Abstract
Aim: To assess the safety and efficacy of the second generation DIOR paclitaxel drug-eluting balloon (DEB) for in-stent restenosis in a real world setting in a prospective single-arm registry with 8-month clinical outcomes.
Methods and results: In this “real world”, international prospective registry, patients with in-stent restenosis (bare-metal stent and drug-eluting stent) were enrolled- in a unique study design- with a one week enrolment period, spread over 104 centres worldwide. Patients underwent predilatation with a regular balloon, with subsequent DEB inflation in the target lesion. Additional stenting of the target lesion was left to the operators discretion in case of suboptimal angiographic success (TIMI flow <3 and/or residual stenosis >30%). The primary endpoint was 6-9-month major adverse cardiac events (MACE: all cause death, myocardial infarction, and target vessel revascularisation). A total of 250 evaluable patients were enrolled in a large web-based clinical research form and treated with the second generation DIOR DEB. Of these, 244 had 6-9 month clinical follow-up, with a mean follow-up time of 7.5 months. The cumulative MACE rate at follow-up was 11.1%, with 3 (1.2%) cardiac deaths, 1 (0.4%) non-cardiac death, 5 (2.0%) myocardial infarctions of which 2 (0.8%) periprocedural, 21 (8.6%) target vessel revascularisations, of which 18 (7.4%) target lesion revascularisations.
Conclusions: In-stent restenosis treatment with the second generation DIOR DEB is safe and feasible, with high angiographic success and low target lesion revascularisation and overall MACE rates. Moreover this new and unique method of high speed and short duration multicentre study enrolment was very successful.
Introduction
With the introduction of drug-eluting stents (DES) the treatment of bare-metal stent (BMS) in-stent restenosis (ISR), improved significantly with respect to standard balloon angioplasty, with a clinical reduction of recurrent ISR and target lesion revascularisation rates.1-3 However, concerns about double metal layers and increased stent thrombosis rates have been raised in DES treated patients.4,5 Hence, prolonged (at least one year) dual-antiplatelet therapy (DAPT) is required with the use of DES. Moreover, stent for ISR-treatment causes multiple layers of metal (“double burger”) with increased stiffness of the vessel and limited repeatability of the procedure. Therefore, an alternative treatment strategy is warranted in order to replace the necessity of stent for ISR placement, combined with reduced DAPT. The use of a drug-eluting balloon (DEB) seems very promising. Preclinical and clinical data so far showed superior results for DEB in comparison to regular angioplasty balloon and also superior results with respect to a DES.6-8 However, all of these studies were relatively small and performed by one dedicated group with two different devices (Sequent® Please [B. Braun Melsungen AG, Berlin, Germany] and Paccocath® [Medrad Inc., Warrendale, PA, USA] an investigational device). The present study was performed with a different device, the second generation DIOR® DEB (Eurocor GmbH, Bonn, Germany), in a large population of ISR patients treated in more than 100 hospitals worldwide. In this unique registry all patients were enrolled in just nine days, with a worldwide on-site monitoring.
Methods
The Valentines I Trial is an international multicentre “real world” prospective registry, designed to assess the safety and efficacy of the second generation DIOR DEB, in patients treated for ISR. Four novelties were introduced: 1) a unique first of its kind call for investigators from all over the globe via CRTonline.org and PCRonline.com homepages, 2) no pre-selection of sites was performed, 3) rather than a predefined number of patients a predefined time frame for enrolment was determined (one week), 4) the study enrolment completion and subsequent first results were linked to a scientific congress, the CRT 2010 & 2011.9 The study was carried out in accordance with international healthcare guidelines, local laws and regulation. An electronic data capture system was designed and deployed and a data monitoring structure was organised to ensure that more than 50% of the data was verified. Baseline clinical and angiographic characteristics and in-hospital outcomes were entered into the electronic database within 28 days. Clinical follow-up was performed between six and nine months.
Patient selection
Patients were recruited in 104 centres from 26 countries around the globe between February 14th, 2010 (Valentine’s day) and February 23rd, 2010 (the last day of the CRT 2011 congress).
Included patients were older than 18 years of age, presenting with ISR (>50%) of a previously placed stent (DES or BMS). Eligible patients had stable or unstable angina pectoris and/or documented ischaemia. All treated lesions were in native vessels, with a maximum of two lesions per patient. Exclusion criteria were life expectancy less than 12 months, acute myocardial infarction within the last 48 hours, lesion requiring additional stenting with either BMS or DES prior to the DEB treatment, previous therapeutic radiation to the target vessel, and patients who were unable to take DAPT for at least 3-month. Vessel size (diameter) was no exclusion criteria.
Drug-eluting balloon
The DEB used in this study was the second generation DIOR coronary angioplasty balloon catheter. This device is coated with 3 micrograms of paclitaxel per square millimeter of balloon surface using a shellac coating method with paclitaxel as active drug (available lengths: 15, 20, 25, and 30 mm; available diameters: 2.0, 2.25, 2.5, 2.75, 3.0, 3.5, 4.0 mm). The un-inflated three folded balloon protects the drug from an early wash-off effect during insertion into the guiding catheter, during tracking in the coronary vasculature and during crossing of the lesion. The inflation time of the balloon has to be at least 30 seconds (this time is halved with respect to the first generation DIOR DEB) in order to achieve adequate drug concentrations into the vessel wall.10
The first generation DIOR DEB had a roughened balloon surface, containing a crystalline coating. The currently available second generation DIOR has a coating consisting of a 1:1 mixture of paclitaxel with shellac applied to the balloon by a micro-pipetting procedure. Shellac is a natural coating layer. In the second generation DIOR DEB the hydrophilic shellac-network, once in contact with body tissues, swells and opens the structure for the pressure-induced fast release of paclitaxel on the inflated balloon. Due to this new shellac coating and higher loading dose, the advised inflation time in order to deliver the adequate amount of drug to the vessel tissue is 30-45 seconds in the second generation DIOR DEB instead of the previously prescribed 60 second (first generation DIOR DEB).11
Interventional procedure
All patients enrolled in the study were treated with acetylsalicylic acid (80-325 mg per day) and clopidogrel (300-600 mg loading-dose before the procedure, if needed, followed by 75 mg per day). Heparin was administered intravenously in order to maintain an activated clotting time ≥250 seconds during the procedure. Administration of glycoprotein IIb/IIIa inhibitors was left at the physician’s discretion. Acetylsalicylic acid was continued indefinitely after the procedure, and clopidogrel was continued for at least three months.
After obtaining coronary angiograms, patients underwent regular balloon pre-dilatation of the lesion. Pre-dilatation with balloons with a diameter 0.7-0.8:1 balloon to original stent ratio and short length was advised. In case of good angiographic result (TIMI flow >III and residual stenosis < 30%) a DEB was inflated for at least 30 seconds in the stent with an overlap of at least 2 millimetres on each edge compared to the pre-dilatation balloon. The DEB-diameter was sized with a 1.0-1.1:1 DEB to original stent ratio. In case of haemodynamic or ischaemic instability multiple inflations were allowed in 2-3 inflation cycles of 15 seconds using the same DEB. Special care was taken to position each DEB, in order to avoid potential geographic miss (area treated by standard balloon but not covered by DEB). Additional stenting of the target lesion was left to the operators discretion in case of suboptimal angiographic success (TIMI flow <3 and/or residual stenosis >30%).
Follow-up and study endpoints
Clinical follow up was performed between six and nine months. The primary endpoint was the occurrence of major adverse cardiac event (MACE), defined as all cause death, myocardial infarction, and target vessel revascularisation. Definitions for stent thrombosis were according the Academic Research Consortium criteria.11 Spontaneous myocardial infarction was defined as any elevation of troponin (or other cardiac enzymes if troponin was lacking) combined with ischaemic chest pain. Periprocedural myocardial infarction was defined as an elevation of myocardial markers >3 times the upper limit of normal (depending on the hospital, it could be troponin or creatine kinase-MB or creatine kinase). Relationship of the myocardial infarction to the target vessel was based on electrocardiographic changes. Target vessel revascularisation (TVR) was defined as any repeat percutaneous or surgical coronary intervention in any segment of the treated vessel.
Target lesion revascularisation (TLR) was defined as any repeat percutaneous or surgical coronary intervention due to a restenosis in the treated segment (including the whole DEB treated segment plus 5 mm proximal and distal of the treated segment). Angiographic success was defined as achievement of TIMI 3 flow and final residual stenosis <30%, after using the DEB device. Adverse outcomes were adjudicated by an independent clinical events committee.
Statistical analysis
Given the design of the study, only descriptive statistics are presented. Continuous variables are presented as means ±standard deviations, categorical variables are presented as counts and percentages. The diabetic sub-study presented in the results section was not pre-specified, but rather performed as a post hoc analysis for descriptive purposes.
Results
Patient and procedural characteristics
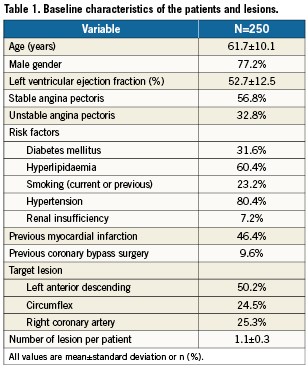
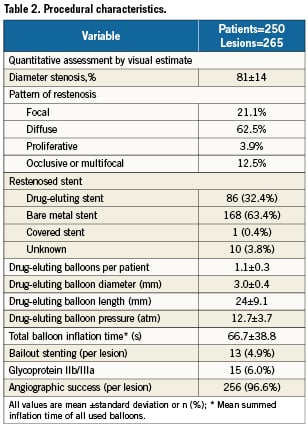
A total of 276 patients with suitable ISR lesions were both screened and treated with a DEB between the 14th and the 23rd of February 2010 (Figure 1). Of the initial 276 enrolled patients, 26 were excluded because of: violation of inclusion/exclusion criteria (13 patients), and unanswered procedural and/or discharge queries (13 patients). In the end 250 evaluable patients and treated according to protocol were enrolled in the database. Baseline clinical characteristics are shown in Table 1. Procedural characteristics are shown in Table 2.
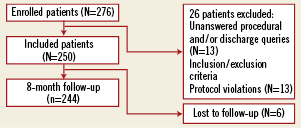
Figure 1. Enrolment of patients.
In-hospital adverse events
Angiographic success was reached in 256 of 265 treated lesions (96.6%). A total of 2 (0.8%) periprocedural myocardial infarctions occurred during the initial hospitalisation. In the first patient minor elevations of cardiac markers occurred due to occlusion of septal branches, no additional treatment was required. The second patient had a myocardial infarction in the target lesion, for which a re-PCI was performed. This TLR was not reported as a stent thrombosis by the operators, but due to a flow limiting dissection, not seen during the index procedure.
Adverse events between discharge and follow-up
The clinical events are shown in Table 3. Follow-up between six and nine months was available in 244 patients (97.6%). After discharge one non-cardiac death (0.4%) due to a hepatic carcinoma and three cardiac deaths (1.2%) were recorded.
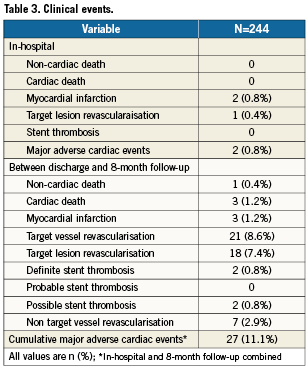
The three cardiac deaths were at 10, 11 and 20 weeks of follow-up. The first and second patient had a myocardial infarction, and consequently died. Unfortunately, no further details were recovered. However, both were defined as possible stent thrombosis according to ARC. The third patient had a myocardial infarction of a non-target vessel, for which he underwent primary PCI. Nevertheless, the patient died during the interventional procedure.
In two (0.8%) patients a late definite stent thrombosis occurred, at 11 and 12 weeks, respectively. Both patients underwent a successful re-PCI in the target lesion for this stent thrombosis.
A further 16, resulting in a total of 18 clinically driven TLR (7.4%) and three clinically driven TVR-non-TLR (1.2%) were performed. Of these TVR-non-TLR, two were by PCI (0.8%) and one by CABG (0.4%). Finally a total of seven non-TVR (2.9%) were performed, of which one CABG (0.4%) and six PCI (2.5%). The cumulative MACE rate at 8-month follow-up was 11.1%.
Adverse events in the bare-metal stent and drug-eluting stent groups
In 10 of the 250 available patients at baseline the originally implanted stent was unknown or a covered stent. Of the remaining 240 patients the originally implanted stents were BMS in 157 (62.8%) patients and DES in 83 patients (33.2%), counting 168 and 86 lesions in the BMS and DES groups, respectively. The TLR were eight (5.1%) in the BMS group and nine (10.8%) in the DES group, and in one the originally implanted stent was unknown. For TVR-non-TLR this was two (1.2%) and one (1.2%), respectively. Two (1.2%) myocardial infarctions were recorded in the BMS group and one (1.2%) in the DES group. Definite stent thrombosis occurred in two patients, one in the BMS group, one (1.2%) in the DES group, and in another, the originally implanted stent was unknown. Finally there were two (1.2%) cardiac deaths in the BMS group and one (1.2%) in the DES group.
Adverse events in diabetics and non-diabetics
At 8-months, 78 patients with diabetes mellitus (DM) and 166 patients without (non) diabetes mellitus (NDM) were followed-up. In both groups, nine TLR were recorded (11.5% and 5.4% in DM and NDM groups, respectively). Furthermore, three (1.8%) TVR-non-TLR, three (1.8%) myocardial infarctions, two (1.2%) definite stent thrombosis, three cardiac deaths (1.8%), and one (0.6%) non-cardiac death were recorded in the NDM group. In the DM group no additional MACE was recorded next to the TLR.
In both groups the left anterior descending artery was most often the culprit lesion. Considering the lesion types, in the DM group the lesion types were numerically more often diffuse than in the NDM group (47.4% versus 37.4%).
Discussion
The main findings of the Valentines I Trial registry were: 1, high acute angiographic success; 2, low overall MACE rate at follow-up; 3, low clinical TVR rate at follow-up; 4, results suggesting clinical safety in a “real world” ISR population (i.e., treatment of BMS and DES restenosis); 5, successful multicentre, worldwide-based, study enrolment and completion in a very short time frame.
Previous small randomised studies showed superior results for DEB when compared to a normal angioplasty balloon and a DES for the treatment of BMS ISR.7,8 The DEB used in these studies was either the Paccocath (investigational device) or the Sequent Please. The Sequent Please showed better results than the first generation DIOR DEB in one animal study.13 It is believed that the pharmacokinetics and bioavailability of the first generation DIOR was not sufficient to successfully inhibit the restenotic process within the prescribed 60 seconds inflation time.14
The Valentines I Trial registry is the first study using exclusively the second generation DIOR DEB. This second generation DIOR DEB has a delivery dose of paclitaxel up to 100 times higher than the first generation DIOR DEB, and around the same delivery dose of the Sequent Please DEB.10,15 Hence, this all-comer study gives us insight on the effect of higher delivery dose DEB.14
This study showed in terms of absolute numbers at least similar clinical results in ISR as the first published studies with a Paccocath or Sequent Please DEB.6,7 However, a 1-to-1 comparison between these studies is difficult, as this study did not use stringent exclusion criteria as used in randomised trials. This is reflected by worse prognostic baseline patient characteristics in this study, such as a high rate of diabetics (>30%), patients with renal insufficiency (>7%) and patients with long diffuse ISR lesions (>60%).
When comparing BMS-ISR vs. DES-ISR, the clinical outcomes seem to be better in the originally BMS treated patients, with figures suggesting lower revascularisation rates in the BMS restenosis treated patients. However, baseline patient characteristics in the DES group were less favourable than the BMS group (e.g., diabetes mellitus 39.8% vs. 28.0% and previous CABG 14.5% vs. 7.0% in the DES and BMS groups, respectively). Nevertheless, DEB treatment yielded good results in both initially DES and BMS treated patients.
Results for DEB treatment in diabetics and non-diabetics suggest no additional safety hazard for diabetics. However, there is a trend towards a higher TLR rate in patients with diabetes mellitus. Nevertheless, it has been shown that restenosis rates in diabetics remain higher than in non-diabetics. Hence, the current findings are in line with these earlier results.16,17
Furthermore, it is interesting that a cross-over rate from DEB to stent was just <5% in the present study. This number is comparable to the cross-over rate in the Pepcad-II (9.1%) and much lower than the PICCOLETO.6,14 Several factors may explain the lower cross-over rate when compared to the PICCOLETO study: 1, The PICCOLETO studied small vessel disease, instead of ISR like the Pepcad-II and the present study. The likelihood of accepting suboptimal angiographic results in small-de novo lesions is very low, while much higher for ISR. 2, In the PICCOLETO study, only 28 patients were in the DEB arm, thus each individual patient that crossed-over to the stent arm had a higher impact in percentages.
Finally, the Valentines I Trial was the first study using a new and unique study design. Instead of enrolling large number of patients at a few, usually high-skilled centres, small numbers of patients in 104 centres across the globe were included in this study. And rather than setting a predefined number of enrolments, a strict enrolment time was chosen. There was no selection of sites, investigators volunteered and were approved to participate after completing an on-line web survey. This is probably a better “real world” reflection of patients presenting with ISR by minimising bias due to treatment by few but highly skilled operators and high enrolment rates per centre. On-site data monitoring during study follow-up was performed in 56% of cases, which is high compared to other large multicentre registries.
Conclusion
Treatment of ISR with the second generation DIOR DEB is feasible and safe with high angiographic success and low target lesion revascularisation and overall MACE rates at mid-term follow-up. Moreover this new and unique method of high speed and short duration multicentre study enrolment was very successful. This registry showed a marked increase in efficiency with a previously defined time period for enrolment but also outcomes. This may potentially increase data generation and enhance implementation of new findings in clinical practice. Hence, it will be interesting to see to what extent the Valentines I Trial will impact future studies.
Study limitations
All limitations as accounted for other registries also apply to this registry. Although in principle almost all patients with ISR should have been enrolled in this registry, selection bias may have occurred in individual cases. Second, although clinical outcomes are very good, the nature of this registry does not allow for 1-to-1 comparison with a reference technique. Third, no angiographic corelab analysis was performed on index interventions. Fourth, the effect of including very limited number of patients per centre is unknown, hence, validation of this newly explored enrolment technique needs still to be taken into consideration. Fifth, cardiac enzymes were not systematically obtained. Finally, when patients were free of complaints, angiographic follow-up was not mandatory, which could lead to an underestimation of (silent-) re-occlusions.
Sources of funding
Eurocor supported all centres by providing free of charge DIOR DEB devices and providing on-site data monitoring. Moreover, data verification was performed by Eurocor. Furthermore, web-based data collection and data analysis was performed by MedStar Health Research Institute (Washington D.C, USA).
Conflict of interest statement
St. Stahnke and R. Pogge von Strandmann are employees of Eurocor GmbH. Pieter R. Stella is member of the scientific advisory board of Eurocor GmbH. The other authors have no conflict of interest to declare.

