- drug-eluting balloon
- drug-eluting stent
- bare metal stent
- in-stent restenosis
- revascularisation
- late lumen loss
Abstract
Aims: Drug-eluting balloons (DEB) have recently emerged as a valuable treatment option for patients with coronary in-stent restenosis (ISR). However, little information exists on results of DEB therapy of in-stent restenosis from drug-eluting stents (DES).
Methods and results: The Pantera Lux balloon catheter, a novel DEB releasing paclitaxel with a BTHC matrix was studied in a prospective, multicentre first-in-man trial of patients with ISR either from bare metal stents (BMS) or drug-eluting stents (DES). Here we report on the clinical and angiographic follow-up of the first 45 Pantera Lux patients after six months. The mean age (±SD) of the studied ISR patients was 69±9 years (82% males, 18% females). The distribution of BMS and DES in these patients was 53% and 47%, respectively. Success of deployment of the device was 100%. After six months, the major adverse cardiac event (MACE) rate was 7.7% including one post-procedural non-fatal myocardial infarction, one target lesion- and one target vessel- revascularisation (each clinically driven). Angiographic in-stent late lumen loss was 0.03±0.35 mm (BMS: –0.08±0.37 mm, DES: 0.15±0.28 mm) as assessed by an independent angiographic core laboratory.
Conclusion: Treatment of coronary in-stent restenosis with the Pantera Lux paclitaxel-releasing balloon catheter shows very promising preliminary 6-month results, irrespective of whether the initially implanted stent was a bare metal- or a drug-eluting stent.
Introduction
In-stent restenosis (ISR) continues to be a clinical problem despite the increasing rate of use of drug-eluting stents (DES) in coronary artery disease1. Increased late thrombotic events in DES compared to BMS leaves bare metal stents (BMS) or plain angioplasty as alternative treatment options in ISR, albeit with only modest intermediate- or long-term efficacy2-4.
Brachytherapy for ISR therapy was explored, but the TAXUS V ISR trial studying paclitaxel-eluting stents and the SISR trial of sirolimus eluting-stents suggested a marked reduction risk of restenosis with either one compared with brachytherapy5-7. Subsequently, Ellis et al8 reported on the 2-year clinical outcomes of the TAXUSV trial, the continued superiority of the paclitaxel-eluting stent for BMS ISR versus brachytherapy. However, the TAXUS V trial showed that ischaemia-driven target lesion revascularisation (TLR) and target vessel revascularisation (TVR) rates were relatively high at 10.1% and 18.0%, respectively at 24 months. The SISR trial, in contrast, showed TLR and TVR rates of 8.5% and 12.4%, respectively at nine months9. DES-ISR may behave differently than BMS-ISR, which may be a further limitation to the stent-in-stent approach for ISR10.
An alternative strategy for ISR therapy is the drug-eluting balloon (DEB) technology. Intra-coronary delivery of paclitaxel by means of contrast medium or a drug-coated balloon catheter in swine resulted in drug concentrations in vascular tissue that were high enough to have antiproliferative effects, thus leading to a significant reduction in neointimal proliferation11. A pivotal randomised clinical trial conducted by Scheller et al studying an uncoated versus a paclitaxel-coated balloon in 52 patients with ISR provided evidence for the proof of concept of a DEB in humans12.
The safety and effectiveness of the Pantera Lux catheter has been demonstrated in several pre-clinical experimental settings. The Pantera Lux balloon catheter showed results at least comparable to other paclitaxel-releasing balloons in established ISR animal models of neointimal plaque burden [unpublished data].
Here we present the preliminary first in-man results of a new paclitaxel-releasing balloon catheter combining paclitaxel with butyryltri-n-hexyl citrate (BTHC) releasing matrix in patients with coronary in-stent restenosis.
Methods
Device description
The Pantera Lux paclitaxel-releasing PTCA balloon catheter (Biotronik, Berlin, Germany) is designed for balloon dilatation in coronary arteries and simultaneous release of paclitaxel to the vessel wall during balloon inflation in order to reduce reoccurrence of in-stent restenosis (ISR). The balloon surface of the Pantera Lux PTCA catheter is homogeneously coated with 3µg paclitaxel per mm2 incorporated in a proprietary delivery matrix of butyryltri-n-hexyl citrate (BTHC). BTHC is a lipophilic excipient keeping paclitaxel in a microcrystalline structure for rapid drug absorption by the vessel wall, and optimal subsequent local drug availability (Figure 1). A removable sheath protects the balloon prior to insertion into the guiding catheter in order to retain its factory-made profile and drug coating.
Figure 1. The Pantera Lux balloon surface (as shown by the milky colour) features butyryltri-n-hexyl citrate (BTHC) as an excipient for retaining paclitaxel in a microcrystalline structure for improved local drug availability.
Study design
Patients who presented with a single in-stent restenotic lesion (diameter stenosis ≥50% and <100%, target reference vessel diameter of 2-4mm; target lesion length of 8-28mm) in an area of acoronary artery previously stented with either a BMS or DES were included in a non-randomised European multicentre feasibility and safety trial (PEPPER) with a 1-month clinical, 6-month angiographic and 12-month clinical follow-up. The angiograms of the first 45 patients enrolled in the PEPPER trial (completed with 81patients) were analysed after a six month follow-up period by an independent, external angiographic core laboratory as a preliminary interim analysis (Figure 2).
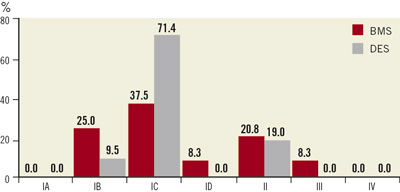
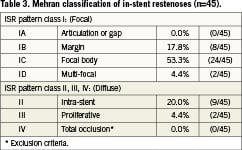
Figure 2. Mehran classification of ISR by stent type of 45 patients prior to Pantera Lux treatment. Mehran IV class represents an exclusion criterion for treatment of bare metal stent (BMS) or drug-eluting stent (DES) in-stent restenosis.
The inclusion criteria were a single coronary in-stent restenosis in patients with stable coronary artery disease. Patients with left ventricular ejection fraction <30%, angiographically visible thrombus in the target vessel, or myocardial infarction (STEMI/NSTEMI) within 72 hours of the intended treatment were excluded.
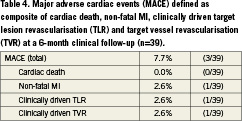
The primary endpoint of this study was angiographic in-stent late lumen loss (LLL) at 6-month follow-up. Secondary endpoints were in-segment LLL, in-stent and in-segment diameter stenosis, binary in-stent and in-segment restenosis at 6-month follow-up, cumulative MACE as composite of cardiac death, non-fatal myocardial infarction (MI), clinically driven target lesion revascularisation (TLR), clinically driven target vessel revascularisation (TVR) rate at 1, 6, and 12 months, new in-stent restenosis according to the Mehran classification13, device success (defined as exact deployment as documented by two different angiographic projections), and technical success in terms of deliverability and feasibility (defined as a composite endpoint of successful lesion passage, completion of the endovascular procedure and immediate anatomic success of <30% residual diameter stenosis by angiography).
Predilatation using a standard PTCA balloon was mandatory, with a recommendation for predilation balloon to be at least 5mm shorter than the paclitaxel-releasing balloon. The predilatation balloon size had to be equal or 0.25-0.5mm smaller than the treatment balloon. Cutting balloons were not allowed to be used for predilatation per protocol in order to standardize the type of vascular injury during balloon pre-dilatation.
All adverse events were assessed by an independent Clinical Events Committee (CEC). Administration of 100-500mg acetylsalicylic acid was recommended prior to intervention and subsequently 75-325 mg daily lifelong, as well as clopidogrel 300-600mg or ticlopidine 500mg daily prior to intervention and subsequently clopidogrel 75mg daily or ticlopidine 500mg daily for three months post-intervention.
The study was performed according to the Declaration of Helsinki (Seoul version 2008), ISO 14155 “Clinical Investigation of Medical Devices”, ICH Topic E 6 (Guideline for Good Clinical Practice) as well as all applicable national requirements.
Quantitative coronary angiography analysis
The CAAS II Research System (Pie Medical Imaging, Maastricht, The Netherlands) was used for automated contour detection and quantification. The system and validation data are described elsewhere14. The measurement procedure was previously published in detail15. Angiography was performed before and after all interventions and at angiographic follow-up using identical projections and analyses. Frames were selected as recommended by Harrington and Walford16. Analyses followed the guidelines proposed by Reiber et al17. Lesion length was the distance along a vessel segment characterised by a diameter stenosis >50%, as compared to the reference segment. Lesion length, mean diameter within the lesion (mean stenosis diameter), and minimum lumen diameter (MLD) were calculated for the target vessel segment. In addition, mean diameter stenosis and minimum lumen diameter were measured at a distance of 5mm from each stent edge. Restenosis was defined as >50% in segment diameter stenosis at angiographic follow-up.
Statistics
The data are given as mean ±SD. Patient demographics and percentages were entered from the quantitative coronary angiography workstation (CAAS; Pie Medical Imaging) into aExcel and SPPS software database. The angiographic preprocedural data were compared with post-procedural and follow-up data using a paired t-test with a Bonferroni’s correction for multiple testing. A p-value of <0.05 was considered significant.
Results
Patients and lesions
Forty-five patients with in-stent restenoses were treated with the Pantera Lux paclitaxel-releasing balloon between August 2009 and February 2010. The mean age (±SD) of the patients was 69±9 years (82% males,18% females). Pantera Lux treatment was performed in 24 BMS (53%) and in 21 DES (47%) patients. Table1 summarises patient demographics, medical history as well as indication for treatment.
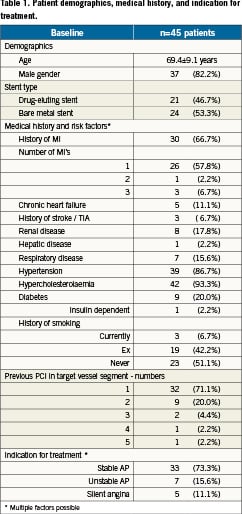
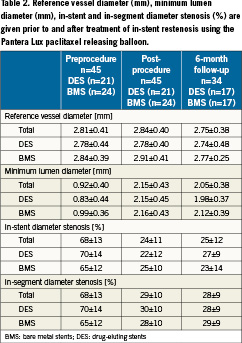
The reference vessel diameter (RVD) was 2.81±0.41mm and the minimal lumen diameter (MLD) was 0.92±0.40mm in the target vessel. Preprocedural diameter stenosis (DS) was 68±13% (Table2). The angiographically determined preprocedural in-stent restenosis pattern was classified according to Mehran13. The overall distribution is shown in Table 3.
Procedural data
The mean length of the previously stented target lesion was 18.2±6.3mm (DES: 17.5±6.6mm, BMS: 18.6±6.1mm) with range being 9mm to 38mm. Predilatation was performed in all patients before application of Pantera Lux with 16±5 bar (range 8 to 30 bar) using a mean balloon length of 12.9±3.8mm (range 8 to 20mm). Following predilatation, the Pantera Lux was applied using a mean balloon length of 20.4±6.2mm. The difference in length of predilatation vascular injury during standard balloon and Pantera Lux treatment length was 7.8±4.7mm. Maximal inflation pressure of Pantera Lux was 12.4±2.2 bar (6 to 17 bar) applying an continuous balloon inflation period of 43±11 seconds (30 to 65 seconds). In all patients, procedural and device success was documented. Post-procedural MLD increased to 2.15±0.43mm resulting in a residual diameter stenosis >50% (p<0.05 versus pre-procedure). No difference in post-procedural results was observed between patients with BMS or DES (p=n.s., Table2).
Clinical follow-up at six months
At six months, 39 of the first 45 patients were seen in a regular clinical follow-up (one patient withdrew consent, one patient died and four patients were returned out of schedule), of which 34 had angiographic follow-up (angiographic results from two patients are pending and three patients did not consent to repeat angiography).
The rate of major adverse cardiac events (MACE) rate after six months was 7.7%, consisting of one post-procedural non-fatal MI after the index procedure, one TLR and one TVR, each clinically driven (Table4). One of the two patients was asymptomatic, but he presented with two moderate lesions. The investigator estimated a75% diameter stenosis which met the criteria for clinically driven TLR per the protocol. However, independent angiographic core lab assessment detected a 48% diameter stenosis in both in-stent and in-segment.
Angiographic core laboratory quantitative coronary analysis (QCA) at 6-months
Minimal lumen diameter (MLD) was 2.05±0.38mm, representing a decrease of 4.7% compared to the post-procedural angiographic PCI result. Diameter stenosis data at six month follow-up was essentially unchanged compared to post-procedure diameter stenosis data. Diameter stenosis in-stent was 25.0±12.0% and in-segment 28.0±9.0%. Late lumen loss (LLL) was evaluated in the stent of the target lesion, and “in-segment” which was measured as the in-stent length plus 5mm proximal and 5 mm distal to the stent. At six months, LLL in-stent was 0.03±0.35mm (DES: 0.15±0.28mm, BMS: –0.08±0.37 mm) and in-segment was –0.01±0.27mm (DES: –0.02±0.27mm, BMS: 0.0±0.28 mm, Figure3).
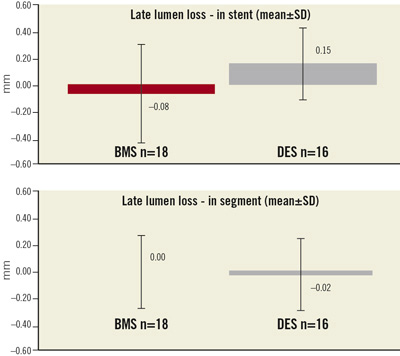
Figure 3. Six-month angiographic follow-up of 34 patients showing a low late lumen loss (mm) in the stent (upper graph) and also in the treated coronary segment 5 mm proximal and 5 mm distal of the stent (lower graph).
There were no statistically significant differences between the DES and BMS treated patients at six month for any of the aforementioned criteria.
Discussion
The Pantera Lux PTCA drug-releasing balloon catheter features apaclitaxel delivery from a matrix of butyryltri-n-hexyl citrate (BTHC) for drug uptake of the vessel wall during balloon dilatation. The choice of BTHC is based on its lipophilicity and its effect of keeping paclitaxel in microcrystalline structure for rapid drug absorption by the vessel wall and local drug availability. The balloon surface of the Pantera Lux PTCA balloon catheter is homogeneously coated with 3µg paclitaxel/mm2 incorporated in the BTHC matrix.
This paper reports on the first 45 patients suffering from coronary ISR treated by means of the Pantera Lux balloon that were followed for 6-months in the first-in-man PEPPER trial currently under evaluation. In PEPPER, the efficacy of the Pantera Lux balloon is studied for treatment of in-stent restenosis (ISR) of either bare metal or drug-eluting stents during a one year follow-up.
The preliminary results of the first patients treated with the Pantera Lux appear to be very similar to previously reported bare metal ISR data where at least one group of patients was treated with paclitaxel released from a balloon at a dose of 3µg/mm2. Similar MACE rates and late lumen loss results were obtained in the In.Pact Coro ISR study, the PACCOCATH I/II trials and the PEPCAD II trial18-20.
While most of the published drug-eluting balloon data were obtained from patients with BMS ISR, this trial reports on a nearly equal distribution of BMS and DES ISR patients (BMS: n=24 [53%], DES: n=21 [47%]) with almost identical treatment outcomes.
Mehran type I ISR focal lesions were reported to occur at a rate of 47% in PEPCAD II and only 20% in PACOCATH I//II19,20. Sirolimus- and paclitaxel-eluting stents are known to produce more focal Mehran typeI lesions21. We also found predominantly focal in-stent restenotic lesions in DES, which were fairly straightforward to treat with a drug-eluting balloon.
As indicated by this trial, a low recurrence rate of in-stent restenosis after Pantera Lux treatment may be encountered because avery low in-stent late lumen loss (LLL) of 0.03±0.35mm was observed by QCA at 6-months in 34 patients. The mean in-stent LLL is slightly lower compared to the corresponding LLL results of the PACCOCATH I/II, the PEPCAD II, and the In.Pact Coro ISR trials, with similar standard deviations (Figure 4) indicating at least equivalence to prior study results.
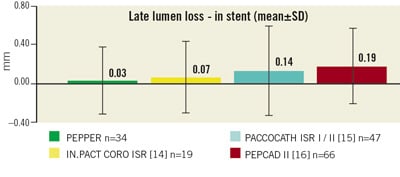
Figure 4. Six month angiographic follow-up with in-segment late lumen loss of the first 34 patients of the PEPPER trial measured by quantitative coronary angiography in comparison to published results of IN.PACT14, PACCOCATH ISR I/II15, and PEPCAD II16 trials. Note the similar standard deviation of LLL in the drug-eluting balloon studies.
We await for the final six month and one year follow-up results of the PEPPER trial studying the Pantera Lux balloon in 81 consecutive patients, but based on these preliminary data we conclude that the Pantera Lux balloon may yield excellent long-term follow-up results in the treatment of coronary in-stent restenosis of either bare metal stents or drug-eluting stents.
Acknowledgements
The study was sponsored by BIOTRONIK AG, Switzerland.
Conflict of interest statement
C. Hehrlein received an institutional research grant for this study from Biotronik. The other authors have no conflict of interest to declare.
References

