Abstract
Aims: Although paclitaxel-coated balloon (PCB) angioplasty has been reported to be effective for in-stent restenosis (ISR) lesions, the optimal treatment for recurrent ISR lesions caused by PCB failure remains unclear. This study compared clinical and angiographic outcomes after everolimus-eluting stent (EES) implantation and repeat PCB angioplasty for PCB failure.
Methods and results: From November 2008 to July 2012, we performed PCB angioplasty for 599 ISR lesions, of which 93 recurrent ISR lesions underwent EES implantation (53 lesions, 52 patients) or repeat PCB angioplasty (40 lesions, 37 patients). The choice of treatment strategy was decided at the operator’s discretion. Angiographic outcomes were evaluated by follow-up angiography at six to eight months after procedure. The baseline characteristics were similar between the two groups. At follow-up angiography (93.5% of all lesions), minimum lumen diameter was significantly larger and the binary restenosis rate was significantly lower after EES implantation than after repeat PCB angioplasty (2.08±0.79 mm vs. 1.45±0.68 mm, p<0.001; 20.0% vs. 54.1%, p=0.001; respectively), whereas late lumen loss was not different between the two groups (0.49±0.62 mm vs. 0.59±0.74 mm, p=0.47). At two years, the incidences of both target lesion revascularisation (TLR) and clinically driven TLR were significantly lower after EES implantation than after repeat PCB angioplasty (17.9% vs. 57.5%, p=0.001; 5.9% vs. 18.1%, p=0.01; respectively).
Conclusions: EES implantation was more effective for PCB failure in preventing subsequent TLR than repeat PCB angioplasty because of better angiographic results.
Introduction
In-stent restenosis (ISR) has been an important clinical problem in percutaneous coronary intervention (PCI) with stents. Although the optimal management of ISR lesions remains to be established, drug-eluting stent (DES) implantation has been reported to be effective1-5. Several randomised trials have recently shown that paclitaxel-coated balloon (PCB) angioplasty was superior to uncoated balloon angioplasty in the treatment of both bare metal stent and DES restenosis6-11. Furthermore, PCB angioplasty was equivalent to repeat DES implantation for the treatment of DES ISR lesions11. However, PCB failure has emerged as recurrent ISR after PCB angioplasty for ISR lesions. Currently, there are no data available to evaluate PCI for PCB failure, in particular regarding whether DES implantation or repeat PCB angioplasty is better for PCB failure.
In this study, we compared the clinical and angiographic outcomes of the implantation of an everolimus-eluting stent (EES), the most widely used DES, with those of repeat PCB angioplasty for the treatment of ISR lesions resulting from PCB failure.
Methods
STUDY POPULATION
From November 2008 to July 2012, 1,744 consecutive ISR lesions were treated with PCI, of which 599 were treated with PCB angioplasty. Among these lesions, 121 recurrent ISR lesions after PCB angioplasty were treated with PCI. We enrolled 89 patients with 93 lesions who underwent EES implantation or repeat PCB angioplasty for PCB failure in this single-centre study after excluding 28 lesions which were treated with other modalities. There were 52 patients with 53 lesions treated with EES implantation (EES group) and 37 patients with 40 lesions treated with repeat PCB angioplasty (repeat PCB group). A study flow chart of this study is shown in Figure 1. All patients provided informed consent for the procedure and for subsequent data collection.
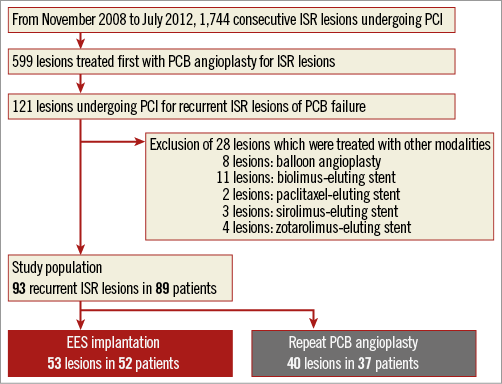
Figure 1. Study flow chart. EES: everolimus-eluting stent; ISR: in-stent restenosis; PCB: paclitaxel-coated balloon; PCI: percutaneous coronary intervention
TREATMENT STRATEGY
The choice of treatment strategy was at the operator’s discretion. A paclitaxel-coated PTCA balloon (SeQuent Please balloon catheter; B. Braun Melsungen AG, Vascular Systems, Berlin, Germany) was used as the PCB. The recommended inflation time for the PCB was at least 30 seconds. During PCI for PCB failure, predilatation was performed in all lesions. High-pressure balloon dilatation with a non-compliant balloon or scoring balloon dilatation using the Lacrosse NSE balloon catheter (Goodman Co., Ltd., Nagoya, Japan), the AngioSculpt® PTCA balloon catheter (AngioScore Inc., Fremont, CA, USA), or the Cutting Balloon catheter (Boston Scientific, Marlborough, MA, USA) was recommended at predilatation to obtain the optimal dilatation of restenotic lesions. Before PCI, loading doses of aspirin (200 mg) and clopidogrel (300 mg) were administered unless patients had previously received antiplatelet therapy. After PCI, only patients in the DES group were maintained on aspirin (100 mg once daily) and ticlopidine (200 mg twice daily) or clopidogrel (75 mg once daily) for at least one year. Patients in the repeat PCB group were maintained on aspirin and ticlopidine or clopidogrel for at least three months.
CLINICAL FOLLOW-UP
We collected clinical data on all-cause death, cardiac death, non-fatal myocardial infarction, stent thrombosis, and target lesion revascularisation (TLR). Clinical outcomes were evaluated at two years on a per patient basis. Clinical information was obtained either by reviewing hospital records or by telephone interviews with the patients, their family members, or their primary care physicians. Stent thrombosis was defined as definite/probable stent thrombosis according to the Academic Research Consortium definitions12. Myocardial infarction was defined as ischaemic symptoms and/or ischaemic change of electrocardiogram plus elevation of creatine kinase levels to twice the upper limit of normal, with a rise in creatine kinase-MB fraction. TLR was defined as either repeat PCI or coronary bypass grafting for restenosis or thrombosis of the target lesion that included the proximal and distal edge segments in coronary angiography. Clinically driven TLR was defined as TLR with symptoms or objective signs of ischaemia by functional assessment.
ANGIOGRAPHIC FOLLOW-UP
Follow-up coronary angiography was scheduled at six to eight months after PCI for PCB failure, but was performed earlier if ischaemia was clinically indicated. Patients who underwent unscheduled follow-up angiography for clinical reasons within one year were included for serial angiographic analysis. Quantitative coronary angiographic analysis was performed using QCA-CMS (Medis Medical Imaging Systems, Leiden, The Netherlands). All angiograms were analysed in a random sequence by two experienced observers who were blinded to the clinical characteristics of the patients. Angiographic measurements were obtained in multiple views following intracoronary nitrate injection. Reference diameter, minimum lumen diameter (MLD), and diameter stenosis (DS) were measured before and after PCI. Acute gain was the difference between post-procedural MLD and pre-procedural MLD, and late lumen loss was the difference between post-procedural MLD and MLD at follow-up. Binary ISR was defined as DS >50% by quantitative coronary angiographic analysis. ISR was classified as focal (≤10 mm in length), diffuse (>10 mm in length), proliferative (>10 mm in length and extending outside the stent), or occlusive according to the Mehran classification13.
STATISTICAL ANALYSIS
Data are expressed as mean±standard deviation for continuous variables. Values are reported as numbers with relative percentage or standard deviation. Continuous values were compared using an unpaired Student’s t-test or Mann-Whitney U test, on the basis of the distribution. Categorical variables were compared using a chi-square test or Fisher’s exact test. A p-value of <0.05 was considered significant. The cumulative incidence of clinical events was estimated by the Kaplan-Meier method, and differences were assessed using the log-rank test. The IBM SPSS statistical software, Version 20 (IBM Corp., Armonk, NY, USA) was used for all statistical calculations.
Results
Baseline patient characteristics are shown in Table 1. The prevalence of diabetes was 50.0% in the EES group and 48.6% in the PCB group (p>0.99). The prevalence of haemodialysis was 11.5% in the EES group and 10.8% in the PCB group (p>0.99). Table 2 shows the baseline lesion and procedural characteristics. Intravascular ultrasound guidance was used during PCI for PCB failure in 84.9% of the EES group and 90.0% of the repeat PCB group (p>0.99). A scoring balloon was used in 41.5% of the EES group and 50.0% of the repeat PCB group (p=0.66). Focal lesions constituted 49.1% of lesions with PCB failure in the EES group, and 45.0% of those in the repeat PCB group (p=0.66). The serial restenotic pattern before and after index PCB angioplasty is shown in Figure 2. The restenotic patterns before index PCB angioplasty included 33 focal lesions, 46 diffuse lesions, seven proliferative lesions, and seven occlusive lesions. The restenotic patterns in PCB failure were 44 focal lesions, 37 diffuse lesions, eight proliferative lesions, and four occlusive lesions. The restenotic pattern improved in 28.0%, remained the same in 57.0%, and became worse in 15.0% of lesions after index PCB angioplasty. No additional stent was implanted after repeat PCB angioplasty for PCB failure.
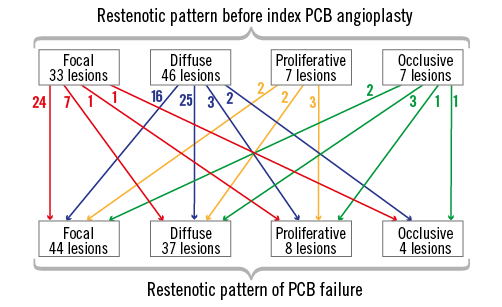
Figure 2. Serial restenotic patterns before and after index PCB angioplasty. PCB: paclitaxel-coated balloon
The serial angiographic results are shown in Table 3. There were no differences in the pre-procedural angiographic data between the two groups. Post-procedural DS was significantly smaller in the EES group (16.2±7.4%) than in the repeat PCB group (31.8±10.3%, p<0.001). Post-procedural MLD and acute gain were significantly larger in the EES group than in the PCB group (2.56±0.54 mm vs. 2.02±0.44 mm, p<0.001; 1.76±0.69 mm vs. 1.06±0.67 mm, p<0.001; respectively). Angiographic follow-up was available in 87 lesions (93.5%). The mean duration from procedure to follow-up angiography was 228±50 days after EES implantation and 227±111 days after repeat PCB angioplasty. At follow-up angiography, MLD was significantly larger and DS was significantly smaller in the EES group than in the repeat PCB group (2.08±0.79 mm vs. 1.45±0.68 mm, p<0.001; 16.2±7.4% vs. 51.6±20.6%, p<0.001; respectively), whereas late lumen loss was similar between the two groups (0.49±0.62 mm vs. 0.59±0.74 mm, p=0.47). Furthermore, the binary restenosis rate was significantly lower in the EES group (20.0%) than in the repeat PCB group (54.1%, p=0.001). We divided the lesions into focal (44) lesions and non-focal (49) lesions. The binary restenosis rate was similar between the focal and non-focal lesions in both EES (16.0% vs. 24.0%, p=0.73) and repeat PCB groups (56.3% vs. 52.4%, p>0.99) (p for interaction=0.50).
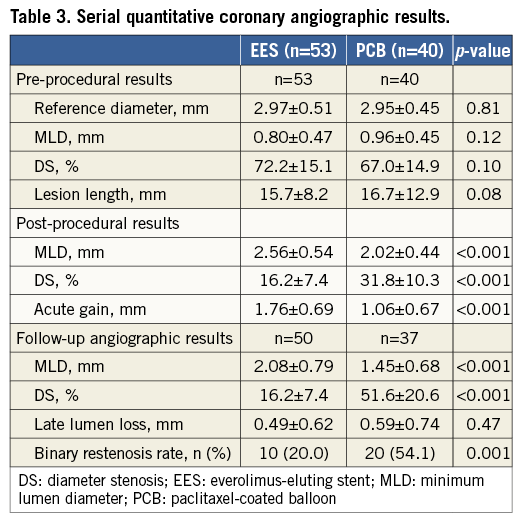
Clinical follow-up was performed on all patients, and the median clinical follow-up duration was 699 days (interquartile range 406 to 927 days). Table 4 shows the cumulative incidences of clinical outcomes at two years. The mortality rate was 8.4% in the EES group and 10.7% in the repeat PCB group (p=0.72) (Figure 3). Among six patients who died during the study period, cardiac death occurred in two patients, one due to sudden death after EES implantation and the other due to myocardial infarction after repeat PCB angioplasty. During the study period, myocardial infarction due to definite stent thrombosis occurred in one patient at 524 days after repeat PCB angioplasty for PCB failure. It occurred immediately after the surgical procedure with discontinuation of antiplatelet therapy. The cumulative incidence of TLR at two years was significantly lower in the EES group (17.9%) than in the PCB group (57.5%, p=0.001). Among 24 patients who had undergone TLR, two (8.3%) were for unstable angina (repeat PCB two), six (25.0%) for effort angina (EES two, repeat PCB four), one (4.2%) for silent ischaemia detected by non-invasive tests (repeat PCB one), and 15 (62.5%) for restenosis at follow-up angiography (EES five, repeat PCB 10). In addition, the incidence of clinically driven TLR was significantly lower in the EES group (5.9%) than in the PCB group (18.1%, p=0.01).
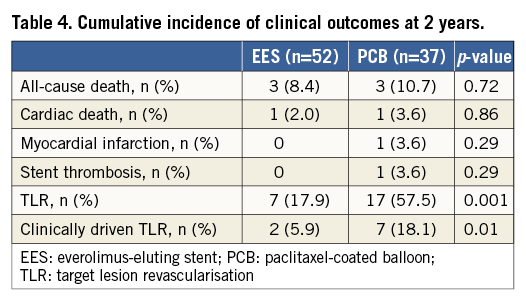
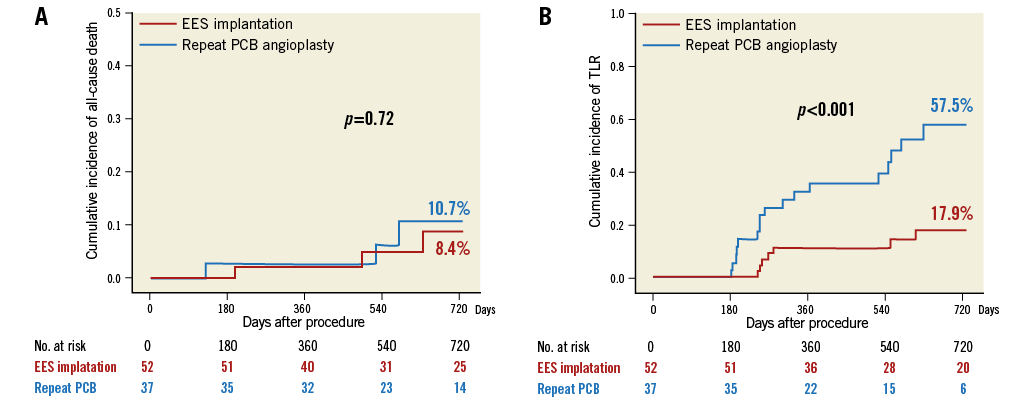
Figure 3. The Kaplan-Meier curves of clinical outcomes at two years after treatment for PCB failure of in-stent restenosis lesions. A) The cumulative incidence of all-cause death after EES implantation and repeat PCB angioplasty. B) The cumulative incidence of TLR after EES implantation and repeat PCB angioplasty. EES: everolimus-eluting stent; PCB: paclitaxel-coated balloon; TLR: target lesion revascularisation
Discussion
The main findings of this study were the following. 1) For PCB failure of ISR lesions, EES implantation was more effective in preventing angiographic restenosis than PCB angioplasty, whereas late lumen loss was similar between the two groups. 2) EES implantation for PCB failure had better clinical outcomes than repeat PCB angioplasty because of the lower TLR rate.
PCB angioplasty could be a standard treatment for ISR lesions because the similar outcomes were obtained without the need for an additional metal layer compared with DES implantation. With the expansion of PCB treatment, PCB failure has increased and has become a clinically important issue. Therefore, it is necessary to investigate the optimal treatment for PCB failure of ISR lesions. This study was the first report to evaluate the clinical and angiographic outcomes after PCI for PCB failure, although it was a non-randomised comparison of the treatment selection. The binary restenosis rate after PCI for PCB failure was 20.0% in the EES group and 54.1% in the repeat PCB group. Previous studies have reported that the restenosis rate after the first PCB angioplasty for DES ISR lesions was 4 to 17%8-11. Repeat PCB angioplasty for PCB failure had a much higher restenosis rate than the first PCB angioplasty for DES ISR lesions. Late lumen loss in the repeat PCB group was 0.59 mm in this study, which also tended to be large compared with the first PCB angioplasty8-11. Repeat PCB may not provide the reduction in neointimal proliferation that has been shown by PCB for ISR lesions14. The lower efficacy of the repeat PCB would be associated with the difference in angiographic results between EES implantation and repeat PCB angioplasty. A different approach from that selected for the first ISR lesion would be recommended for the lesions with PCB failure.
The concept of drug resistance to paclitaxel has been previously reported15. In fact, previous studies have focused on a different DES approach for DES ISR lesions16-22. Among these studies, some reported that implantation of a different type of DES for DES ISR lesions had a more favourable outcome than implantation of the same type of DES, supporting a role of drug resistance in restenosis18,20-22. This factor may affect the poorer response of repeat PCB for lesions with PCB failure.
In the current study, EES implantation had much higher acute gain than repeat PCB angioplasty. Only with balloon angioplasty would it be difficult to obtain a sufficient lumen area, even after using high-pressure balloon dilatation or scoring balloon dilatation. Therefore, additional stenting may be necessary to obtain the optimal dilatation. Generally, late lumen loss is not a suitable endpoint to make a comparison between stenting and balloon angioplasty because additional stenting with high acute gain tends to increase late lumen loss11. In a previous study comparing stenting with balloon angioplasty for recurrent ISR lesions, late lumen loss was similar, and acute gain was an important factor leading to favourable angiographic results of stenting23. Similarly, in this study, because late lumen loss was similar between the two groups, larger acute gain by EES implantation may also provide better angiographic results than repeat PCB angioplasty. PCB was reported to be associated with a lower TLR rate than EES for initial DES ISR lesions24. In this previous study, despite the similar pre-procedural angiographic data, post-procedural MLD was also similar between the two groups. Therefore, no mechanical advantage of additional stenting may lead to the inferiority of EES. On the other hand, the RIBS V trial showed that EES and PCB for bare metal stent restenosis lesions provided similar rates of clinical and angiographic recurrences25. However, EES had better late angiographic results compared with PCB, probably because of larger acute gain and similar late lumen loss. This superiority of EES in the RIBS V trial was consistent with our study. Furthermore, in recurrent ISR lesions after PCB angioplasty compared with initial ISR lesions, optimal acute results of EES may be more likely to provide favourable angiographic outcomes. Because this mechanical advantage of larger acute gain is common to all DES, DES implantation may generally show more angiographic benefits to PCB failure lesions compared with PCB angioplasty.
In the treatment of PCB failure, the more favourable clinical outcomes after EES implantation, compared with repeat PCB angioplasty, were driven by the lower TLR rate. The current study showed that EES implantation also reduced clinically driven TLR compared with repeat PCB angioplasty. Several large registries have shown that repeat DES implantation is safe and does not increase adverse cardiac events, including stent thrombosis4,26, but the adverse events after PCI for PCB failure have not been investigated. Despite the relatively small population of our study, no stent thrombosis or myocardial infarction was observed after EES implantation for PCB failure. From the results of this study, EES implantation is attractive for PCB failure of ISR lesions considering adverse cardiac events. However, re-recurrent restenosis is a further problem after EES implantation for PCB failure. To keep implanting additional metal layers may not be routinely advocated in terms of multiple metal layers and a prolonged dual antiplatelet therapy27. If restenosis recurs after PCB angioplasty for an ISR lesion, PCB angioplasty can be used multiple times without the necessity of additional stenting, and this point is the chief advantage of repeat PCB angioplasty. The best method for the treatment of PCB failure remains unclear. This study result is expected to be confirmed by randomised trials or larger prospective studies.
Limitations
The present study has several important limitations. First, this is a retrospective, observational, single-centre study. Second, selection of treatment strategies for PCB failure was not randomised and depended on the operator’s discretion. EES implantation had the potential to be performed preferentially for PCB failure lesions at the stent edge and those with stent fracture. Furthermore, repeat PCB angioplasty may be performed preferentially for long ISR lesions. Because a selection bias related to the treatment strategy cannot be adjusted, it may have influenced our study results. Third, because the angiographic follow-up rate was 93.5% in this study, not all lesions were included in the angiographic analysis. Fourth, there was the possibility of a type II error in the current analyses because of the limited number of patients with PCB failure. Finally, imaging analyses using intravascular ultrasound and optical coherence tomography were not performed. Because stent underexpansion may be an important mechanical factor in recurrent ISR lesions after PCB angioplasty, the assessment of stent underexpansion by intravascular imaging could be important to decide whether to implant an additional DES. However, we have no systematic data of intravascular imaging to evaluate the mechanical factors which may affect the recurrent ISR lesion.
Conclusions
EES implantation was more effective for PCB failure in preventing subsequent TLR than repeat PCB angioplasty because of better angiographic results.
| Impact on daily practice PCB has been an effective treatment for ISR lesions. Recurrent ISR lesions caused by PCB failure do not occur frequently, but these lesions sometimes become clinically important problems. The current study clarified that EES had the lower binary restenosis and TLR rate than repeat PCB in the treatment of PCB failure. These findings may be due to the suboptimal acute gain of PCB angioplasty compared with additional stent implantation. Although the advantage of PCB angioplasty is that it can be used multiple times without the necessity of additional metal layers, our study results would be helpful for considering the next optimal treatment for recurrent ISR lesions caused by PCB failure. |
Acknowledgements
We appreciate the secretarial assistance of Miho Kobayashi, Makiko Kanaike, and Yoshimi Sano.
Conflict of interest statement
The authors have no conflicts of interest to declare.

