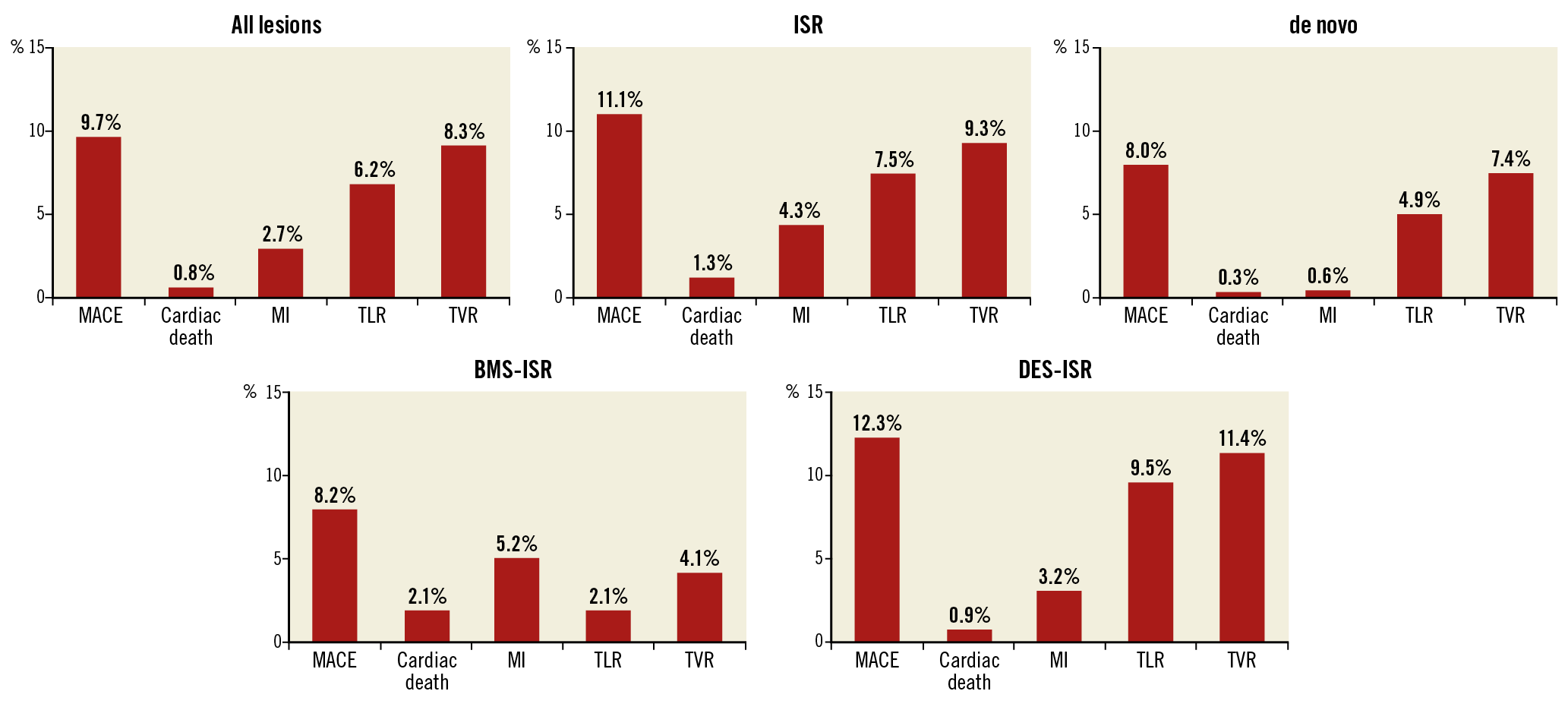
Figure 2. 12-month clinical outcome. Data are shown as % of patients. BMS: bare metal stent; DES: drug-eluting stent; ISR: in-stent restenosis; MACE: major adverse cardiac events; MI: myocardial infarction; TLR: target lesion revascularisation; TVR: target vessel revascularisation
Discussion
The FALCON Registry reveals several important findings. 1) The IN.PACT Falcon paclitaxel-coated balloon with urea as inert excipient is safe and efficient in a real-world setting. 2) Treatment of ISR with the IN.PACT Falcon shows good clinical results, with TLR/TVR and MACE rates comparable with the outcomes of iopromide-, butyryl trihexyl citrate- or other excipient-based paclitaxel-coated balloon trials. 3) The high rate of successful treatment of de novo lesions provides further evidence that the concept of “DCB-only therapy” for de novo lesions is nowadays commonly used and provides good results with the urea-based paclitaxel-coated balloon IN.PACT Falcon.
DCB are coated with a layer of antirestenotic drug mixed with an excipient. The mitotic inhibitor paclitaxel was identified as the primary drug for DCB due to its rapid uptake and prolonged retention in the vessel wall; therefore, most commercially available DCB are coated with paclitaxel. The excipient is needed to facilitate the uptake of the active drug to the vessel wall13. Various excipients are used with the contrast agent iopromide, butyryl trihexyl citrate, the film-forming agent shellac or urea. However, evidence exists from preclinical testing that tissue levels of drug following DCB treatment are different among various excipients14,15,16. Therefore, it cannot be presumed that there is a class effect for clinical performance of DCB. The IN.PACT Falcon DCB uses the natural product urea as inert excipient. Urea, due to its hydrophilic nature, dissolves rapidly once hydrated by the blood and water in tissue, facilitating the release of the paclitaxel from the balloon’s surface into the vessel wall. The present FALCON Registry proves that the IN.PACT Falcon DCB using urea as excipient provides good clinical 12-month efficacy results with an overall clinical TLR rate at 12 months of 6.2% and is safe with a MACE rate of 9.7% in a real-world setting. Compared to previous registries with other paclitaxel-coated DCB, the FALCON Registry had a different distribution of treated lesion types. While in the SeQuent Please World Wide Registry and the DELUX registry more BMS vs DES-ISR were present17,18, the ratio of BMS vs DES-ISR shifted to less BMS-ISR (~1/3) and more DES-ISR (~2/3). Furthermore, a higher percentage of patients was treated with a DCB-only strategy for de novo lesions in the FALCON Registry. The change of ratio of BMS vs DES-ISR is not surprising given that in recent years more DES have been implanted and, according to the European clinical practice guidelines in 2014, no indication for the use of BMS remains4. The first clinical application of DCB was for treatment of BMS-ISR by Scheller et al in the PACCOCATH-ISR trial, demonstrating superiority over plain balloon angioplasty. Other randomised clinical trials and registries comparing DCB vs PES or DCB vs DES followed, confirming the results5,7,18,19. The success rate in treatment of ISR might however be dependent on the excipient used, as indicated by data from an optical coherence tomography study which suggested that urea was more efficient than shellac, the coating of the Dior DCB (Eurocor GmbH, Bonn, Germany)20. The very low TLR rate at 2.1% in patients treated for BMS-ISR in the present FALCON Registry confirms these initial results seen with the Falcon DCB11,12. Treatment of DES-ISR is more challenging, as is known from previous trials17,18,21,22,23. The FALCON Registry with a urea-based paclitaxel-coated balloon shows comparable good results with a TLR rate of 9.5% and a MACE rate of 12.3% (Supplementary Table 3).
Compared with other published all-comers DCB registries, the FALCON Registry has the highest percentage (43.1% of all patients) treated for de novo lesions with a DCB-only strategy. Furthermore, over 50% of these lesions were complex (type B2 26.5%, type C 25.3%). However, the procedural success rate with this strategy was good (92.3% optimal result, 6.9% suboptimal and only 0.9% device failure) and safe (MACE 8.0%), with good clinical success after 12 months with a TLR rate of 4.9%. Previous data for treatment of small de novo lesions with the IN.PACT Falcon balloon in the randomised BELLO trial showed a low TLR rate of 4.4% and a MACE rate of 10% at six months, though bail-out stenting was carried out in 20%11. A significant difference in MACE was reported in favour of DCB therapy in the BELLO trial also after three years24. However, the PICCOLETO study with the Dior I paclitaxel-coated balloon showed a higher incidence of MACE in patients who underwent DCB therapy as compared to those who received a first-generation paclitaxel-DES. This divergent result might be due to the lack of excipient used by the first-generation Dior balloon25. In the PEPCAD I trial, the procedural success rate with the iopromide-based paclitaxel-coated SeQuent® (B. Braun, Melsungen, Germany) was also high in de novo lesions; however, 27% received additional stenting26. Numerous observational studies have investigated the DCB-only strategy, mostly for small vessel disease. The rate of additional stenting ranges from 3% to 36%, with evidence that bail-out stenting should be avoided as it seems to have worse outcome27,28. In the FALCON Registry, only in 17 cases (4.8%) was a combination with a stent implantation felt to be necessary. This is probably explained by the experience of the centres and the knowledge gained and recommendations for treatment of de novo lesions by a DCB-only strategy29. The largest registry to date, a prospective “real-world” registry for the use of a “PCB-only” strategy in small vessel de novo lesions with the SeQuent Please, had 6% additional stenting and a TLR rate of 3.6% at 9.4±1.7 months30. When comparing these previous results, one has to keep in mind that in the FALCON Registry 20.8% of the de novo lesions were bifurcation lesions and in 75.1% the lesion had a lumen less than 2.75 mm while in 24.9% the vessel lumen was greater than 2.75 mm, reflecting real-world treatment (Supplementary Table 4).
Study limitations
The FALCON Registry is a clinical registry with clinical follow-up data from office or telephone interviews. No regular angiographic follow-up data after 12 months were obtained and there was no quantitative core laboratory analysis performed on index interventions; therefore, no data on late lumen loss exist. When comparing the outcomes in relation to previous registries and trials, one needs to consider that a possible bias might exist for several reasons. Experience in the use of DCB has increased over recent years, reflected, for example, by the nearly 100% predilation rate in the FALCON Registry influencing the outcome. Further, the characteristics of the cohorts probably vary, as in more recent registries a higher rate of DES-ISR is present and in this group one expects a higher rate of second- or later-generation DES now presenting with ISR.
Conclusions
Treatment with the urea-based IN.PACT Falcon paclitaxel-coated balloon results in good 12-month outcomes in a European-wide all-comers real-world setting. Efficacy and safety are demonstrated by low revascularisation, myocardial infarction and cardiac death rates. The high proportion of successful treatment of de novo lesions indicates that the concept of balloon angioplasty with DCB only confirms previous clinical results of this device in small vessels and that using urea as an inert excipient only is nowadays common and safe.
|
Impact on daily practice Treatment of de novo and ISR coronary lesions with the IN.PACT Falcon urea-based paclitaxel-coated balloon is safe and efficient with low clinical TLR/TVR rates. |
Acknowledgements
We would like to thank all other participating centres and all study nurses involved in the FALCON Registry as well as the Hannover Clinical Trial Center for their support.
Funding
This registry was supported by an unrestricted grant from Medtronic to the Hannover Medical School.
Conflict of interest statement
J. Widder is a consultant for Medtronic. S. Eccleshall reports grants and personal fees from B. Braun. A. Roguin is a consultant for Medtronic. B. Scheller is a shareholder of InnoRa GmbH, Berlin, Germany and is named as co-inventor on patent applications by Charite Hospital, Berlin, Germany. J. Bauersachs is a consultant for Medtronic. The other authors have no conflicts of interest to declare.

