Abstract
Aims: Treatment of bare metal in-stent restenosis with the paclitaxel-coated balloon catheter based on the PACCOCATH® technology has yielded superior six-month angiographic and one-year clinical results compared to a paclitaxel-eluting stent. The three-year clinical follow-up is presented.
Methods and results: One hundred and thirty-one patients with coronary bare metal in-stent restenosis (>70%, length: <22 mm, vessel diameter: 2.5-3.5 mm) were randomly treated with the paclitaxel-coated balloon (DCB) (3 µg/mm²) or a paclitaxel-eluting stent (DES). Clinical follow-up information was requested from the patients and from their physicians. Quantitative angiographic and demographic baseline data were statistically not different between the groups. Per intention-to-treat analysis at 12 months, the lesion-related rates of major adverse cardiac events were 7.6% and 16.9% (p=0.11) while at 36 months the respective numbers were 9.1% and 18.5% (p=0.14). These differences were primarily due to reduced target lesion revascularisation (TLR) in DCB 4/66 (6.2%) compared to DES patients 10/65 (15.4%) (p=0.10). From 12 to 36 months, 1/65 (1.5%) DCB patients experienced a myocardial infarction while neither TLR nor death occurred in any study patient in either group during that period.
Conclusions: The six-month superiority of the paclitaxel-coated balloon compared to the paclitaxel-eluting stent in the treatment of bare metal coronary in-stent restenosis persisted throughout the three-year clinical follow-up period indicating stability of the lesions treated. (ClinicalTrials.gov Identifier: NCT00393315)
Introduction
Drug-coated balloon catheters (DCB) based on the PACCOCATH® technology (Bayer Schering Pharma AG, Berlin, Germany) have yielded good angiographic and clinical outcome of up to one year in indications such as coronary bifurcations and small vessel coronary artery disease1,2 and in the latter clinically up to three years3. In bare metal in-stent restenosis the DCB exhibited superior six-month angiographic and one-year clinical results compared to conventional balloon catheters and a paclitaxel-eluting stent in randomised controlled trials4,5. In the latter indication, drug-coated balloon catheters avoid the disadvantages associated with stenting such as adding an additional layer of metal to the vessel with the ensuing reduction in vessel flexibility and the prolonged need for dual antiplatelet therapy to reduce the occurrence of late thrombotic events6-9. Two-year and the recently published five-year clinical data with a predicate device confirmed the good one-year outcome with only one target lesion revascularisation being reported in the coated balloon group10,11. Since late complications such as thromboses may occur during longer follow-up with drug-eluting stents, the PEPCAD II ISR study was designed to analyse the clinical outcome of up to three years following the index procedure with either the second-generation paclitaxel-coated SeQuent® Please balloon catheter (B. Braun Melsungen AG, Vascular Systems, Berlin, Germany) or with the paclitaxel-eluting TAXUS® Liberté® stent (Boston Scientific, Natick, MA, USA) in the treatment of bare metal coronary in-stent restenosis.
Methods
The methods have been described in detail previously5.
STUDY DESIGN
The study was a randomised, non-blinded trial conducted at ten German cardiology departments5 (see Appendix for participants). The study was sponsored by B. Braun Melsungen AG, Vascular Systems, Berlin, Germany, the manufacturer of the drug-coated balloon catheter. The sponsor had a role in the design of the study, but not in the analysis of the results, the decision to publish, nor in the preparation of the manuscript. An independent clinical research organisation and core lab took responsibility for the accuracy and completeness of the data.
The study was performed according to the Declaration of Helsinki and World Health Organization guidelines and approved by the centres’ ethics committees. The requirements of sections 20 to 22 of the German Medical Device Law and of the European standard EN 540 were followed. Patients provided written informed consent prior to enrolment.
Eligible patients had clinical evidence of stable or unstable angina or evidence of ischaemia, and exhibited a single bare metal in-stent restenosis of any type. Exclusion criteria comprised factors such as acute myocardial infarction within the preceding 48 hours, severe renal insufficiency (GFR <30 ml/min), hypersensitivity or contraindication to long-term anti-aggregation, and a life expectancy of less than three years. Angiographic exclusion criteria encompassed stented segments longer than 22 mm, vessel diameters below 2.5 mm, stenoses below 70% of the luminal diameter, unprotected left main stenosis, and stents covering a major side branch (>2 mm).
STUDY DEVICES
The drug-coated coronary angioplasty balloon catheter (SeQuent® Please; B. Braun Melsungen AG) is covered with 3 µg paclitaxel/mm2 of balloon surface area with a more than 90% drug release upon single balloon inflation12. The balloons used were 17 mm to 30 mm long with diameters ranging from 2.5 mm to 3.5 mm. Patients in the control group were treated with the paclitaxel-coated TAXUS® Liberté® drug-eluting stent 16 mm to 28 mm long with diameters from 2.5 mm to 3.5 mm (Boston Scientific).
INTERVENTIONAL PROCEDURE
Patients were administered 250 mg of aspirin IV, heparin as an initial bolus of 70-200 IU/kg body weight adjusted according to the activated clotting time with a target of 200 to 250 seconds, and a loading dose of 300 mg of clopidogrel the day prior to the procedure or 600 mg immediately before the intervention. Glycoprotein IIb/IIIa antagonists were administered at the operator’s discretion. Intracoronary injection of 100 µg to 200 μg nitroglycerine preceded baseline angiography. Eligible patients were randomised by envelope to either of the treatment options.
Predilation of the target lesion was recommended for both groups using a conventional balloon. The recommended inflation time for the drug-coated balloon was ≥30 seconds13. Post procedure, the vascular sheaths were removed according to usual hospital practice.
QUANTITATIVE CORONARY ANGIOGRAPHY
Angiography was performed at the index procedure, at six months, and when clinically indicated. Automated quantitative analysis of the coronary angiographic images was performed by an independent core laboratory (Clinical Research Institute, Rotenburg/Fulda, Germany) by two operators using the CAAS II system (Pie Medical, Maastricht, The Netherlands). Manual adjustments corrected for obvious cases of machine error. Restenosis was defined as a diameter stenosis of ≥50%.
FOLLOW-UP AND ENDPOINTS
All patients received ≤100 mg aspirin daily for life. Clopidogrel (75 mg/day) was administered for three months following stand-alone drug-coated balloon angioplasty and for six months after drug-eluting stent deployment. Patients underwent clinical observation for a total of 36 months. All endpoints and adverse events were adjudicated by an independent clinical events committee.
Late lumen loss was the primary endpoint assessed at scheduled six-month or any clinically driven follow-up before that time point. Secondary endpoints encompassed the three-year combined clinical event rate, stent thrombosis, target lesion revascularisation, myocardial infarction, and death. Stent thrombosis was defined according to the ARC14. Revascularisation was based on symptoms and angiographic findings. Myocardial infarction was assumed to have occurred if at least two of the following five criteria were present: chest pain lasting longer than 30 minutes; electrocardiography (ECG) diagnostic of acute myocardial infarction (ST-segment elevation of ≥0.1 mV in at least two adjacent ECG leads or the new occurrence of a complete left bundle branch block); increase in the level of creatine kinase or its MB isoform of at least three times the upper normal limit; new, clinically significant Q-waves; and chest pain necessitating angiography up to six hours after the onset of the pain, with angiographic evidence of an occluded vessel. Serious adverse events were defined according to international (ICH) guidelines14.
STATISTICAL ANALYSIS
It was estimated that enrolment of 130 patients would be needed for the study to achieve a statistical power of 90% for a difference in the primary endpoint. Estimates of late lumen loss for this power calculation were based on data from trials of paclitaxel-coated stents and drug-coated balloons for treatment of bare metal in-stent restenosis4,15,16.
The intention-to-treat analysis describes the outcome of the procedures with the products whereas as-treated analysis assesses the performance of the study products. Following testing for normal distribution using the Kolmogorov-Smirnov test, continuous variables are expressed as mean±standard deviation. Categorical variables were compared with the Fisher´s exact test, continuous variables with the two-sided Student’s t-test or the Welch’s test for unequal variances. Confidence intervals for the difference between proportions were calculated with a normal approximation of the binomial distribution with correction for continuity (software: StatView 5.0 and Bias 8.05). Event-free survival was compared by Kaplan-Meier analysis with the Mantel-Cox log-rank test constructed by SPSS software, version 15.0 (SPSS Inc., Chicago, IL, USA). P-values of <0.050 were considered statistically significant.
Results
PATIENTS
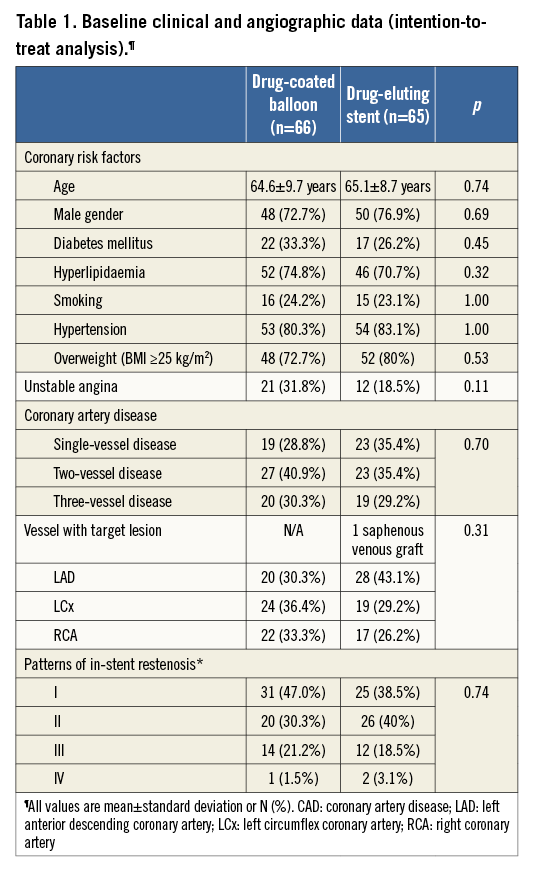
Of the 131 patients (64.9±9.2 years, 75% male) enrolled in the study between January and December 2006, 66 (50.4%) were randomly assigned to drug-coated balloon and 65 (49.6%) to drug-eluting stent treatment. Baseline characteristics of the patients were statistically not different between the two groups (Table 1).
ANGIOPLASTY
Procedural data were statistically not different between the two groups. In the drug-coated balloon group, five patients required additional bare metal stent deployment following dissections. Details are given in Table 2.
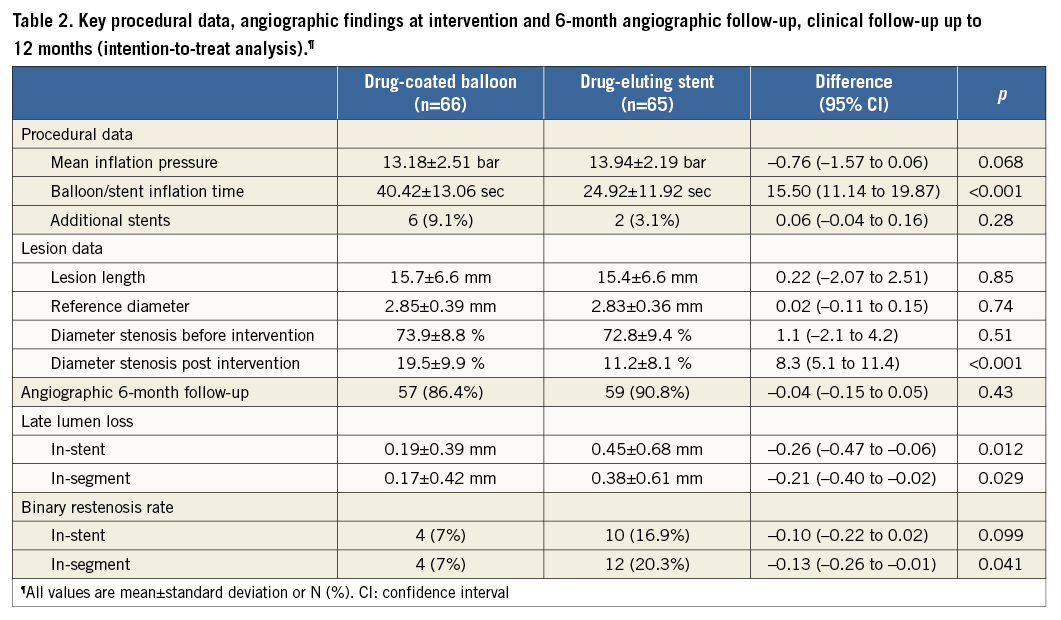
Predilation was performed in 62/66 (93.9%) patients treated with the drug-coated balloon and in 49/65 (75.4%) patients of the DES group. In the latter, two patients required an additional TAXUS® stent for dissection. In all patients, the DCB crossed the lesion while the TAXUS® stent failed in 5/65 (7.7%) patients despite predilation with a conventional balloon. One patient was excluded from the as-treated analysis for erroneously not being treated with a paclitaxel-eluting device. Since four patients in whom the DES failed to cross the lesion were treated with the DCB, both the intention-to-treat and the as-treated analyses will be presented.
ANGIOGRAPHIC FOLLOW-UP
Follow-up angiography after 6.1±1.1 months in 116/131 (89%) patients showed a superior outcome in the DCB group compared to DES in mean in-segment late lumen loss of 0.17±0.42 mm (median [IQR, 25th, 75th percentile] 0.09 [0.42,-0.08, 0.34] mm) vs. 0.38±0.61 mm (median [IQR, 25th, 75th percentile] 0.24 [0.55, 0.00, 0.55] mm) (p=0.032) while the difference in restenosis was borderline (p=0.06) with 4/57 (7%) in the DCB group compared to 12/59 (20.3%) in the DES group.
In the as-treated analysis, all DCB crossed the lesion while four DES failed. In the drug-coated balloon group the mean in-segment late lumen loss (0.18±0.41 mm) was significantly (p=0.04) lower compared with the drug-eluting stent group (0.39±0.63 mm). The advantage of the drug-coated balloon was also reflected by the significantly (p=0.05) lower binary in-segment restenosis rate (6.7% vs. 20.4%). The details were published previously5.
CLINICAL FOLLOW-UP
After one year, for clinical follow-up 65/66 (98.5%) patients were available in the DCB group and 63/65 (96.9%) in the DES group (p=0.62), whereas after three years 65/66 (98.5%) patients were available in the DCB group and 61/65 (93.8%) in the DES group (p=0.21).
During the index procedure one myocardial infarction occurred in the drug-eluting stent group due to a side branch occlusion, while no serious adverse occurred when the drug-coated balloon was used (p=0.50).
Four patients in the drug-coated balloon group died of non-cardiac causes, which were renal failure in two and one each from peritoneal carcinoma and septic shock. Four patients in the drug-eluting stent group died, one each from gastric carcinoma, renal failure, a car accident, and from an accident followed by drowning.
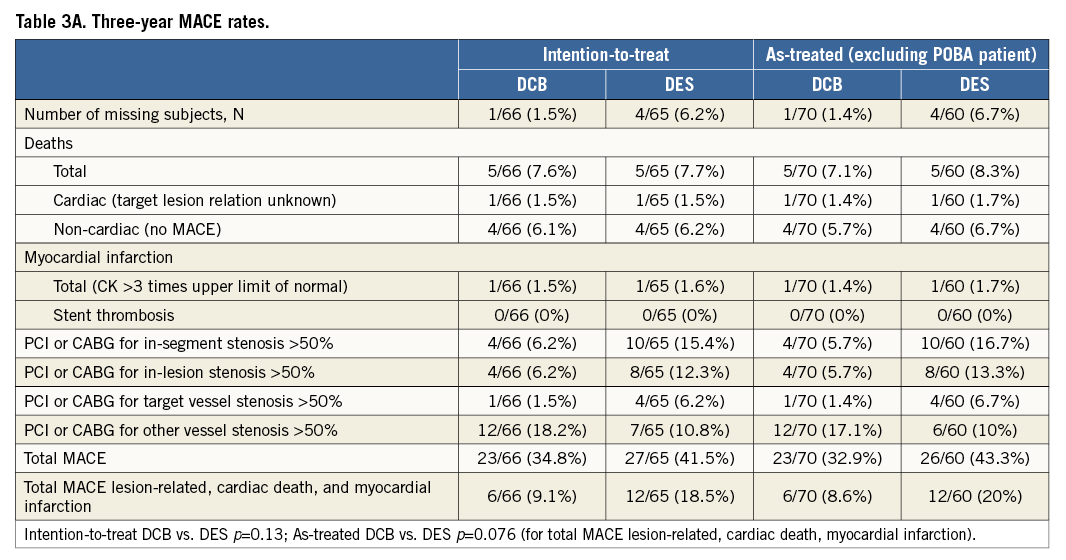
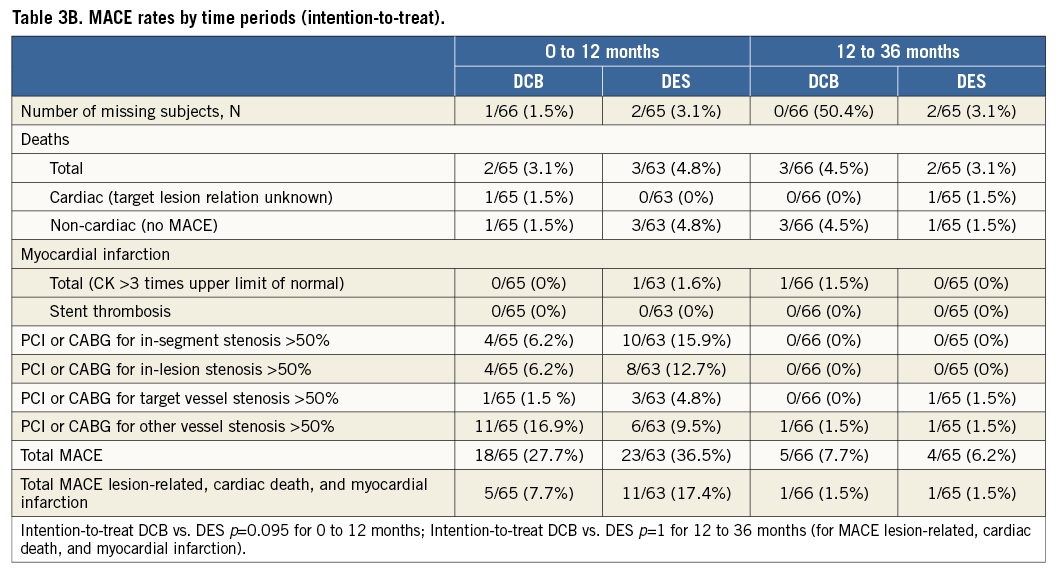
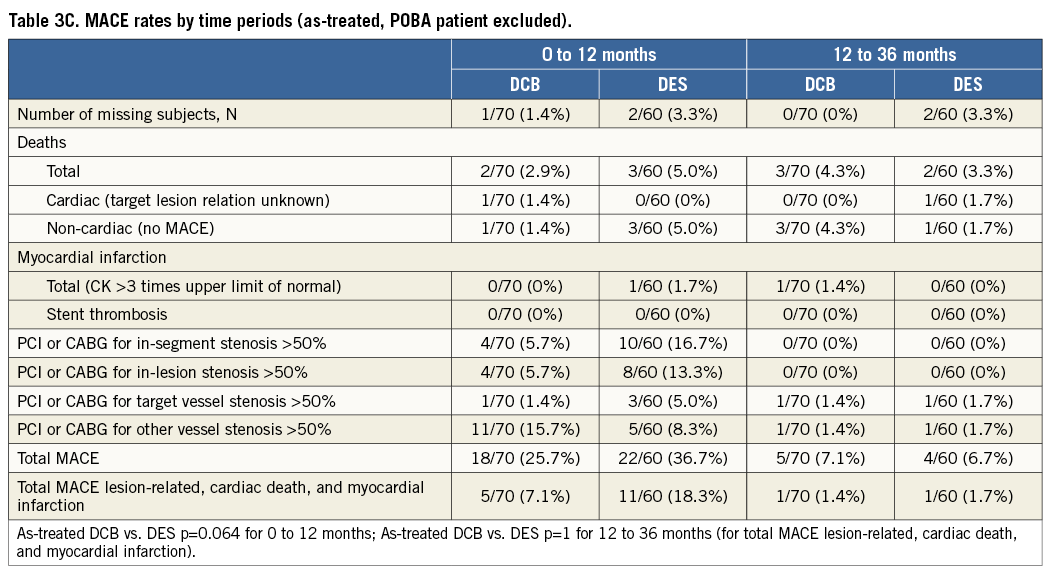
The Kaplan-Meier estimates of survival free from clinical events during the three-year follow-up are shown in Figure 1A and Figure 1B. The differences in event rates were still mainly driven by target lesion revascularisation at scheduled (after six months) and clinically driven angiographic follow-up during the follow-up period. After three years, major adverse cardiac events were twice as high in the drug-eluting stent group compared to the drug-coated balloon group (intention-to-treat 18.5% vs. 9.1%, as-treated 20% vs. 8.6%) with corresponding p-values of p=0.13 and p=0.076, respectively (Table 3A). During the second and third year of follow-up one MACE occurred in each treatment group (intention-to-treat: 1.5% in each group; as-treated: DCB 1.4%, DES 1.7%) (Table 3B and Table 3C).
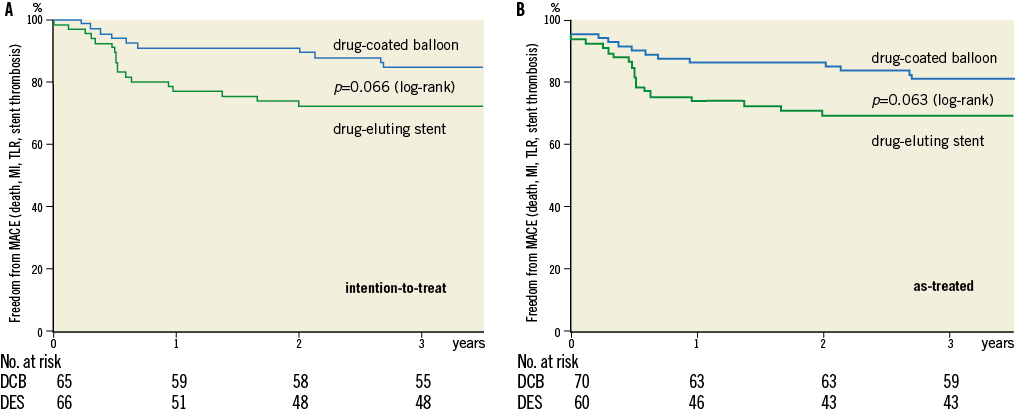
Figure 1. Freedom from stent thrombosis, target lesion revascularisation, myocardial infarction, and death. Log-rank (Mantel-Cox). Intention-to-treat analysis (n=131). As-treated analysis (n=130).
ANTIPLATELET THERAPY DURING FOLLOW-UP
Per study design at six months (p<0.001) and at 12 months (p=0.003), significantly more patients in the DES group were on clopidogrel compared to the patients from the DEB group, whereas at one and three years there was no difference between the treatment groups with dual antiplatelet therapy reaching the level of significance (p<0.001) only after six months (Figure 2A and Figure 2B).
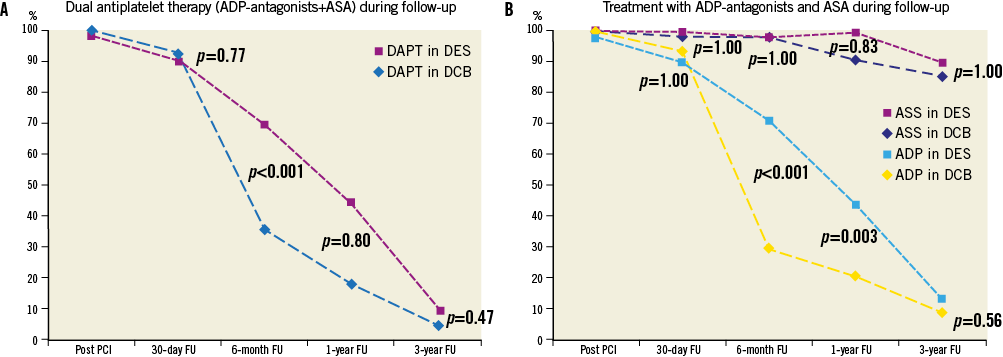
Figure 2. Antiplatelet therapy during the 3-year follow-up. Adherence to dual antiplatelet therapy. Adherence to aspirin and ADP-antagonist separately.
Discussion
Current understanding perceives the development of restenosis after the deployment of coated or uncoated stents as a continuous process instigated through mechanical irritation by the foreign body and the physicochemical reactions between the stent material and the vascular wall leading to sustained neointimal proliferation. Hence, it is assumed that prolonged local drug administration is necessary for effective inhibition of this slow and continuous process that is most effectively accomplished by sustained drug delivery to the vessel wall via a drug-eluting stent. Drug delivery to the arterial wall using any DES platform, however, is inhomogeneous17, since about 85% of the stented vessel wall area is not covered by the stent struts, with ensuing low tissue concentrations of the antiproliferative agent in these areas. Therefore, high drug concentrations on the stent struts are mandatory to achieve antirestenotic efficacy in these areas18 at the cost of delayed and incomplete endothelialisation of the stent struts that increases the susceptibility of thrombosis19. Along this line, the polymeric matrices on the stent embedding the antiproliferative drug are prone to inducing inflammation and thrombosis20. However, despite the fact that to date no stent thromboses have been reported when the drug-coated balloon has been used as a stand-alone device, the relatively limited data do not yet allow a comparison with the low thrombosis rates of the newer drug-eluting stents and CoCr stents21.
Among other catheter-based local drug delivery approaches for restenosis inhibition such as double-balloon catheters22,23 and porous balloons24,25, only the drug-coated balloons based on the PACCOCATH® technology have yielded angiographic and clinical results superior to drug-eluting stents in bare metal in-stent restenosis4,10,11 and trended superior in small vessel coronary artery disease1, and in the side branches of coronary bifurcations2. All of these studies share low neointimal proliferation of below 0.2 mm six months after the intervention when the balloon is used as a stand-alone procedure. The present study fosters the predicate data with in-segment neointimal proliferation of 0.17±0.42 mm, thus being significantly (p=0.03) lower than the 0.38±61 mm with the DES.
Longer clinical follow-up studies with this technology revealed the need for target lesion revascularisation in 1/54 (1.9%) bare metal in-stent restenoses treated with the DCB during the period of 12-24 months post PCI as opposed to none in the conventional balloon patients, leaving a significant difference for total major adverse cardiac events (MACE) of 7% vs. 40% in favour of the DCB10. MACE remained significantly (p=0.009) lower when comparing the drug-coated balloon (27.8%) with conventional balloons in bare metal in-stent-restenosis (59.3%) throughout the five-year follow-up period11.
The results of the current work echo the previous data which indicate target lesion stability from 12-36 months with one non-target lesion-related ST-elevation myocardial infarction (CK 3 x the upper normal) occurring in the DCB group, similar to one target lesion revascularisation in the DES patients. The resulting target lesion-related MACE rates were less than half in the DCB patients compared with those treated with the DES in the intention-to-treat (6/66 [9.1%] vs. 12/65 [18.5%], p=0.13) and as-treated analysis (6/70 [8.6%] vs. 12/60 [20%], p=0.076). These data may be explained by the fact that endothelialisation of the lesion has been shown to be complete after 28 days in all 36 porcine coronary vessels treated with the DCB13. These differences in favour of the DCB were observed despite the shorter clopidogrel administration of three months in DCB patients as opposed to six months in the DES group, the standard therapeutic regimen at the time of study initiation. In the PACCOCATH study, clopidogrel was administered for only four weeks4. Hence, the present clinical data are not indicative of the late catch-up phenomenon that is observed with drug-eluting stents26,27. As the catch-up phenomenon appears to occur earlier in paclitaxel-eluting devices than with sirolimus-coated stents26,28, it could have been expected during the three-year observation period of this study. Moreover, as compared with drug-eluting stents, drug-coated balloons do not expose the treated vessels to long-term irritation through metal, polymers, and delayed drug release, all of which present possible pathomechanisms for late catch-up29. However, as clinical follow-up is less sensitive than angiographic control, a definite statement on late catch-up warrants mandatory angiographic follow-up of at least three years in a larger number of patients. Given the good clinical results, routine angiographic follow-up for the assessment of neointimal proliferation may be hard to justify.
Limitations
Limitations of this study encompass the preponderance of type I or II patterns of in-stent restenosis that are per se associated with favourable outcomes. However, since lesion severity is statistically not different between the two groups, no effect on the outcome difference is expected. Another limitation relates to the treatment of four patients in the drug-eluting stent group with the drug-coated balloon resulting in an advantage for the stent group. In the as-treated analysis the coated balloon group suffered from a systematic shift of difficult-to-treat patients from the drug-eluting stent group to the coated balloon group. The superiority of the angiographic and clinical outcomes is based on the comparison with a drug-eluting stent, the technology of which has undergone some advancement since the start of this trial and, thus, the results may not be transferred to more recently developed stents. Also, the data must not be transferred to the treatment of drug-eluting stent in-stent restenosis, which seems to be pathologically distinct from those in bare metal stents30. Finally, the study was not designed to detect significant differences in MACE and, thus, not powered accordingly.
Conclusions
Overall, the present data show no indication that treating bare metal in-stent restenosis with the drug-coated balloon might be associated with late catch-up. Along with no occurrence of unexpected adverse events, it is suggested that bare metal in-stent restenoses treated with the drug-coated balloon based on the PACCOCATH® technology remain stable during the three years following the procedure.
In conclusion, this study suggests that using the drug-coated balloon catheter based on the PACCOCATH® technology for the treatment of bare metal in-stent restenosis and reducing the time period for dual antiplatelet therapy is clinically superior to deploying a drug-eluting stent.
| Impact on daily practice The evaluation of intracoronary devices and procedures requires clinical follow-up periods of at least three years, as late catch-up has been reported. The drug-coated balloon based on the PACCOCATH technology establishes its critical advantage over a stent coated with the same drug, primarily during the first six months. This benefit is maintained over the three-year follow-up period. In patients with bare metal in-stent restenosis this device may be used universally, based on its superior angiographic and clinical success and easy handling. Moreover, in patients at high bleeding risk, the shorter antiplatelet therapy compared to drug-eluting stents will reduce potential adverse events. |
Appendix
PEPCAD II STUDY GROUP
Principal Investigator: Martin Unverdorben, Institut für Klinische Forschung, Herz- und Kreislaufzentrum, Rotenburg an der Fulda, Germany
CRO and Angiographic Core Lab: Ralf Degenhardt, Institut für Klinische Forschung, Herz- und Kreislaufzentrum, Rotenburg an der Fulda, Germany. Staff: Tina Iffland, Melanie Häußler
Statistical Advisor: Hanns Ackermann, Zentrum für medizinische Informatik, Abteilung für Biomathematik, Universität Frankfurt/Main, Frankfurt/Main, Germany
Kardiologische Klinik, Herz- und Kreislaufzentrum, Rotenburg an der Fulda, Germany: Christian Vallbracht, Manfred Scholz, Henning Köhler, Bernd Abt, Eberhard Wagner; 40 patients
Klinik für Innere Medizin III, Universitätsklinikum des Saarlandes, Homburg/Saar, Germany: Bruno Scheller, Bodo Cremers, Michael Kindermann, Michael Böhm; Nicole Hollinger, Bianca Werner; 37 patients
Medizinische Klinik, Kardiologie, St. Johannes Hospital, Dortmund, Germany: Hubertus Heuer, Norbert Schulze Waltrup, Joachim Weber-Albers, Maritta Marks, Axel Bünemann, Dietmar Schmitz, Mathias Stratmann; Martin Schulz, Claudia Rosendahl, Birgit Laschewski, Alexandra Thrun, Kathrin Euler, Ute Dieckheuer; 17 patients
Klinik und Poliklinik für Innere Medizin II, Universitätsklinikum Regensburg, Regensburg, Germany: Christian Hengstenberg, Andreas Jeron, Andreas Luchner, Daniel Griese, Kurt Debl, Stefan Weber, Roland Wensel; Katrin Pietzsch; 11 patients
Kerckhoff Klinik, Bad Nauheim, Germany: Christian Maikowski, Matthias Rau, Christian Hamm; 9 patients
Medizinische Klinik I, Klinikum Darmstadt, Darmstadt, Germany: Gerald Werner, Werner Jung; 5 patients
I. Medizinische Abteilung, Krankenhaus Bogenhausen, Munich, Germany: Diethmar Antoni, Markus Kasel; 4 patients
Klinik für Innere Medizin, Unfallkrankenhaus Berlin, Berlin, Germany: Franz. X. Kleber, Sascha Rux, Daniel Grund; Heike Bull; 4 patients
Medizinische Klinik mit Schwerpunkt Kardiologie, Campus Virchow-Klinikum, Universitätsklinikum Charité, Berlin, Germany: Wolfgang Bocksch, Martin Steeg; Katrin Dittkrist; 3 patients
Klinik für Kardiologie, Pneumologie und Angiologie, Klinikum Esslingen, Esslingen, Germany: Matthias Leschke, Jean Rieber; Birgit Blaich; 1 patient
Funding
The study was supported by B. Braun Melsungen AG, Berlin, Germany.
Conflict of interest statement
B. Cremers reports obtaining lecture fees from B. Braun and Medtronic. B. Scheller reports having research contracts with AngioScore, B. Braun, and MDT; holding stock in InnoRa GmbH; and being named as co-inventor on several patent applications. M. Unverdorben worked for B. Braun for a limited time while conducting the study and has obtained lecture fees. R. Degenhardt is Head of the Clinical Research Institute that is paid for conducting the study. The other authors have no conflicts of interest to declare.

