Abstract
Aims: Although the outcomes of various treatments for in-stent restenosis (ISR) after drug-eluting stent (DES) implantation have been reported, the optimal treatment of recurrent ISR lesions after DES implantation for ISR lesions was unknown. This study compared the efficacy between DES implantation and balloon angioplasty (BA) for recurrent ISR lesions after DES implantation.
Methods and results: From 2003 to 2010, DES were implanted in 1,101 ISR lesions, of which 148 recurrent ISR lesions (142 patients) were treated with BA (76 lesions, 72 patients) and DES implantation (72 lesions, 70 patients). Clinical outcomes were evaluated for major adverse cardiac events (MACE), including a composite of death, myocardial infarction, and target lesion revascularisation (TLR). Angiographic outcomes were evaluated by follow-up angiography at six to eight months after procedure. At angiographic follow-up (94.4% of all patients), the binary restenosis rate was significantly lower in DES implantation (25.0%) than in BA (64.4%, p<0.001), whereas late lumen loss was similar between DES implantation and BA (0.80±0.78 mm vs. 0.87±0.79 mm, p=0.60). The incidence of four-year MACE was significantly higher in BA (75.2%) than in DES implantation (45.8%, p<0.001), mainly because of the lower TLR rate in DES implantation (60.5% vs. 27.6%, p<0.001). Multivariate analysis revealed that BA is an independent predictor of TLR, followed by non-focal lesion, non-intravascular ultrasound guidance, and dyslipidaemia.
Conclusions: In the treatment of recurrent ISR lesions, DES implantation is markedly more effective with a lower incidence of TLR compared to BA.
Introduction
Percutaneous coronary intervention (PCI) with drug-eluting stents (DES) dramatically reduced the rates of restenosis and target lesion revascularisation (TLR) as compared to bare metal stents1,2. For in-stent restenosis (ISR) lesions after bare metal stent implantation, DES implantation has been reported to have better clinical and angiographic outcomes than balloon angioplasty (BA) in some randomised trials3-5. Although the best management for DES ISR lesions has not been established, repeat DES implantation has been reported to be superior to BA in several studies6-10. Because of immediate feasibility and safety, DES implantation is currently a popular retreatment modality for DES restenosis. However, recurrent ISR after DES implantation for ISR lesions still occurs, and it has become a clinically important problem. There are no data available on the outcomes after PCI for recurrent ISR lesions after DES implantation for ISR lesions, because the circumstances requiring PCI for recurrent ISR lesions are infrequent.
In this study, we compared the clinical and angiographic outcomes between DES implantation and BA for recurrent ISR lesions after DES implantation for ISR lesions.
Methods
From December 2003 to June 2010, 1,101 lesions were treated with DES implantation for ISR lesions. Among these lesions, 169 recurrent ISR lesions were treated by either DES implantation or BA from June 2004 to December 2011. We excluded 21 lesions that had already been included in this study due to PCI for re-recurrent ISR lesions after DES implantation for recurrent ISR lesions. Therefore, 70 patients with 72 recurrent ISR lesions treated with DES implantation and 72 patients with 76 recurrent ISR lesions treated with BA were enrolled in this retrospective single-centre study. The flow chart in Figure 1 describes how patients were selected for this study. In 37 patients with 40 lesions, other treatment modalities were used during the study period. Cutting balloon was used in one patient (one lesion), bare metal stent in six patients (six lesions), and drug-eluting balloon in 30 patients (33 lesions). The first PCI was defined as a previous stent implantation before DES implantation for ISR lesions. The second PCI was defined as DES implantation for ISR lesions. The third PCI was defined as DES implantation or BA for recurrent ISR lesions after the second PCI. All patients provided informed consent for the procedure and subsequent data collection.
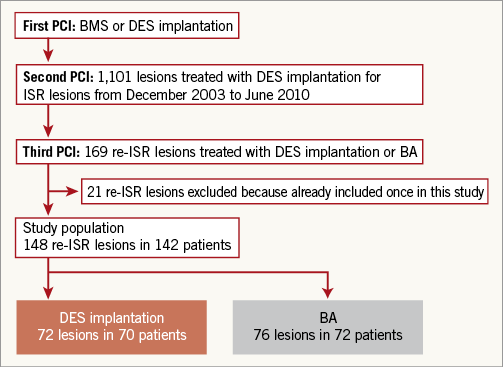
Figure 1. Study flow chart. BA: balloon angioplasty; BMS: bare metal stent; DES: drug-eluting stent; ISR: in-stent restenosis; re-ISR: recurrent in-stent restenosis
The choice of treatment strategy, including additional stent implantation and IVUS usage, was decided at the operator’s discretion. During PCI for ISR lesions, high-pressure balloon dilatation was recommended using a non-compliant balloon to obtain the optimal dilatation of stents. When implanting DES for ISR lesions, predilatations and post-dilatations were recommended. Before the PCI, patients were administered loading doses of aspirin (200 mg) and ticlopidine (400 mg) or clopidogrel (300 mg) unless they had previously received antiplatelet therapy. After PCI, only patients in the DES group were maintained on aspirin (100 mg once daily) and ticlopidine (200 mg twice daily) or clopidogrel (75 mg once daily) for at least one year, and patients in the BA group were maintained on aspirin and ticlopidine or clopidogrel from one to three months. Furthermore, we recommended the continuation of both aspirin and ticlopidine or clopidogrel for more than one year after sirolimus- and paclitaxel-eluting stent implantations to all patients with absence of the risk of bleeding.
We collected clinical data on all-cause death, cardiac death, non-fatal myocardial infarction, and TLR. Clinical outcomes were evaluated on a per-patient basis. Clinical information was obtained either by reviewing hospital records or by telephone interviews with the patients, their family members, or their primary care physicians. Major adverse cardiac events (MACE) were defined as a composite of all-cause death, non-fatal myocardial infarction (MI), and TLR. Stent thrombosis was defined according to the Academic Research Consortium definitions11. MI was defined as ischaemic symptoms and/or ischaemic change of electrocardiogram plus elevation of creatine kinase levels to twice the upper limit of normal, with a rise in creatine kinase-MB fraction. TLR was defined as either repeated PCI or coronary bypass grafting for restenosis or thrombosis of the target lesion that included the proximal and distal edge segments in coronary angiography.
Angiographic follow-up was scheduled at six to eight months after PCI by coronary angiography, but was performed earlier if ischaemia was clinically indicated. Patients who underwent unscheduled follow-up angiography within one year for clinical reasons were included for serial angiographic analysis. Quantitative coronary angiographic analysis was performed using QCA-CMS (Medis Medical Imaging Systems, Leiden, The Netherlands). All angiograms were analysed in a random sequence by two experienced observers who were blinded to the clinical characteristics of the patients. Angiographic measurements were obtained in multiple views following intracoronary nitrate injection. Reference diameter, minimum lumen diameter (MLD), and diameter stenosis were measured before and after PCI. Late lumen loss was the difference between postprocedural MLD and MLD at follow-up. ISR was defined as diameter stenosis >50% by quantitative coronary analysis. Focal ISR lesion was defined as <10 mm in length at the body and edge of the stent, and multifocal lesion was included in non-focal lesions12.
Data are expressed as mean ± standard deviation for continuous variables. Values are reported as numbers with relative percentage or standard deviation. Continuous values were compared with unpaired Student’s t-test or Mann-Whitney U test, on the basis of the distribution. Categorical variables were compared using a chi-square test or Fisher’s exact test. A p-value of <0.05 was considered significant. Predictors of clinical outcomes were assessed using a stepwise Cox proportional hazards model. First, a univariate analysis was performed. Next, variables with a probability value <0.10 were entered into a multivariate model. The cumulative incidence of clinical events was estimated by the Kaplan-Meier method, and differences were assessed using the log-rank test. The IBM SPSS statistical software version 20 (IBM Corp., Armonk, NY, USA) was used for all statistical calculations.
Results
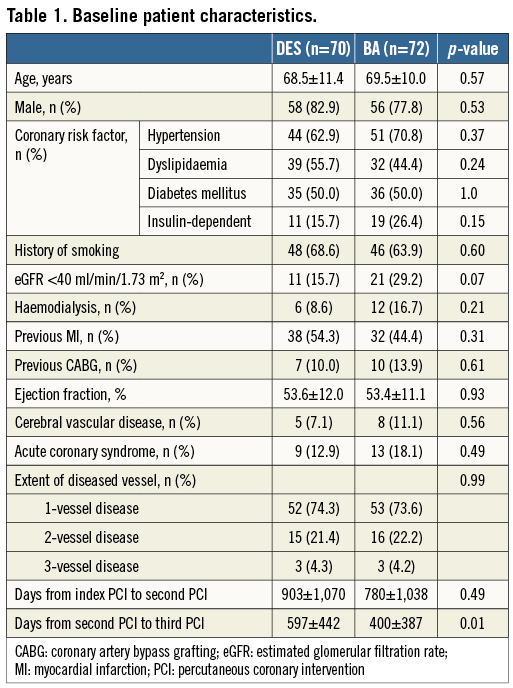
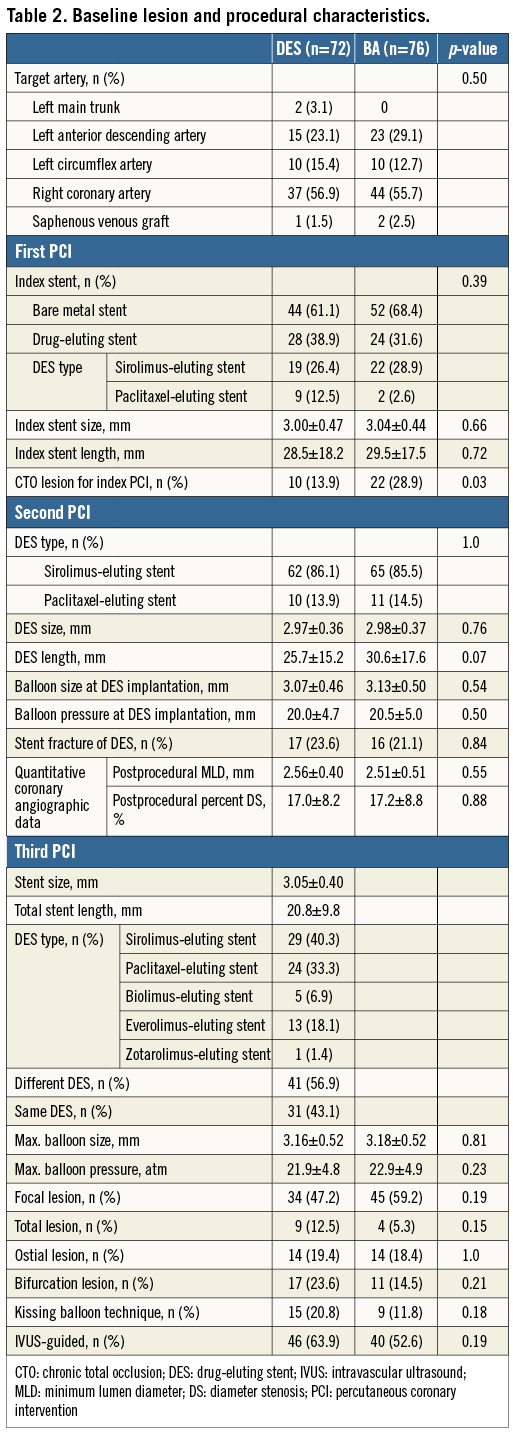
Baseline patient characteristics are shown in Table 1. There was no significant difference in the baseline patient characteristics between the DES and BA groups, except days from second to third PCI. The number of days from the second to the third PCI was significantly more in the DES group than in the BA group. Table 2 demonstrates baseline procedural and angiographic characteristics. In more than half of the patients in both groups, the target lesion was in the right coronary artery. The prevalence of chronic total occlusion at the first PCI was significantly lower in the DES group than in the BA group. Regarding the pre-procedural morphological pattern of the third PCI, the focal lesion was 47.2% in the DES group and 59.2% in the BA group. In DES implantation for recurrent ISR lesions, different DES implantation was performed in 41 lesions (56.9%). DES implantation was performed in 46 lesions (63.9%) and BA was performed in 40 lesions (52.6%) under intravascular ultrasound (IVUS) guidance. At the first PCI, bare metal stent implantation was performed for bare metal stent restenosis lesions in four lesions (5.6%) of the DES group, and seven lesions (9.7%) of the BA group (p=0.53). Therefore, the fourth stent was implanted in these lesions at the third PCI.
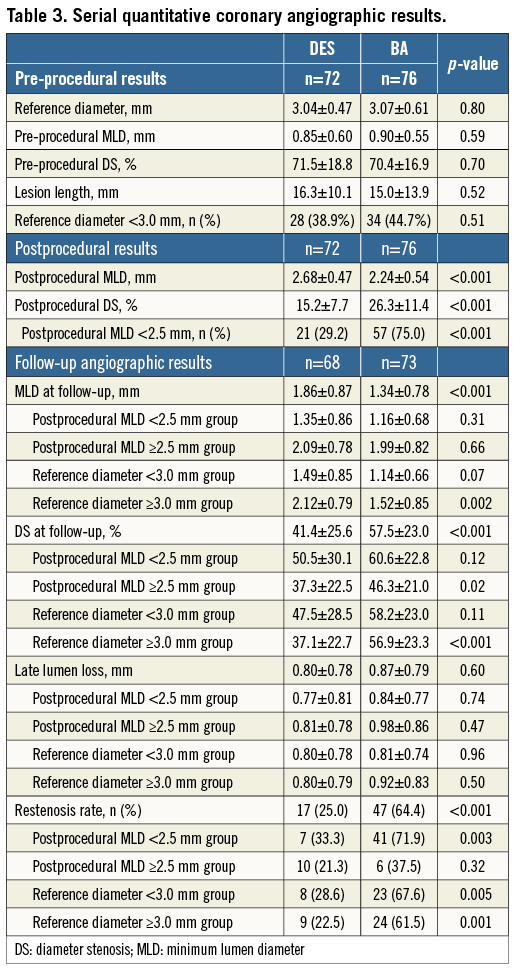
Serial angiographic results are shown in Table 3. Postprocedural MLD was larger in DES implantation than in BA. Postprocedural diameter stenosis was lower in DES implantation. Angiographic follow-up was available for 141 lesions (94.4%). MLD at follow-up angiography was significantly smaller in the BA group than in the DES group (1.34±0.78 mm vs. 1.86±0.87 mm, p<0.001), and diameter stenosis at follow-up angiography was significantly higher in the BA group (57.5±23.0% vs. 41.4±25.6%, p<0.001). Although late lumen loss was similar between the DES and BA groups (0.80±0.78 mm vs. 0.87±0.79 mm, p=0.60), the binary restenosis rate was lower in the DES group than in the BA group (25.0% vs. 64.4%, p<0.001). We divided the 148 lesions into two groups according to the size of postprocedural MLD: <2.5 mm group (78; DES 21, BA 57), and ≥2.5 mm group (70; DES 51, BA 19). The binary restenosis rate in the postprocedural MLD <2.5 mm group was significantly higher in BA than in DES implantation (71.9% vs. 33.3%, p=0.003); however, that in the postprocedural MLD ≥2.5 mm group showed no significant difference between BA and DES implantation (37.5% vs. 21.3%, p=0.32). Furthermore, when we compared DES implantation and BA according to reference diameter, <3.0 mm (62; DES 28, BA 34) and ≥3.0 mm groups (86; DES 44, BA 42), the binary restenosis rate was significantly higher in BA than in DES implantation in both the <3.0 mm (67.6% vs. 28.6%, p=0.005) and the ≥3.0 mm (61.5% vs. 22.5%, p=0.001) groups. The binary restenosis rate was similar between bare metal stent and DES which had been implanted at the first PCI in both BA (64.7% vs. 63.6%, p=1.0) and DES groups (29.5% vs. 16.7%, p=0.38).
Clinical follow-up was obtained for all patients. The median clinical follow-up duration with survival patients was 1,634 days (interquartile range 1,102 to 2,010 days). Figure 2A shows the cumulative incidence of MACE of the DES and BA groups at four years. The incidence of MACE was significantly higher in the BA group (75.2%) than in the DES group (45.8%, p<0.001). There was no significant difference in the incidence of all-cause death at four years between the BA and DES groups (30.4% vs. 21.5%, p=0.16) (Figure 2B). The incidence of cardiac death at four years was also statistically similar between the two groups (13.4% vs. 4.0%, p=0.07) (Figure 2C). The causes of death are shown in Table 4. Among 33 patients who died during the study period, cardiac death occurred in 10 patients (30.3%), including four patients with sudden death, three patients with fatal arrhythmia, and three patients with congestive heart failure. The cumulative incidence of TLR at four years was significantly higher after BA (60.5%) than after DES implantation (27.6%, p<0.001) (Figure 2D). The cumulative incidence of TLR from one to four years was similar between the BA and DES groups according to landmark analysis (26.0% vs. 15.4%, p=0.21). During the study period, target lesion coronary artery bypass grafting was performed in four patients (2.8%). Among 56 patients (BA 40, DES 16) who had undergone TLR, one (1.8%) was for MI (BA 1), eight (14.3%) for unstable angina (BA 6, DES 2), 24 (42.9%) for effort angina (BA 19, DES 5), seven (12.5%) for silent ischaemia detected by non-invasive tests (BA 4, DES 3), and 16 (28.6%) for restenosis at follow-up angiography (BA 10, DES 6). In the DES group, there was no difference in the incidence of TLR between the same DES and the different DES implantations (30.2% vs. 28.0%, p=0.35). The incidence of non-fatal MI was similar between the two groups (1.5% vs. 5.9%, p=0.50). During the follow-up period, definite stent thrombosis occurred in one patient after BA for a recurrent ISR lesion as late stent thrombosis (after 330 days). No stent thrombosis was observed after DES implantation for recurrent ISR lesions.
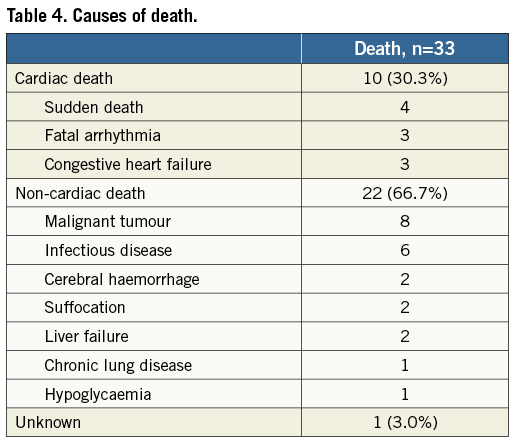
Figure 2. The Kaplan-Meier curves of the four-year clinical outcomes after PCI for re-ISR lesions after DES implantation for ISR lesions.
A) Cumulative incidence of MACE after DES implantation and BA for re-ISR lesions. B) Cumulative incidence of all-cause death after DES implantation and BA for re-ISR lesions. C) Cumulative incidence of cardiac death after DES implantation and BA for re-ISR lesions. D) Cumulative incidence of TLR after DES implantation and BA for re-ISR lesions. BA: balloon angioplasty; DES: drug-eluting stent; ISR: in-stent restenosis; MACE: major adverse cardiac events; PCI: percutaneous coronary intervention; re-ISR: recurrent in-stent restenosis; TLR: target lesion revascularisation
The patient subgroups (focal and non-focal patterns) were evaluated separately. The focal lesion group included 78 patients (DES, 33; BA, 45), and the non-focal group included 64 patients (DES, 37; BA, 27). Among patients with focal lesions, the incidence of TLR at four years was significantly higher in the BA group (53.2%) than in the DES group (19.8%, p=0.005) (Figure 3A). Among patients with non-focal lesions, the incidence of TLR at four years was also higher in the BA group (72.2%) than in the DES group (37.2%, p<0.001) (Figure 3B).
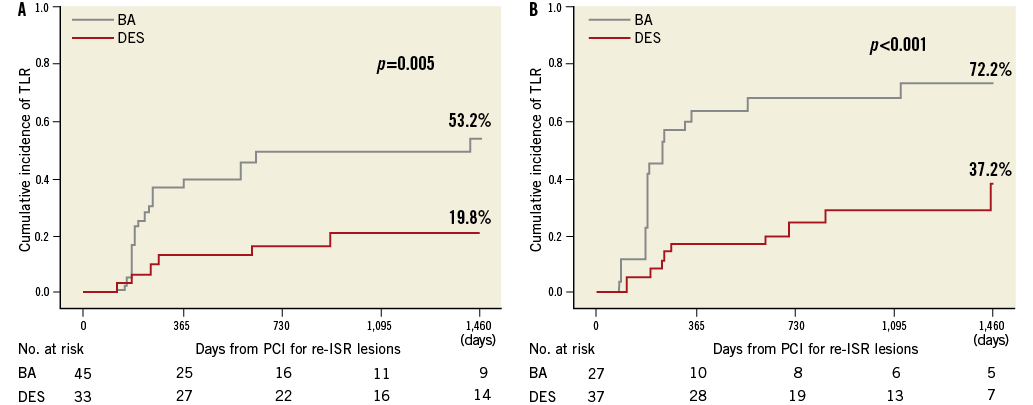
Figure 3. Kaplan-Meier curves of four-year TLR after PCI for re-ISR lesions after DES implantation for ISR lesions according to the morphological patterns. A) Cumulative incidence of TLR after DES implantation and BA for re-ISR lesions in focal lesions. B) Cumulative incidence of TLR after DES implantation and BA for re-ISR lesions in non-focal lesions. BA: balloon angioplasty; DES: drug-eluting stent; ISR: in-stent restenosis; PCI: percutaneous coronary intervention; re-ISR: recurrent in-stent restenosis; TLR: target lesion revascularisation
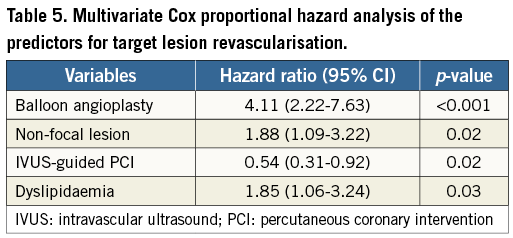
A stepwise Cox proportional hazards model was used to identify independent predictors of TLR after PCI for re-ISR lesions (Table 5). The multivariate analysis shows BA was the most important predictor of TLR (hazard ratio [HR] 4.11, 95% confidence interval [CI]: 2.22-7.63, p<0.001). Other predictors of TLR were non-focal lesion (HR 1.88, 95% CI: 1.09-3.22, p=0.02) and dyslipidaemia (HR 1.85, 95% CI: 1.06-3.24, p=0.03). IVUS guidance was a negative predictor of TLR after PCI for recurrent ISR lesions (HR 0.54, 95% CI: 0.31-0.92, p=0.02). Within the IVUS guidance group, there was no significant difference in the incidence of TLR between the DES and BA groups (43.9% vs. 60.5%, p=0.15). The incidence of TLR was markedly lower in the DES group than in the BA group within the non-IVUS guidance group (61.0% vs. 18.5%, p<0.001).
Discussion
The main findings in this study were as follows: (1) for recurrent ISR lesions after DES implantation for ISR lesions, DES implantation had better outcomes than BA, mainly because of a higher TLR rate in BA whether the recurrent ISR pattern was focal or non-focal; (2) although late lumen loss was similar between the two groups, the angiographic restenosis rate after PCI for recurrent ISR after DES implantation was significantly lower in DES implantation than in BA; (3) IVUS-guided PCI was associated with a lower TLR rate after PCI for recurrent ISR lesions, particularly after DES implantation.
Although the outcomes of various treatments for ISR lesions after DES implantation have been shown in several studies7-9, the outcomes after PCI of recurrent ISR lesions after DES implantation for ISR lesions have not been reported adequately because of the small population of recurrent ISR lesions and difficulties in clinical follow-up. The mortality rate and the incidence of cardiac death following PCI for recurrent ISR lesions were statistically similar between the BA and DES groups in our study, and this trend is the same as that observed in previous studies which evaluated clinical outcomes after PCI for ISR lesions9,10. In this study, the mortality rate was high compared with the previous studies which evaluated the long-term clinical outcomes after PCI for ISR lesions9,10,13. The patients with recurrent ISR lesions tend to have a high prevalence of prior MI, low ejection fraction, severe renal insufficiency, and especially haemodialysis. Therefore, the high mortality rate in our study was influenced by the poorer patient backgrounds of those with recurrent ISR lesions, compared with those with ISR lesions.
Previous studies have shown that repeat DES implantation for DES ISR lesions has lower TLR compared with BA for short or long-term follow-up4,7-10. Our study demonstrated that DES implantation for recurrent ISR lesions had a lower incidence of TLR than BA. Although the incidence of TLR after DES implantation for recurrent ISR lesions in our study was similar to that for ISR lesions in a previous study, the incidence of TLR after BA for recurrent ISR lesions in our study was markedly higher than that for ISR lesions in a previous study9. As TLR repeats any number of times, the efficacy of BA may decrease compared with that of DES implantation due to the difficulty in obtaining sufficient lumen area by only BA, and the advantage of DES for recurrent ISR lesions would be high compared with that for ISR lesions. A previous small study, which had shown favourable clinical and angiographic outcomes after the third stent implantation for recurrent ISR lesions, was consistent with our current findings14 and, consequently, additional stenting for recurrent ISR lesions was superior to BA. On the other hand, repeat DES implantation may be considered to have a higher risk of MI or stent thrombosis compared with BA15. However, no stent thrombosis following DES implantation for recurrent ISR lesions was observed. While our study population was relatively small, DES implantation could be recommended for recurrent ISR lesions after DES implantation for ISR lesions considering the unexpected adverse clinical events. After PCI for recurrent ISR lesions, because it is unclear whether further additional stenting is the right way or not, another alternative modality which obtains results equivalent to or better than DES implantation is eagerly awaited. The efficacy of a drug-eluting balloon for DES ISR lesions has been reported16,17. A recent study reported that a paclitaxel-eluting balloon had a smaller late lumen loss than BA and the same efficacy as a paclitaxel-eluting stent18. Better outcomes can be expected with the use of a paclitaxel-eluting balloon for recurrent ISR lesions compared with BA; however, further studies making comparisons with DES are required. In several previous studies, the clinical outcomes were reported to differ according to the morphological pattern of the lesion8-10,13,19,20. Regarding PCI for recurrent ISR lesions, the superiority of DES implantation over BA was shown to be consistent in the current study, whether the morphological pattern was focal or non-focal. Our study also revealed that non-focal lesions were associated with the incidence of TLR in multivariate analysis. The morphological pattern predicts the outcomes in patients with recurrent ISR, similar to ISR lesions.
The current study showed angiographic results after PCI for recurrent ISR lesions with a high angiographic follow-up rate. In previous studies, late lumen loss and restenosis rate after DES implantation and BA for DES ISR lesions varied greatly in each study7,8,21. In our study, late lumen loss after PCI for recurrent ISR lesions had a higher trend than that for ISR lesions. Interestingly, although reference diameter and late lumen loss after DES implantation were similar to those after BA for recurrent ISR lesions in our study, postprocedural MLD and the binary restenosis rate were markedly lower in the DES group. From these data, the lumen area after PCI for recurrent ISR lesions may be an important factor affecting restenosis and TLR. In fact, although DES implantation had better angiographic results than BA in both smaller and larger reference diameter groups, the restenosis rate was considerably lower in DES implantation in the smaller postprocedural MLD group and was similar in the larger postprocedural MLD group. These findings suggest that a larger acute gain and postprocedural lumen area by DES implantation were associated with a lower restenosis rate regardless of the reference vessel size. DES implantation should be recommended in residual stenosis lesions only after balloon dilatation.
Predictors of TLR after PCI for recurrent ISR lesions were shown in the present study. After PCI for ISR lesions, it was reported that BA, diffuse ISR pattern, and haemodialysis were the independent predictors of TLR in multivariate analysis of previous studies9,13,22. Some predictors are common to the predictors of TLR after PCI for ISR and recurrent ISR lesions. In our study, it was notable that IVUS guidance was beneficial in reducing TLR after PCI for recurrent ISR lesions, particularly in the DES implantation cases. IVUS has been reported to be associated with better early and long-term clinical outcomes23. Regarding ISR lesions, a few case reports have demonstrated the usefulness of IVUS-guided PCI24. The prevention of stent underexpansion by IVUS guidance may lead to better clinical outcomes after DES implantation for recurrent ISR lesions; however, IVUS-guided PCI may be of limited effectiveness in BA for recurrent ISR lesions. A recent study reported that a different DES implantation for DES ISR lesions had a more favourable outcome than the same DES implantation25. However, this factor was not associated with the incidence of TLR in our study.
Limitations
This study has several important limitations. First, this is a retrospective, observational, and single-centre study. Second, selection of treatment strategies for recurrent ISR lesions was not randomised and depended on the operator’s discretion. However, it may be possible that the recurrent ISR lesions at the stent edge and those with stent fracture could be selected for DES preferentially. A selection bias of the treatment strategy might have influenced our study results. Third, although periprocedural IVUS findings may have influenced the treatment strategy, IVUS findings were not evaluated in this study. Finally, the BA group did not include cutting balloon and drug-coated balloon angioplasty. Nonetheless, our study is a novel report about the clinical outcomes of PCI for recurrent ISR lesions.
Conclusion
In conclusion, DES implantation is markedly more effective than BA in the treatment of recurrent ISR lesions after DES implantation for ISR lesions, mainly because of the lower TLR rate, irrespective of the morphological pattern.
Acknowledgements
We appreciate the secretarial assistance of Miho Kobayashi, Makiko Kanaike, and Yoshimi Sano.
Conflict of interest statement
The authors have no conflicts of interest to declare.

