Abstract
Aims: Safety and efficacy of percutaneous coronary interventions using the Pantera Lux paclitaxel-coated balloon have been demonstrated in the PEPPER first-in-man trial. This prospective, multicentre, clinical registry aims to evaluate its safety and efficacy in an international real-world setting in a larger cohort of patients.
Methods and results: Between April 2010 and April 2011, 1,064 patients were treated for predominantly diffuse and proliferative in-stent restenosis of bare metal stents (BMS-ISR) and drug-eluting stents (DES-ISR), or for de novo lesions. Clinical device success was obtained in 98.2% of the patients. The study endpoint was major adverse cardiac events (MACE), defined as a composite of all-cause mortality, non-fatal myocardial infarction and clinically driven target vessel revascularisation, and was 8.5% in the overall, 6.0% in the BMS-ISR, 11.5% in the DES-ISR and 7.0% in the de novo population at six months, and 15.1%, 11.6%, 20.6% and 9.4% at 12 months, respectively. Definitive stent thrombosis occurred in 0.4% of the patients within 12 months.
Conclusions: Safety and efficacy of the Pantera Lux paclitaxel-coated balloon was confirmed in a real-world setting with low major adverse cardiac event rates in patients with in-stent restenosis or de novo lesions. (ClinicalTrials.gov: NCT01081366).
Introduction
Despite the widespread use of drug-eluting stents (DES) and improvements in coronary stent material and design, the occurrence of in-stent restenosis (ISR) remains an area of concern and the optimal treatment strategies of ISR are still under debate1. The concept of paclitaxel-coated balloons (PCB) is interesting, as it avoids several issues of DES, such as biological issues resulting from hypersensitivity and polymer toxicity, issues secondary to stent fractures, non-uniform drug application only at the strut area, and technical issues such as stent underexpansion and incomplete stent coverage. The advantages of PCB include homogeneous and rapid transfer of the drug to the entire vessel wall, absence of polymer which could decrease chronic inflammation, no further stent layer and hence a reduced need for dual antiplatelet therapy2,3.
For bare metal stent restenosis (BMS-ISR), it has been shown in animal studies4,5 and randomised trials6-8 that treatment with PCB is superior to conventional balloon angioplasty6,7, and persistently reduces repeat revascularisation during long-term follow-up6. This was even reproduced in the first randomised trial, where PCB was shown to be at least as efficacious as DES8. In 2010, the Task Force on Myocardial Revascularisation of the European Society of Cardiology and the European Association for Cardio-Thoracic Surgery gave a class IIa level B recommendation for PCB in BMS-ISR, provided that the specific devices have a proven efficacy/safety profile, since there is no class effect in PCBs9. In the meantime, some experts regard PCB treatment in BMS-ISR as the gold standard10, and cost-effectiveness over DES has been demonstrated11.
Compared to treatment of BMS-ISR, ISR of DES generally has higher revascularisation rates12-17.There are obvious differences in the pathophysiologic mechanism of DES-ISR and the optimal treatment remains unclear1,14,17-19. Yet, recent randomised control trials showed superiority of PCB compared to conventional balloon angioplasty20,21,22, and non-inferiority to DES22.
The present registry aimed to provide further evidence for PCB treatment of ISR and to assess the safety and efficacy of the Pantera Lux PCB (BIOTRONIK AG, Bülach, Switzerland) using Butyryl tri-n-hexyl citrate (BTHC) as an inert excipient in a real-world setting, evaluating whether the promising results of the PEPPER first-in-man trial17 can be replicated in an unselected patient population in daily practice.
Methods
DELUX was an international, prospective, non-randomised, multicentre, observational clinical registry to assess the safety and efficacy of the Pantera Lux PCB (BIOTRONIK AG) under real-world conditions. The registry was conducted in accordance with the Declaration of Helsinki. The study protocol and the patient data release form were approved by independent ethical committees as per local regulations. Inclusion and exclusion criteria were based on study specific criteria such as written informed consent, eligibility for percutaneous coronary intervention (PCI) and safety criteria such as exclusion of patients with known allergies to one of the substances applied; adherence to the instructions for use was encouraged. Nevertheless, to allow an assessment under real-world conditions, sites were asked to record all consecutive patients even if they did not comply with the eligibility criteria or instructions for use, under the premise that they provided informed consent.
Procedural strategy and adjuvant medical therapy were left to the discretion of the operator and followed routine clinical practice. Lesion preparation was recommended to be performed with an uncoated balloon at least 5 mm shorter than the PCB. It was left to the operator as to whether it was necessary to implant an additional stent (e.g., in the case of dissection). According to the PCB instructions for use, dual antiplatelet therapy was recommended for three months post index procedure, unless otherwise required.
The balloon surface of the Pantera Lux is homogenously coated with a delivery matrix of 3 µg paclitaxel per mm2 using Butyryl tri-n-hexyl citrate (BTHC) as an excipient. BTHC incorporates paclitaxel into a micro-crystalline structure to improve drug uptake into the vessel wall4,5. It degrades to citric acid and alcohol. Paclitaxel is a lipophilic antiproliferative substance that allows a rapid drug absorption by the surrounding tissue. The PCB was available in lengths of 10 to 30 mm with diameters of 2.0 to 4.0 mm.
Clinical follow-up was performed at one, six and 12 months. Follow-up assessments were conducted according to the standard of care at participating sites. All major adverse cardiac events (MACE) and stent thromboses were adjudicated by an independent clinical events committee (Dr Ralf Birkemeyer, MD, Oberndorf, Germany). Data quality was assured by a minimum of 10% source document verification of randomly selected patients during monitoring visits. A minimum of two patients per site were monitored.
The primary study endpoint was rate of MACE (hierarchical composite of all-cause mortality, non-fatal myocardial infarction and clinically driven target vessel revascularisation) at six months. Secondary study endpoints were MACE at one and 12 months, clinically driven TVR at one, six and 12 months and clinical device success, defined as successful positioning and inflation of the PCB at the intended target lesion with a final residual stenosis below 50%, without use of a device outside the assigned treatment strategy. Myocardial infarction (MI), target vessel revascularisation (TVR), target lesion revascularisation (TLR) and stent thrombosis were defined according to the standardised definitions of the Academic Research Consortium23. Even though, in the treatment of de novo lesions, there was no underlying, previously implanted stent and PCB treatment was not followed by an additional stent implantation in most cases, the traditional and well defined term stent thrombosis was used in the case of a vessel thrombosis in the segment of an earlier PCB treatment. Additionally, target lesion failure (TLF), a device-oriented composite of cardiac death, target vessel MI and TLR, was assessed.
The analysis was performed on the intention-to-treat population on the basis of the available data. Continuous data were expressed as mean±standard deviation and categorical data as frequencies and percentages. Hypothesis tests for categorical data were made using either the chi-square or Fisher’s exact test. For continuous data the t-test was used. All tests had a significance level of 5%, which implies that a probability value (p-value) of less than 0.05 is statistically significant. SAS statistical software version 9.3 (SAS Institute, Inc., Cary, NC, USA) was used for all statistical calculations.
The study was sponsored by BIOTRONIK AG, Bülach, Switzerland. The sponsor was involved in the design of the study, data collection, monitoring and data analysis and interpretation. The corresponding author had full access to all the data in the study and together with the co-authors had final responsibility for the decision to submit for publication. The trial was registered at ClinicalTrials.gov (NCT01081366).
Results
Between April 2010 and April 2011, 1,064 patients with 1,137 lesions were enrolled at 62 sites in 12 countries. Nine hundred and eighteen patients (86.3%) presented with ISR (499 [54.4%] with BMS-ISR, 419 [45.6%] with DES-ISR), 105 patients (9.9%) presented with de novo lesions, and 41 (3.8%) could not be clearly assigned to one of the groups as either several different lesion characteristics were observed per patient or the information was unknown. Notably, two thirds (n=77, 69.4%) of the de novo lesions were ≤2.75 mm (Figure 1).
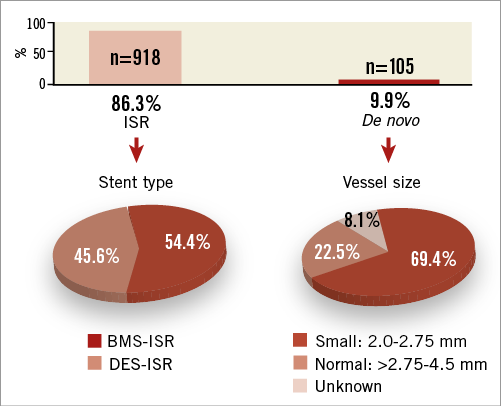
Figure 1. Indications for which patients were treated with PCB. The missing 3.8% from a total of 100% reflects unknown indications. BMS: bare metal stent; DES: drug-eluting stent; ISR: in-stent restenosis
Overall, patients were 66.5±10.7 years old and predominantly male (74.6%) with a high rate of prior myocardial infarction (51.8%), diabetes (34.1%) and congestive heart failure (20.6%), as shown in Table 1. Patients treated for ISR were significantly older and significantly more often had previous MI, hyperlipidaemia and arterial hypertension compared to patients treated for de novo lesions. There was no significant statistical difference between the DES-ISR and BMS-ISR groups except a higher rate of diabetes.
Almost half (47.6%) of the ISR lesions were Mehran class II, defined as a diffuse intra-stent ISR24 (Table 2). Predilatation was performed in 85.3% of the lesions using cutting or scoring balloons in more than one fifth, which resulted in a high clinical device success (98.2%). Of the 105 patients with 111 de novo lesions, 18 lesions were additionally treated with a BMS, six with a DES and one with BMS plus DES. Therefore, 23 lesions were first treated with PCBs followed by a stent and two vice versa. Overall, patients treated for de novo lesions had more bifurcation lesions, a significantly smaller reference vessel diameter, significantly less pressure applied for predilatation, and significantly more patients had pre- and post-procedure TIMI flow <3 than patients with ISR. Due to the nature of de novo lesion treatment there was a significantly higher need for additional stent implantation than in patients treated for ISR. Compared to BMS-ISR patients, patients with DES-ISR had significantly more bifurcation lesions and higher pressure was applied for predilatation (Table 3).
Follow-up data were available for 1,033 (97.1%) of the patients at six months and for 1,008 (94.7%) at 12 months. Hierarchical MACE events were observed in 8.5% of the patients at six months and in 15.1% at 12 months, and TLF in 5.9% and 10.1%, respectively (Table 4, Figure 2). At 12 months, patients with ISR had numerically higher MACE (15.7% vs. 9.4%, p=0.10) and significantly higher TVR rates (10.2% vs. 3.1%, p=0.03) than patients with de novo lesions. Predilatation did not have a significant impact on clinical outcomes at 12 months (14.9% vs. 16.1%, p=0.72, for MACE, and 10.1% vs. 10.5%, p=0.87, for TLF). An additional stent implantation in the de novo group approximately doubled the event rate (15.0% vs. 8.1%, p=0.35, for MACE, and 10.0% vs. 4.1%, p=0.29, for TLF).
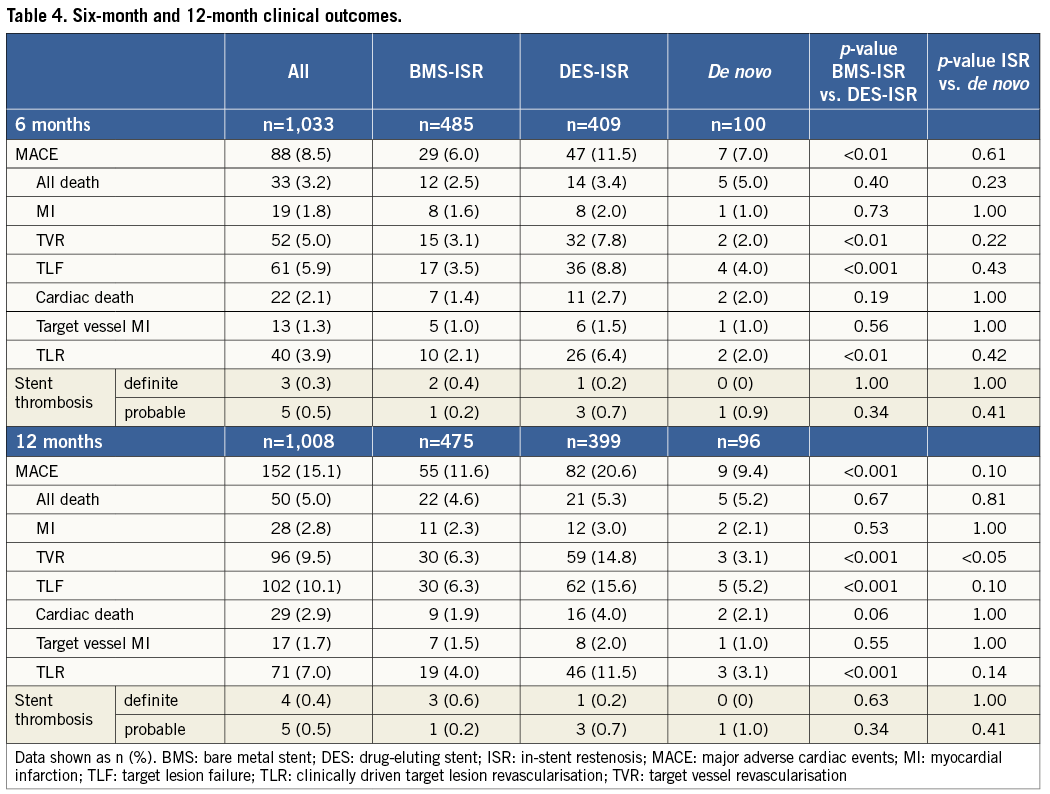
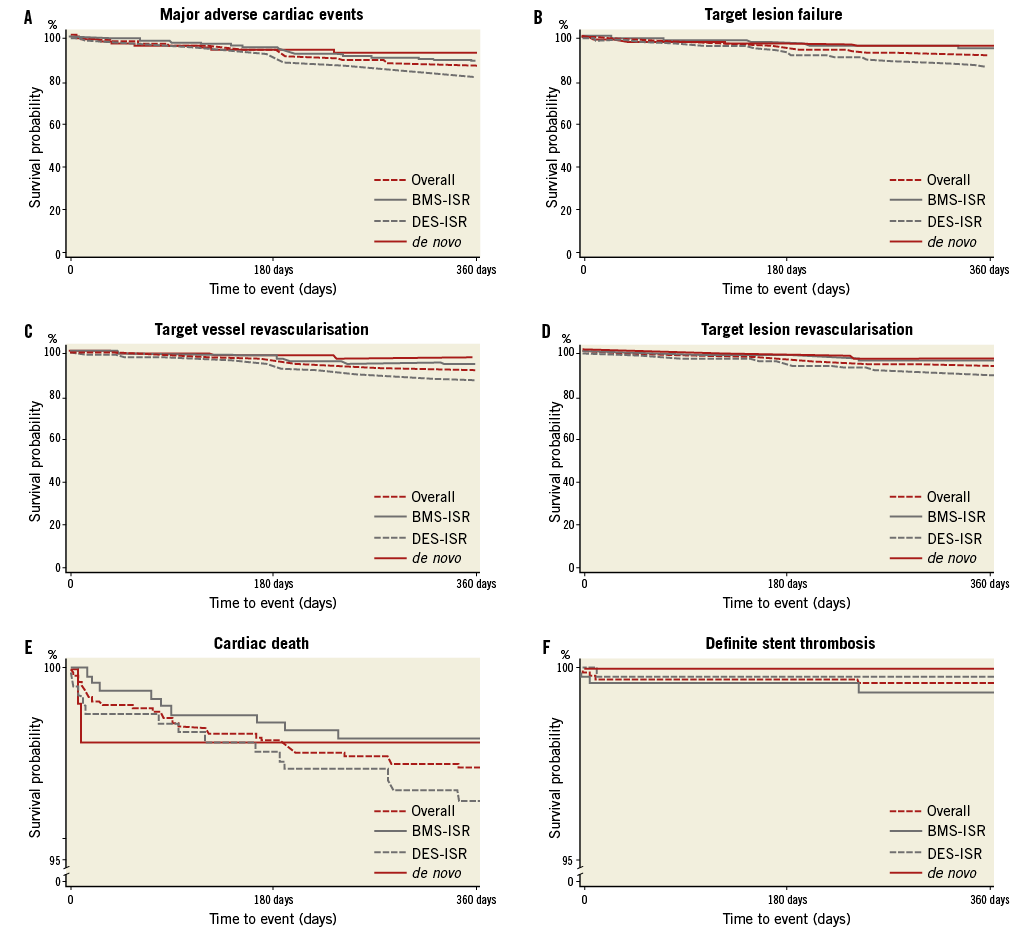
Figure 2. Freedom from major adverse cardiac events (A), target lesion failure (B), target vessel revascularisation (C), target lesion revascularisation (D), cardiac death (E) and definite stent thrombosis (F).
Comparing DES-ISR and BMS-ISR, significantly more DES-ISR patients experienced MACE (11.5% vs. 6.0%, p<0.01, at six months, and 20.6% vs. 11.6%, p<0.001, at 12 months), which was mainly due to the difference in TVR rates. Probable and definite stent thrombosis according to the ARC definitions occurred in 0.5% and 0.4% of the patients at 12 months (Table 4). In the de novo group, only one probable stent thrombosis occurred. A 91-year-old patient with several comorbidities and reduced left ventricular function, who was treated for myocardial infarction with a PCB followed by BMS, experienced sudden death seven days after intervention. Hence, this was classified as probable stent thrombosis according to the Academic Research Consortium definitions23.
Discussion
The DELUX registry reveals several important findings. 1) The results of the PEPPER first-in-man study, a clinical study assessing the safety and efficacy of the Pantera Lux balloon, can be replicated in a large all-comers population in daily practice. 2) The DELUX results compared well with outcomes of other paclitaxel-coated balloon and DES trials. 3) Outcomes in de novo lesions suggest that PCB could be an option in these patients.
Similarly to the PEPPER trial, BMS-ISR and DES-ISR were almost equally distributed, but the DELUX population additionally included almost 10% of patients with de novo lesions. As expected under real-world conditions, the DELUX population was more complex. In the PEPPER trial, ISR treated using the Pantera Lux were predominantly classified as focal (Mehran class I) lesions17, whereas in DELUX they were predominantly diffuse and proliferative (Mehran class II and class III). Furthermore, the DELUX population had fewer patients with a history of an MI than PEPPER (51.8% vs. 63.0%), and more patients suffered from unstable angina (32.9% vs. 14.8%) and congestive heart failure (20.6% vs. 8.6%)25. Clinical outcomes were similar between both trials. Focusing on MACE at 12 months, hierarchical rates were 15.1% (4.6% all death including 2.6% cardiac, 2.1% MI and 8.4% TVR) vs. 11.8% (0% cardiac death, 1.3% MI, 10.5% TVR)17. Notably, the MACE definitions of PEPPER included only cardiac-related death, whereas in DELUX MACE included all-cause mortality. Applying the same definitions, 12-month MACE rates were nearly identical (13.1% in DELUX versus 11.8% in PEPPER). Similar to PEPPER, the incidence of MACE was higher in DES-ISR compared to BMS-ISR, predominantly due to differences in revascularisation rates. Assuming that merely lesions with a low risk for restenosis have initially been treated with a BMS and conversely lesions with a high risk have been treated with DES, this may count for a bias against DES-ISR. This hypothesis is supported by the fact that in DELUX we found a significantly higher rate of bifurcation lesions and diabetes in the DES-ISR group, which by itself is a predictor for MACE and revascularisation14. Furthermore, we cannot rule out that there are cases of DES-ISR treated in DELUX where the DES was only the last layer of (maybe several) previously implanted stents which may count for an additional higher intrinsic risk for restenosis. DES-ISR may also represent a failure of the antiproliferative drug in contrast to BMS-ISR, which is naïve with respect to drug treatment. All these aspects may trigger a higher late lumen loss, leading to more TLR and consequently MACE in the context of DES-ISR.
It is well known that there are major differences between different PCBs due to different excipients. Its role as a drug carrier to the tissue has a large influence on effectively applying paclitaxel, hence on restenosis4,5. The SCAAR registry showed a conspicuous difference in restenosis rates which were 12.5% without carrier and 3.4% with carrier26. In line with animal studies4,5, the clinical results of the DELUX registry compared favourably with those of different PCBs and even with DES. As an overview, revascularisation rates in DES-ISR patients were 14.8% in DELUX versus 14.8%-24.2% for other PCBs15,22 and 8%-22.2% for DES12,22,27,28 at 12 months. For BMS-ISR, revascularisation rates at 12 months were 6.3% versus 4%-9.2% for other PCBs7,8,15 and 10.3%-15.4% for DES8,12,29. Further, the results are in line with the recently published SeQuent Please World Wide Registry16 (10.1% TVR for DES-ISR and 5.2% for BMS-ISR at nine months) and the Valentines registry13 (8.6% TVR at 7.5 months in a mixed ISR population with only 32% DES-ISR).
Regarding safety aspects after treatment of BMS-ISR, we found 2.3% MI and 1.9% cardiac deaths in DELUX at 12 months, which is similar to the 1.2% MI and 1.1% cardiac deaths in the SeQuent Please World Wide Registry16 after a shorter period of nine months follow-up. In DES-ISR there were 3.0% MI and 4.0% cardiac deaths in DELUX at 12 months, which was similar to the SeQuent Please World Wide Registry with 3.2% MI but lower for cardiac deaths with 0.7%. The Valentines registry13 had reported a rate of 2.0% MI and 1.2% cardiac deaths at a mean follow-up of 7.5 months for its overall collective including BMS-ISR and DES-ISR patients, which compares well with DELUX with 1.8% MI in the total cohort at six months, but shows a slightly lower cardiac death rate than DELUX with 2.1%. In summary, we see comparable rates in MI in other PCB trials but higher levels of cardiac death, mainly driven by death events in the DES-ISR subgroup. A potential explanation for the higher cardiac death rates could be the higher rate of baseline comorbidities with respect to DES-ISR in the DELUX population. Definite stent thrombosis was observed in only 0.4% at 12 months, which was less than the 0.8% in the Valentines Trial13 at 7.5 months, but did not reach the excellent outcomes of only 0.1% at nine months in the SeQuent Please World Wide Registry16. Overall, the DELUX results compared well with the outcomes of other PCB registries, including the results of the PEPPER study, so that it can reasonably be concluded that the class IIa level B recommendation of the guidelines on myocardial revascularisation can also be applied to Pantera Lux9.
For patients with de novo lesions, outcomes were promising, with non-hierarchical 12-month rates of 5.2% overall mortality, 2.1% MI and 3.1% TVR compared to a prospective multicentre German registry with DES treatment in 3,973 de novo lesions resulting in 4.2% mortality, 3.1% MI and 10.5% TVR30. Despite small vessel diameters (2.5±0.5 mm) and a high rate of bifurcation lesions (26.1%) in DELUX, only one (1.0%) probable stent thrombosis occurred in a patient additionally treated with BMS, whereas 0.7% definite stent thrombosis in the above-mentioned registry with DES treatment was observed. Of the five deaths (5.2%) during follow-up, two were cardiac (one sudden death and one death of unknown cause), mirroring the complexity of these patients. Our results are consistent with the ones observed in 491 patients of the SeQuent Please World Wide Registry16 and the BELLO study31, which randomised 182 patients to either PCB or DES and found comparable rates of restenosis in PCBs. Overall, these results suggest that treatment with PCB may offer a treatment option in patients with de novo lesions in small vessels when stents are difficult to place.
Limitations
The DELUX registry is a clinical registry and contains no angiographic follow-up data and no quantitative core laboratory analysis was performed on index interventions. It would have been interesting to see if the lower TLR rates in DELUX compared to ISAR-DESIRE 322, the first randomised study comparing PCB and DES in DES-ISR (11.5% in DELUX versus 22.1% for PCB and 13.5% for DES treatment in ISAR-DESIRE 3), would have been supported by angiographic parameters. Further, some relevant variables, such as the number of vessels affected or the number of previous PCI or stent layers, which could have confirmed the findings of the PEPPER trial17, have not been captured. There was no comparator, hence the validity of comparisons to PCB and DES treatment is limited. The comparison of outcomes to previously published trials and registries can only give landmark information, as the characteristics of the cohorts as well as the trial designs including endpoint assessments may vary.
As the use of PCB in de novo lesions was not encouraged by the instructions for use of the Pantera Lux balloon, de novo lesions account for only 10% of the total collective. There might have been a selection bias in favour of simpler de novo lesions in which an angioplasty, albeit with PCB only, appeared satisfactory. On the other hand, because predominantly de novo lesions in small vessels were treated using PCB, a contrary bias may have occurred. Further randomised studies are needed to elucidate the role of PCB in certain lesion settings. Several trials are currently underway, such as the BASKET small trial (NCT01574534), comparing PCB and third-generation DES in small de novo lesions, and the BIOLUX-RCT (NCT01651390) and RIBS IV (NCT01239940) trials, com-paring PCB with DES for DES-ISR treatment.
Conclusion
Treatment with the Pantera Lux paclitaxel-coated balloon showed good 12-month outcomes in an international real-world setting in a predominantly difficult ISR population. Efficacy and safety are demonstrated by low revascularisation, MI and cardiac death rates and confirm previous clinical results of this device using Butyryl tri-n-hexyl citrate (BTHC) as an inert excipient. Results are favourable both in the overall population and in the de novo lesion subgroup.
Funding
The study was supported by BIOTRONIK AG, Bülach, Switzerland.
Conflict of interest statement
R. Toelg reports having received lecture honoraria and consulting fees from BIOTRONIK and Abbott Vascular. R. Kornowski reports having received a research grant and lecture honoraria from BIOTRONIK. G. Richardt reports having received lecture honoraria from BIOTRONIK. C. Hehrlein reports having received lecture honoraria and consulting fees from BIOTRONIK. All other authors have no conflicts of interest to declare.

