Abstract
Aims: Recently, drug-eluting balloons have received a guideline class IIa recommendation in the treatment of in-stent restenosis after bare metal stent implantation. It is not known if different balloons perform equally. Using a large real world registry, restenosis frequency was reported for two drug-eluting balloons.
Methods and results: From April 2009 until September 2011, 1,129 patients were treated with paclitaxel-eluting balloons in Sweden. Mean follow-up was 328±210 days. Nine hundred and nineteen patients were treated with a balloon using a contrast agent as a drug-carrier and 217 with a balloon without a contrast agent as a drug-carrier. The indications were predominantly de novo (45.4%) or in-stent restenotic (51.8%) lesions. The overall incidence of restenosis at six months was 3.4% with the paclitaxel balloon using a contrast agent as carrier, compared with 12.5% with the paclitaxel-eluting balloon without a carrier (risk ratio: 0.42; 95% confidence interval [CI] [0.26-0.68]). After adjusting for indications, lesion types and procedural factors, the risk ratio was 0.39; 95% CI (0.24-0.65).
Conclusions: This observational study from a large real world population shows a major difference between two paclitaxel-eluting balloons. The findings suggest that there are no class effects for drug-eluting balloons and factors other than the drug may be important for the clinical effect.
Introduction
Restenosis has always been the Achilles’ heel of percutaneous coronary intervention (PCI). Drug-eluting stents (DES) have reduced the rates significantly but at the cost of a limited increase in stent thrombosis1,2. In order to further optimise results after PCI, the use of biodegradable polymers and stents have entered the market3,4. Another option for reducing restenosis is through the use of drug-eluting balloons (DEB). A DEB is capable of delivering a highly lipophilic drug such as paclitaxel5, to the vessel wall in adequate concentrations during balloon inflation and it has also been shown in preclinical trials that coronary restenosis can be inhibited at the onset of injury6,7. Following successful clinical trials using DEB to treat in-stent-restenosis, DEB have now achieved a class IIa recommendation in the European Society of Cardiology (ESC) guidelines with a B-level of evidence8.
However, one cannot assume a class effect for all drug-eluting balloons, although they use the same cytostatic drug to inhibit restenosis. The use of an excipient such as iopromide as a carrier to facilitate drug delivery may be one reason; how the coating is applied, such as by spraying the drug onto the balloon in a crimp or dilated state is another. However, the entire delivery system plays an important role, such as trackability, drug concentration, adherence and deliverability, as well as the time the balloon needs to be inflated for sufficient delivery of the drug.
In Sweden, five new DEB have been introduced since 2009 and all use paclitaxel as the effective drug. Two of these DEB have now been used in more than 200 patients and been entered into the SCAAR database. The aim of the present analysis was to investigate the use of these two DEB and to elucidate how they are performing in terms of restenosis in real life clinical practice.
Materials and methods
The Swedish coronary and angioplasty registry (SCAAR/Swedeheart9) record online, through a web interface, consecutive patient data from all (n=29) PCI performing centres in Sweden. The internet-based system provides each centre with immediate and continuous feedback on processes and quality of care measures. Since 2001, monitoring and verification of registry data have been periodically performed in at least one third of the hospitals by comparing 20 of the entered variables in 20 randomly-selected interventions per hospital and year, with the patients’ hospital records. Automatic quality control is also continuously performed on the SCAAR registry interface. The recordings of clinical and angiographic data are indicated as complete and the case can be closed only if all the mandatory variables have been inserted. (For further information with regard to the database please visit www.ucr.uu.se/en/).
Use of DEB in Sweden started in 2009 with the SeQuent® Please (B. Braun AG., Melsungen, Germany), and also in 2009 the first generation of the ELUTAX® (Aachen Resonance GmbH., Aachen, Germany) was introduced. Three more balloons entered the market in 2010/11 but have so far been used in less than 100 patients and therefore not included in the analysis as data would be uncertain.
Restenosis as registered in the SCAAR registry is defined as a stenosis assessed by angiographic visual estimation (>50%) or by fractional flow reserve (FFR) ≤0.80 in a previously stented segment identified by coronary angiography for any clinical indication performed anywhere in the country. The clinical relevance of restenotic lesions was detected by symptoms, routine non-invasive functional testing (exercise test, myocardial scintigraphy), and/or invasive functional evaluation by FFR.
Being alive, or date of death was obtained from the National Population Registry. The merging of the registries was performed by the Epidemiologic Centre of the Swedish National Board of Health and Welfare and approved by the local ethics committee at the Uppsala University.
Baseline characteristics are summarised with means and standard deviation for continuous variables and percentages for discrete variables. Cumulative event rates were estimated with the Kaplan-Meier method as time to event. Primary endpoint was the registration of restenosis, with clinical presentation in the SCAAR/Swedeheart registry. All individual DEB-treated patients were followed either until September 2011, until death occurred or until restenosis/re-occlusion occurred in the reported segment. Restenosis in the SCAAR registry is evaluated at the discretion of the operator predominantly by eye balling during angiography. In general it is agreed that restenosis should include 5 mm to the ends of a treated segment. The relative risk (RR) of the primary endpoint (time to restenosis) was calculated using the Cox proportional hazards method. For calculation of the adjusted hazard ratio (HR), all factors were forced into the model. The following factors, predicted as being the most important in influencing the risk of restenosis were selected: diabetes, bifurcations, DEB diameter, type of lesion (restenotic or not), acute coronary syndrome (ACS) or not, and if an adjunctive stent was used in the lesion.
To test the statistical interaction between the different types of DEB and type of stenosis (restenotic and de novo) an interaction term “type of DEB”* restenotic/de novo was entered into a separate Cox model.
Only DEB with no missing data are presented. In patients with more than one DEB used in the procedure only one of these DEB were randomly included in the analysis.
All analyses were performed with the use of SPSS statistical software, version 19.0 (SPSS Inc., Chicago, IL, USA).
The drug-eluting balloons evaluated
The Braun SeQuent® Please (BSP) balloon (B. Braun AG., Melsungen, Germany) is a second generation DEB from B. Braun, with an improvement in the Paccocath® technology10. It uses paclitaxel in a concentration of 3 µg/mm2 in a matrix/excipient with the hydrophilic contrast media, iopromide. The matrix enhances the release and dissolution of the drug and possibly also the adherence to the vessel wall. The matrix facilitates almost complete release of the drug during the 30-60 seconds inflation leaving as little as 4.5% of the paclitaxel dose on the balloon after the procedure10.
The Aachen ELUTAX® (ARE) balloon (Aachen Resonance GmbH., Aachen, Germany) evaluated in the study was a first generation DEB from the manufacturer. This balloon has a drug configuration with a concentration of 2 µg/mm2 paclitaxel, without any excipient. No data could be found in the literature concerning the delivery dose of the ARE balloon.
Results
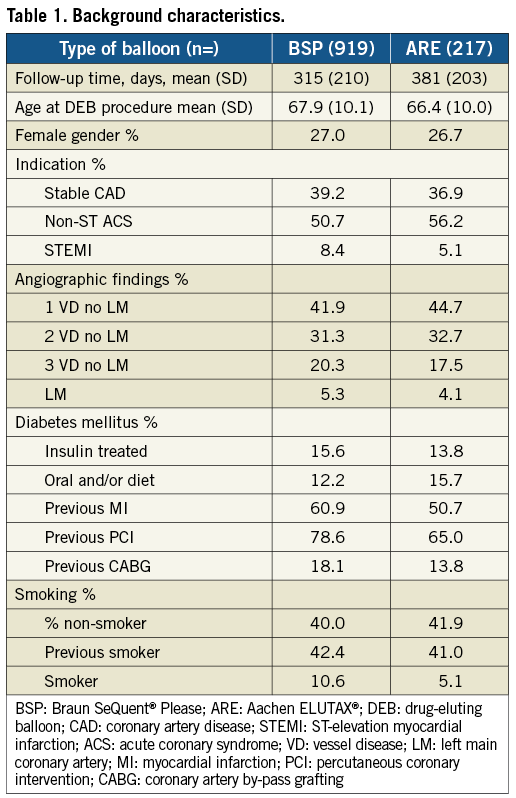
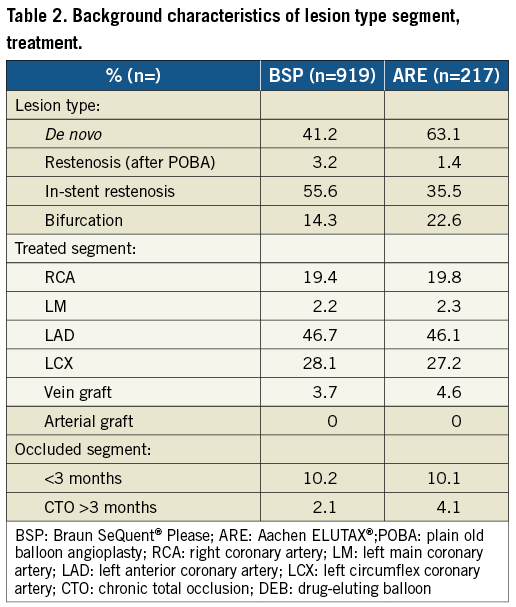
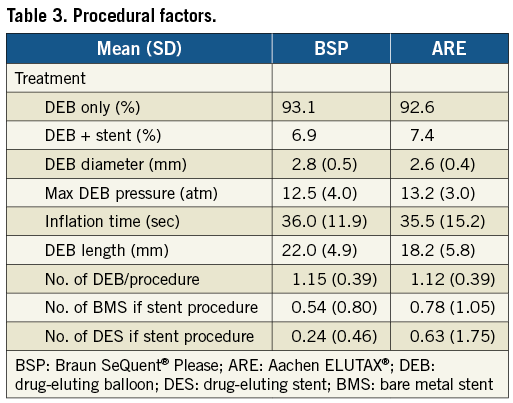
From April 2009, when the first DEB was used until September 2011, 43,998 PCI procedures were performed in Sweden. During this period a total of 1,336 DEB were used, and 1,305 (98%) of these were the ARE or the BSP paclitaxel-eluting balloons. After excluding six patients, with a use of nine DEB, due to missing data, 1,129 patients were treated with 1,236 DEB. Nine hundred and nineteen (80.9%) patients were treated with the BSP and 217 (19.1%) patients with the ELUTAX balloon. The background characteristics, lesion and procedure characteristics were similar between the two types of drug-coated balloons as presented in Table 1, Table 2 and Table 3. ARE balloons were more frequently used in de novo lesions as compared with BSP balloons.
The mean follow-up time was 328 days (SD±210) during which time 71 cases of restenosis were reported (44 in BSP and 27 in ARE balloons). At six months, the reported rate of restenosis was 3.4% (26) in BSP and 12.5% (22) in ARE balloons. Over the whole follow-up period, the risk rate of restenosis was lower in BSP compared ARE balloons (0.42 [0.26-0.68], HR [95% CI]). After adjustment the risk remained lower in the BSP group as compared to the ARE balloon group (0.39 [0.24-0.65], HR [95% CI]). The occurrences of restenosis are illustrated unadjusted in Figure 1 and adjusted in Figure 2.
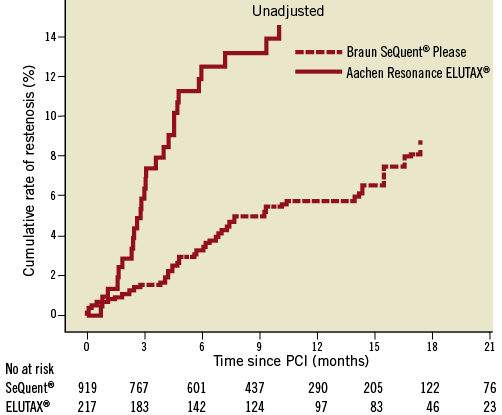
Figure 1. Cox regression analysis of the cumulative risk of restenosis in two different types of drug-eluting balloons. Unadjusted for the differences in background and procedure characteristics, the cumulative probability is calculated at the mean level of each covariate in the model.
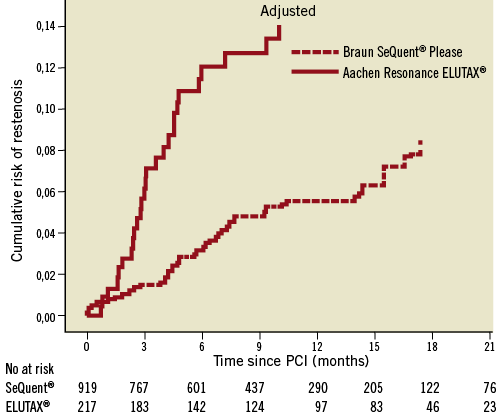
Figure 2. Cox regression analysis of the cumulative risk of restenosis in two different types of drug-eluting balloons. Adjusted for the differences in background and procedure characteristics, the cumulative probability is calculated at the mean level of each covariate in the model.
In the multivariable analysis the strength of the variables of restenosis is presented in a Forest plot (Figure 3).
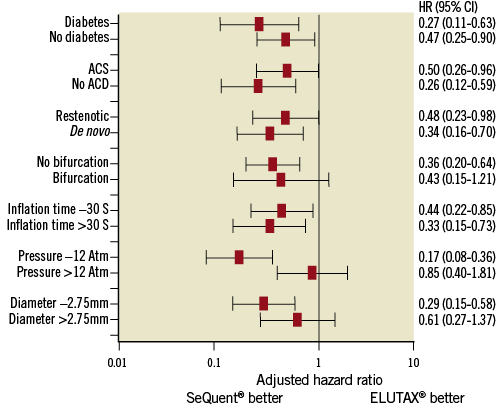
Figure 3. Forest plot showing the strength of different variables on the risk of restenosis at six months.
The risk of restenosis was numerically higher after dilatation with any DEB in a restenosis lesion versus a de novo lesion with an adjusted HR of 1.44 (0.83-2.52) (p=0.20) for a restenotic versus de novo lesion.
The difference in crude restenosis rate between the DEB types was similar for de novo and restenotic lesions at the index revascularisation procedure with an unadjusted HR of 0.46 (0.23-0.95) for the BSP balloon versus the ARE balloon in the restenotic group and 0.32 (0.15-0.66) in the de novo group. Due to the small number of events with the two types of DEB we were unable to perform adjusted subgroup analyses based on the type of stenosis at the index PCI procedure. However, in order to evaluate a possible differential effect between the two types of drug-coated balloons in de novo lesions and in restenosis lesions we performed an interaction test. There was no statistically significant interaction between the type of stenosis at the index procedure and the type of DEB (p=0.546).
Discussion
This hypothesis-generating registry study demonstrated a noticeable difference in terms of restenosis between two commonly used DEB. The BSP demonstrated much lower restenosis rates compared to the ARE balloon but the background characteristics and procedural data such as the number of de novo lesions, in-stent restenosis and bifurcations, as well as balloon length, were different and may in part account for the improved outcome of the BSP balloon. Despite the guideline-recommended use of drug-eluting balloons in restenosis lesions, approximately half of the balloons were used in de novo lesions in small coronary arteries. As expected, the overall risk of restenosis tended to be lower after dilatation in a de novo lesion than in a restenotic lesion. The different restenosis rates between the drug-eluting balloon types appeared to be consistent in de novo and restenosis lesions with a negative interaction test.
Our results may thus indicate different treatment outcomes between different DEB. This variation may in fact be even larger than for DES as the drug delivery to the vessel wall is crucial during balloon inflation and differs between manufacturers. Following multiple animal studies, instructions for use from the manufacturers recommend an inflation time of 30-60 seconds in order to deposit adequate amounts of paclitaxel in the vessel wall11. That almost no drug is found on the delivery balloon catheter after its use indicates that a significant amount has been released at least into the systemic circulation. The mean diameter of the treated arteries was smaller in patients treated with the ARE balloon compared to the BSP balloon and may have influenced the results, as vessel size correlates to restenosis rates, and because the magnitude of late loss is an independent predictor of the reference artery size12. Balloon diameter was however included in the multivariable adjustment model.
The crossover from DEB to include the use of a stent in the same segment was low (around 7% for both DEB). A similar rate of 5% was seen in another clinical trial13.
Current guidelines recommend the use of DEB to treat in-stent restenotic lesions because major adverse cardiac events (MACE), target lesion revascularisation (TLR) and restenosis rates have been shown to be significantly lower compared to ordinary bare balloons or Taxus® stents5,14. The in-stent restenosis indication for the use of the BSP balloon in our study was 55% compared to 35% for the ARE balloon, which may have affected the results. In other complex lesions such as bifurcations and chronic total occlusion (CTO), the ARE balloon was used more frequently than the BSP balloon. Other background characteristics known to influence the rate of restenosis and included in the model, such as gender, indication, angiographic findings, diabetes and previous coronary artery disease were similar.
As in all areas in PCI, constant improvement of methods and materials occurs on almost a day-to-day basis. Similarly, DEB are currently in a developing stage with expected improvements in coming generations. The difficulty to succeed with this new technology was exemplified recently in the comparison of the Dior® DEB (Eurocor GmbH., Bonn, Germany) vs. a Taxus® stent (Boston Scientific, Natick, MA, USA) were the DEB failed to show equivalence in angiographic endpoints in small coronary arteries15. As a real life observational registry, this study indicates that DEB do not behave equally with respect to efficacy. Therefore, current and improved DEB need to be evaluated and compared in prospective randomised head-to-head trials before entering the larger market.
In this hypothesis-generating, descriptive, real life registry, we found the overall risk of restenosis after six months was lower in a paclitaxel balloon using iopromide as a carrier (3.4%), compared with a paclitaxel-eluting balloon without any carrier (12.5%). The results of the present study suggest that there is no class effect for DEB and motivates further investigation in appropriately designed studies comparing DEB.
Limitations
In this relatively small observational study, large differences between the groups were observed, and there may be concealed confounders that could not be adjusted for. Also due to the size of the study even known confounders left limited possibility for adjustment in the model.
Another important factor impacting on the results was the reporting hospital in which the procedure (and probably also the follow-up was performed). Due to low numbers of events, this confounder could not be included in the statistical adjustment model. However, we tested whether hospitals that mainly used the ARE balloon also had a higher rate of restenosis in other settings such as in procedures with bare metal stenting (BMS). In six out of 29 hospitals, the majority of DEB used were ARE balloons (193 out of 242 DEB were ARE balloons). The rate of restenosis using BMS in these six hospitals during the follow-up time was lower than in the rest of the hospitals, which mainly used BSP balloons (0.75 [0.66-0.86], HR [95% CI]). Thus, the six hospitals using the ARE balloons had in general lower rates of restenosis in PCI using BMS, but when the same hospitals used the ARE balloon, restenosis rates were higher compared to hospitals predominantly using the BSP balloon. We interpret this as due to differences in the DEB and not as an operator-dependent factor.
Due to the retrospective nature of the study one needs to be cautious when comparing these two devices, as various lesion subsets have been included in the final analysis and it therefore remains unclear whether every subset accounts for the positive effect of the BSP. Angiographic follow-up was not conducted, which may make it difficult to compare the result with already conducted clinical trials based on angiographic follow-up. However, restenosis in the SCAAR registry represents clinically-relevant cases of restenosis, which makes the results clinically more applicable.
Conclusions
Our observational study from a large real world population suggests that there are major differences between two clinically available paclitaxel-eluting balloons. The findings furthermore suggest that there are no class effects for drug-eluting balloons and factors other than that the drug may be important for the clinical effect. Therefore new drug-coated balloons should be evaluated in prospective randomised trials to prove their performance, safety and efficacy before approval and widespread clinical use.
Conflict of interest statement
The authors have no conflicts of interest to declare.

