Abstract
Aims: Due to the widespread use of drug-eluting stents (DES), in-DES restenosis is increasing. The aim of this study is to evaluate the clinical outcome of patients undergoing repeat percutaneous coronary intervention (PCI) for DES restenosis.
Methods and results: One hundred patients with 108 restenotic lesions using DES were consecutively enrolled in a single-arm 2-centre registry. The repeat-PCI was performed either with balloon angioplasty (POBA) or with DES implantation (homo-stent or hetero-stent). Major adverse cardiac events (MACE) occurrence was assessed long-term. Of the 108 DES restenotic lesions, 34 were treated with balloon angioplasty, 43 with homo-stent and 31 with hetero-stent implantation. Of the patients, 37% had diabetes mellitus, while 30% peripheral or carotid artery disease. Over a mean follow-up of 16.0±6.0 months, the rates of death, myocardial infarction (MI), and ischaemic driven target lesion revascularisation (IDTLR) in hetero-stent, homo-stent and POBA groups were respectively 0% vs. 5% vs. 3% (p=NS), 2% vs. 5% vs. 0% (p=NS) and 17% vs. 23% vs. 25% (p=NS). When comparing patients treated with POBA to those receiving hetero-/homo-DES, no significant difference in terms of IDTLR (25% vs. 20%; p=NS), MI (0% vs. 4%; p=NS) and overall MACE (25% vs. 23%, p=NS) appeared. The presence of previous MI (OR 0.05; 95% CI 0.01-0.3), first DES implanted for BMS restenosis (OR 0.16; 95% CI 0.02-0.99) and peripheral or carotid disease (OR 0.09; 95% CI 0.01-0.67) were negative independent predictors of freedom from IDTLR.
Conclusions: Repeat balloon angioplasty for DES restenosis showed similar clinical outcome compared to re-DES (homo- or hetero-) implantation and could be considered as first treatment strategy in this setting.
Introduction
A large body of data unequivocally supports the superiority of drug-eluting stents (DES) compared to bare metal stents (BMS) in terms of restenosis reduction and, consequently, the need for repeat revascularisation1-3. However, it is gradually emerging that rates of late restenosis after the use of drug-eluting stents are higher than initial experience suggested, particularly in diabetic patients4-7 as well as in those with complex lesions (chronic total occlusion, bifurcation)8-11. With the increasing replacement of BMS with DES as the index procedure device of choice, the focus is shifting from the optimal strategy for in-stent restenosis (ISR) within BMS to that occurring within DES. However, the effective treatment of restenosis within a DES has not yet been adequately addressed12,13.
The aim of this prospective registry was to evaluate the outcomes of patients who developed coronary in-stent restenosis inside DES; focusing on the different strategy, balloon angioplasty (POBA), same DES or different DES as applied.
Materials and methods
Patients and study protocol
The study was designed as a prospective, single-arm, 2-centre registry (San Donato Hospital, Arezzo and Le Scotte Hospital, Siena, Italy) to evaluate the long-term clinical outcome of patients with DES restenosis treated with POBA or repeat DES implantation.
The study population consisted of 100 consecutive patients, enrolled from October 2004 to March 2006, with 108 restenotic lesions inside DES, implanted for a de novo lesion or a BMS restenotic lesion of a native vessel or bypass graft, with objective evidence of ischaemia and without clinical contraindication to prolonged double antiplatelet therapy. There were no exclusion criteria, either related to clinical presentation, stable or unstable patients, or to angiographic characteristics such as vessel diameter or lesion length.
All patients gave written informed consent. The trial was approved by the institutional ethics committee of the two participating centres.
Study protocol and data analysis
All patients received a bolus of unfractionated heparin at dose of 70 IU/Kg before starting the procedure. Balloon predilatation was performed in all target lesions with a balloon of at least 2.5 mm in diameter. The repeat PCI was performed either with POBA, including cutting balloon, or with DES implantation, same type (homo-stent) or different type (hetero-stent) according to operator discretion. During the study period, two DES (Cypher; Cordis Corporation, Miami, FL, USA or Taxus; Boston Scientific Natick, MA, USA) were available. The length of DES implanted was intended to fully cover the restenotic DES, even if the pattern of restenosis was focal. In case of restenosis in a bifurcation lesion, PCI was performed in both branches of the bifurcation but another DES was implanted only in the main vessel.
DES were always implanted at high pressure (>12 atm). Stent post-dilatation with a larger balloon was performed only in case of suboptimal results judged by visual estimation.
Combined antiplatelet therapy with aspirin (at least 100 mg daily) and ticlopidine 500 mg daily (or clopidogrel 75 mg daily) was started at least 48 hours before procedure and continued for at least 12 months in patients treated with re-DES implantation, and one month in those treated with POBA. Clopidogrel was administered with a loading dose of 300 mg. In those patients who were already taking clopidogrel before hospital admission, a re-loading of the drug was not done. The use of glycoprotein IIb/IIIa inhibitors was left to operator choice. Plasma concentrations of creatine kinase and its MB isoenzyme were systematically determined for 12 hours after the intervention. Relevant data were collected and entered into a computer database.
Angiographic analysis
Coronary angiograms were analysed by a semi-automated edge contour detection computer analysis system (MEDIS QCA CMS version 4). In-stent restenosis was classified accordingly to the angiographic patterns reported by Mehran et al14. Reference diameter (RD), minimal lumen diameter (MLD), percentage diameter stenosis (DS) and lesion length were measured before and at the end of the procedure.
Follow-up
All patients were asked to return to outpatient clinic for evaluation by one of the investigators at 1, 6, 12 and 24 months after hospital discharge. For those patients who did not return to the clinic at the designated time, follow-up information was collected by telephone interview. All patients reporting symptoms of chest pain were requested to come to the outpatient clinic for clinical, electrocardiographic, laboratory, and, eventually, angiographic assessment.
Definitions and outcome measures
Single vessel disease was defined as the presence of single or multiple stenosis > 50% in a single coronary vessel. Multivessel disease was defined as the presence of single or multiple stenosis > 50% in more than one coronary vessel. ISR was defined as > 50% diameter stenosis within the first DES or within 5 mm of its edges.
Procedural success was defined as stenosis < 10% in the target segment with the presence of TIMI grade III flow. Major adverse cardiac events (MACE) were defined as death from any cause, myocardial infarction (MI), and ischaemic driven target lesion revascularisation (IDTLR). MI was defined as the presence of new Q waves in ≥ 2 contiguous ECG leads or an elevation of creatine kinase, or MB isoenzyme to ≥ 3 times the upper limit of normal in two samples during hospitalisation, or to two times the upper limit of normal after discharge. IDTLR was defined as any repeat percutaneous coronary intervention or aorto-coronary bypass surgery due to lumen re-narrowing within the stent, or in the 5 mm distal or proximal segments associated with symptoms, or objective signs of ischaemia. Stent thrombosis (ST) was classified, according to the Academic Research Consortium (ARC) definition15, as definite, probable, or possible and as early (0 to 30 days), late (31 to 360 days), or very late (> 360 days). The definition of definite ST required the presence of an acute coronary syndrome with angiographic or autopsy evidence of thrombus or occlusion. Probable ST included unexplained deaths within 30 days after the procedure, or acute myocardial infarction involving the target-vessel territory without angiographic confirmation. Possible ST included all unexplained deaths occurring at least 30 days after the procedure.
Statistical analysis
Values are reported as numbers with relative percentage or standard deviation. Chi-Square or Fisher exact tests were used to compare nominal variables; continuous variables were compared with t-Test. Logistic regression analysis was used to identify predictors of IDTLR. All baseline variables shown in Tables 1 and 2 were entered into the multivariate models.
Odds ratio (OR) and 95% confidence intervals (CI) were reported with 2-tailed probability value: a value of P<0.05 was considered statistically significant. Survival analysis was performed with Kaplan–Meier method. All statistical computations were performed by resorting to StatView version 6 procedures (Figure 1).
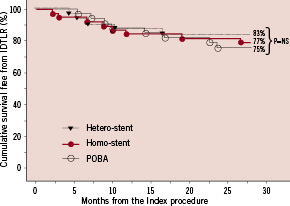
Figure 1. Kaplan-Meier survival analysis for freedom from ischaemic driven target lesion revascularisation (IDTLR) in the three different groups. POBA: Plain Old Balloon Angioplasty.
Results
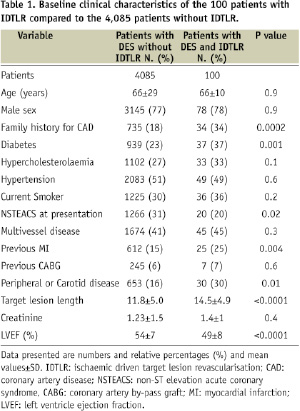
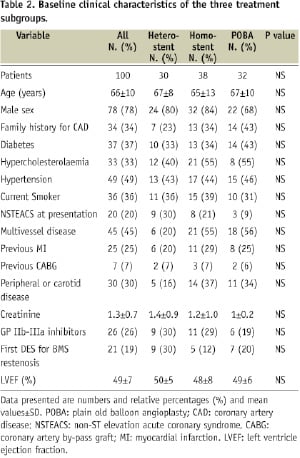
During the study period, 4,185 patients with 6,320 lesions were treated with DES at least in one lesion. During follow-up, 100 patients with 108 restenosis lesions in a DES underwent IDTLR. The clinical characteristics of patients with and without IDTLR is reported in Table 1. Patients with IDTLR for DES restenosis had a high prevalence of diabetes mellitus, family history of coronary artery disease (CAD), previous MI, peripheral or carotid disease, longer lesion, low left ventricle ejection fraction and less non-ST elevation acute coronary syndrome (NSTEMI) at presentation as compared to patients with DES and no IDTLR. Among the 108 restenotic lesions, 34 were treated with balloon angioplasty, 74 with re-implantation of a DES, 43 with homo-stent (six paclitaxel and 37 sirolimus) and 31 with hetero-stent (19 sirolimus in paclitaxel, 12 paclitaxel in sirolimus). No de novo lesions were treated during the index procedure. Clinical and angiographic characteristics of the three treatment subgroups are reported in Tables 2 and 3.
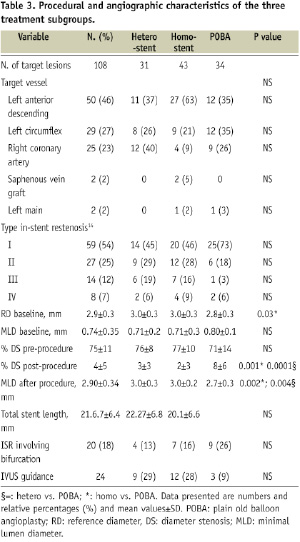
No statistical differences were noted among the three patient subgroups for all the clinical variables evaluated. Among patients treated with DES re-implantation, there was no difference between the diameter of the DES used in the index procedure and the one used in the repeat PCI and no angiographic evidence of discrepancy between the vessel and stent diameters, by visual estimation, was observed.
Clinical outcome
No MACE occurred during hospital stay. Outcome data were available for all patients. Over a mean follow-up of 16.0±6.0 (10 - 38) months, the overall rates of death, MI, and IDTLR were 3%, 3%, and 22%, respectively. Of the three patients who died after hospital discharge, one died of end-stage heart failure, one died after CABG and one died for acute renal failure after surgical revascularisation for critical limb ischaemia. Definite stent thrombosis occurred in two patients of the homo-stent group resulting in MI at 67 and 700 days after the procedure. Both patients were taking only aspirin at the time of stent thrombosis (“early discontinuation” in one patient). No possible and probable stent thrombosis occurred. The cumulative rate of in-hospital and long-term MACE are summarised in Table 4.
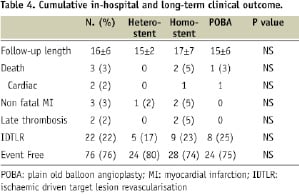
Clinical outcomes among treatment subgroups were similar without significant differences in terms of IDTLR, death and MI. Furthermore, even when comparing patients treated with POBA to those receiving another DES (same type or different type), no significant difference in terms of IDTLR (25% in POBA vs. 20% in re-DES group; p=NS) MI (0% vs. 4% respectively; p=NS) and overall MACE (25% vs. 23%, p=NS) appeared.
Twenty-one patients in whom BMS restenosis was the indication for the initial DES implantation, had a higher IDTLR occurrence compared with patients in whom the initial DES was implanted in a de novo lesion (IDTLR 43% vs. 15%, p=0.01).
Logistic regression analysis demonstrated that the presence of previous MI (OR 0.05; 95% CI 0.01-0.3), first DES implanted for BMS restenosis (OR 0.16; 95% CI 0.02-0.99) and peripheral or carotid disease (OR 0.09; 95% CI 0.01-0.67) were negative independent predictors of freedom from IDTLR.
Discussion
The major findings of the present study are as follows: 1) there were no differences in outcomes among the available treatment strategies for DES restenosis, 2) the rate of major cardiac adverse events is acceptable considering the complexity and the risk profile of the study cohort, 3) repeated DES implantation for DES restenosis in our experience is associated with a 3% risk of late stent thrombosis.
Due to the increasing patient and lesion’s complexity as well as the widespread use of DES as the index procedure device of choice, in-stent restenosis inside DES is becoming an important clinical entity and a challenging task for the interventional cardiologist. Restenosis in DES did not appear as a benign phenomenon in our study, leading to NSTEACS in 20% of the patients.
In the clinical arena, revascularisation options are basically restricted to stand-alone angioplasty, cutting balloon angioplasty, coronary brachytherapy or placement of another drug-eluting stent12,13,16. Currently, a paucity of data have been published to guide decision making. The present registry is one of the first reports of the “real-world” performance of the available treatment strategies in a consecutive, unselected series of patients with DES ISR. Our results suggest that the currently available treatment strategies for DES restenosis are associated with similar clinical outcomes and acceptable adverse event rates according to the risk profile of the study cohort. Moreover, POBA showed similar clinical outcome in terms of IDTLR compared to repeat DES implantation, homo- or hetero- DES. On the other hand, DES in DES technique is associated with a higher risk of late stent thrombosis and side branch entrapment that may increase the rate of myocardial infarction (4% in our study). In addition, DES re-implantation needs a prolonged dual antiplatelet treatment (at least 12 months), which exposes the patients to a higher bleeding risk and increases the overall economical cost. Therefore, POBA could be considered as first treatment option for DES ISR, leaving the strategy of another DES implantation only in cases of suboptimal result after simple balloon angioplasty. Dedicated trials will finally address this issue in a randomised fashion.
No significant difference in terms of IDTLR was observed between the hetero-stent and the homo-stent groups and this observation stands against the issue of the potential role of drug resistance in the pathophysiology of DES restenosis. Obviously, our data cannot address this issue directly. Due to the small number of patients in the present study, in-stent restenosis after sirolimus and paclitaxel-eluting stents were grouped together. However, it is likely that resistance to paclitaxel and to sirolimus has different pathways. Dedicated head-to-head trials of DES–in–DES restenosis, with the same vs. a different antiproliferative agent are needed, and, indeed, they are on-going17.
Our findings confirm and expand the observations of Cosgrave et al12 who found a MACE rate of 28% in the long-term in patients treated with the same or different DES for DES restenosis. No significant difference in outcomes between the same or different DES strategy was found, even if a higher incidence of non-focal restenosis was observed in the hetero-stent group. No other treatment strategy for DES restenosis was evaluated in that study. In contrast, a worse scenario was depicted by Mishkel et al13, who found a high rate of MACE (43%) with current treatment strategies of DES restenosis. The higher frequency of MACE in that report may have been due to the higher risk characteristics of that population, which included also a significant number of patients (8.7%) with stent thrombosis at presentation.
Another important finding of the present study is that patients in whom BMS restenosis was the indication for the initial DES implantation had a higher IDTLR occurrence as compared with patients in whom the initial DES was implanted in a de novo lesion (IDTLR 43% vs. 15%, p=0.01). The question whether DES failures might be more resistant than BMS to re-implantation with DES.
Indeed, the IDTLR rate in the present registry is higher that that observed in the TRUE (Tuscany Registry of sirolimus for Unselected in-stent rEstenosis) study (27% vs. 5%)18, a registry performed in the same centres and focused on the performance of DES implantation for the treatment of BMS restenosis. This difference seems to be related to the different risk profile of the study population, rather than to the pattern of baseline in-stent restenosis that was predominantly focal in the present study compared with the TRUE registry (54% vs. 12%). On the other hand, we cannot exclude that the underlying biological mechanisms responsible of restenosis after BMS and DES may be different.
In our experience, a strategy of repeated DES implantation was associated with 3% late stent thrombosis (two episodes, both in the homo-stent group). Theoretically, the delayed vascular healing and re-endothelialisation that may contribute to the phenomenon of late stent thrombosis in drug-eluting stents could potentially be further exacerbated by additional drug-eluting stent treatment. Optimal DES sizing and expansion in the vessel wall is a major issue to prevent stent thrombosis19,20 and restenosis21. In our study, the DES diameter did not differ from the index PCI and the repeat PCI, and we did not observe by visual estimation DES under-sizing in respect to the reference vessel diameter in the index PCI, as well as during repeat PCI. This fact is probably due to the technique for DES implantation adopted in both study centres, which uses in-stent postdilatation at high pressure with a non-compliant balloon, ensuring the full expansion of the stent. However, IVUS guidance was performed in less than one third of the patients and thus we cannot exclude some cases in which, although an optimal angiographic results, the stent diameter was under sized compared to vessel diameter.
Study limitation
The prospective, non-randomised design of this study with the choice of the interventional strategy left to operator discretion, the number of patients not sufficient for a valid statistical power, the lack of angiographic data on binary restenosis and lumen late loss, these are the major limitations of this study and might have influenced the detection of a significant difference among the three treatment strategies. However, while data coming from randomised trials will hopefully clarify the best strategy to treat DES restenosis and BMS-DES re-restenosis, our experience, together with those previously reported, might contribute to clinical decision making in everyday practice.

