Abstract
Aims: Drug eluting stents (DES) have had a great impact in reducing in-stent restenosis (ISR) in de novo lesions. However, long-term data regarding effectiveness and safety of these stents in treating bare metal stent (BMS) ISR are limited. We report long-term clinical outcomes in a cohort of patients with BMS-ISR treated with DES between April 2002 and December 2003 at our institution.
Methods and results: Sixty-nine consecutive patients with significant BMS-ISR were treated with DES implantation. Sirolimus DES were used in 43 patients and paclitaxel DES in 26. All patients were followed up to determine the incidence of major adverse cardiac event (MACE) rates (all-cause death, myocardial infarction, or target vessel revascularisation [TVR]), angina class and the need for clinically driven angiography.
The mean age of the cohort was 58.6±10.8 years; 68% were male, 33% were diabetic, 50% had hypertension, 78% were on statin therapy and 59% were current (19%) or previous (41%) smokers. The clinical presentation of ISR was with chronic stable angina in 54 patients, 12 had a non-ST elevation acute coronary syndrome and three presented with ST-elevation myocardial infarction. Multivessel stenting was performed in 21 patients and bifurcation stenting in seven patients. Over a mean follow period of 4.9 years, the first event MACE rate was 20% (17 events in 14 patients - eight deaths of which three were cardiac, two non-fatal myocardial infarctions and seven TVR). Excluding non-cardiac death, the adjusted MACE rate was 14.5% (12 events in 10 patients). At long-term follow-up, mean Canadian angina class decreased from 2.3±0.7 pre-procedure to 1.2±0.4, 65% of patients were angina free and 80% were free of MACE. No differences in long-term outcomes were observed between patients receiving paclitaxel and sirolimus DES.
Conclusions: The use of DES for the treatment of BMS-ISR is safe and effective over a mean follow-up period of nearly five years. To our knowledge, this represents the longest follow-up data of real world patients treated in a single interventional centre.
Introduction
Percutaneous coronary artery stent implantation is an effective strategy for the treatment of de novo obstructive lesions. In-stent restenosis (ISR), luminal re-narrowing at the site of the previous intervention which occurs as a result of proliferative neointimal healing, remains a major limitation to successful PCI with bare metal stents (BMS). It is not a benign phenomenon with 9.5% of patients presenting with an acute myocardial infarct and 26.4% with unstable angina in one small registry1. Indeed patients with significant in-stent restenosis in BMS at six month angiographic follow-up have a poorer prognosis at four years compared to those without2. The introduction of drug eluting stents (DES) has led to a marked reduction in restenosis rates, primarily by reducing neointimal proliferation.
Vascular brachytherapy (VBT) has proved a valuable treatment for ISR3 and a recent meta-analysis suggested that VBT improves the medium term outcomes of binary restenosis, late lumen loss, revascularisation and MACE compared to balloon angioplasty and selective BMS placement4. In studies comparing DES with VBT, patients receiving DES demonstrated a reduced need for revascularisation, reduced MACE and reduced binary restenosis at nine months follow-up. This result is in keeping with the conclusion of an earlier meta-analysis involving four randomised controlled trials of DES treatment for ISR with a maximum 12 months follow-up. In addition, there have been a number of small published registries, some with angiographic follow-up, which have all reported promising short-term outcomes (median nine months) in patients treated with DES5-10. Thus, promising interim data and the absence of any widely available and effective alternative has resulted in DES becoming the primary strategy for ISR despite a lack of information concerning long-term safety and efficacy.
As DES become the default treatment for ISR, and with current concerns regarding late stent thrombosis, we conducted a retrospective observational study on the clinical outcomes of unselected patients receiving DES for symptomatic ISR at our institution.
Methods
DES were introduced at our institution in April 2002 and subsequently became the treatment of choice for BMS-ISR. Consecutive patients treated with DES for symptomatic BMS-ISR between May 2002 and December 2003 were identified from the hospital database. There were no specific exclusion criteria to DES implantation and all patients were included. The study was approved by NRES (National Research Ethics Service, United Kingdom).
Patients were followed up to evaluate the incidence of major adverse cardiac events (MACE), the requirement for clinically driven angiography and symptomatic status. Angina was classified according to the Canadian Cardiovascular Society Functional Classification. MACE was defined as death from any cause, myocardial infarction (MI), and target-vessel revascularisation (repeat PCI or coronary artery bypass grafting [CABG]). MI was defined as an admission to hospital with chest pain and a significant rise in cardiac enzymes, with or without ST segment elevation. All clinical information was collected between 48 and 67 months after the index procedure with a mean follow-up of 58.5 months. Data collection involved interrogation of the hospital database for procedural information, the evaluation of hospital notes and routine outpatient follow-up. Any patients no longer under routine follow-up, or those who had not been reviewed within the preceding 12 months were cross checked for survival and events via the hospital database and the patient’s general practitioner before being contacted by post. A telephone enquiry/questionnaire was then conducted.
Angiographic analysis
Angiography during follow-up was undertaken on clinical grounds alone. In those subjects who required coronary angiography, this was performed in a standard manner. Angiographic restenosis was defined as ≥50% diameter stenosis within the target lesion. The proximal and distal edge segments included up to 5 mm on either side of the stent segment. The target lesion was defined as the in-stent segment.
Procedural details
A CYPHER® (Cordis, Johnson & Johnson, Warren, NJ, USA) sirolimus-eluting stent was used in 43 of the patients and a TAXUS® (Boston Scientific, Natick, MA, USA) paclitaxel-eluting stent in 26 patients. Cypher stents used were 2.25, 2.5, 2.75, and 3 mm in diameter (mean 2.9±0.2 mm) and 8, 13, 18, 23 and 33 mm in length (mean 23.6±8.0 mm). The Taxus stents used were 2.5, 2.75, 3 and 3.5 mm in diameter (mean 3.1±0.3) and 12, 16, 20, 24, 28, and 32 mm in length (22.8±5.6 mm). All procedures were performed using standard techniques and the interventional strategy used, and any periprocedural adjuvant pharmacotherapy given, was at the operator’s discretion. All patients were preloaded with aspirin and clopidogrel (300 mg) followed by 75 mg once daily for a minimum of three months after stent implantation according to guidelines in place at the time. Aspirin therapy was continued indefinitely assuming no contraindications. Angiographic success was obtained in all the lesions treated.
Statistics
Descriptive statistical analysis was conducted with continuous data presented as means±SDs and categorical data as percentages. Univariate and multivariate regression analysis was performed on appropriate data sets (age, gender, diabetes, smoking history, hypertension, multivessel disease, previous CABG, target vessel, bifurcation disease, stent types, stent diameter, stent length) to determine predictors of MACE or restenosis. Event free survival was computed using Kaplan-Meier method. A commercially available package was used (SPSS). All p values <0.05 were considered statistically significant.
Results
Patient demographics
A total of 69 patients were treated with DES for BMS ISR with a mean follow-up period of 58.5 months. Baseline characteristics are shown in Table 1.
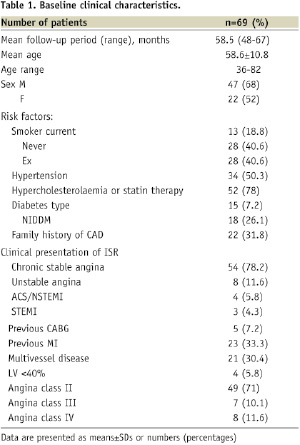
Of these, 47 patients were male, with an average age of 58.6±10.8 years (range 36-82 years). Twenty-three patients had sustained a previous myocardial infarct, and five had previously undergone coronary artery bypass grafting. The clinical presentation leading to the investigation and diagnosis of BMS-ISR was acute in 21.7% of cases.
Angiographic and procedural details
Seventy one lesions were treated, as two patients had co-existing left anterior descending (LAD) and circumflex (Cx) ISR. The procedural and angiographic characteristics are shown in Table 2.
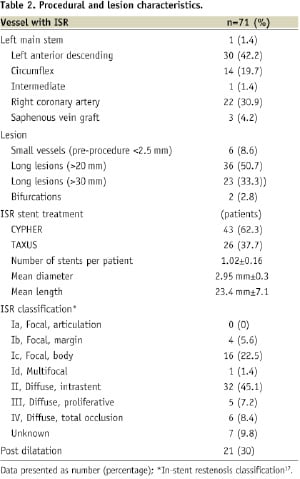
Diffuse, intrastent restenosis (type II) was the pattern of ISR seen in the majority of patients. Lesions less than 2.5 mm in diameter accounted for a small proportion only (8.6%) whereas long lesions, greater than 20 mm, accounted for 50.7% of cases. More than one stent was required to treat the ISR lesion in 2.9% of patients. Glycoprotein IIbIIIa inhibitors were administered in 50.7% of cases. Procedural success was achieved in all patients.
Clinical follow-up
Clinical follow-up was complete for all patients. No events occurred in-hospital or within 30 days of the procedure. Subsequent MACE events are shown in Table 3.
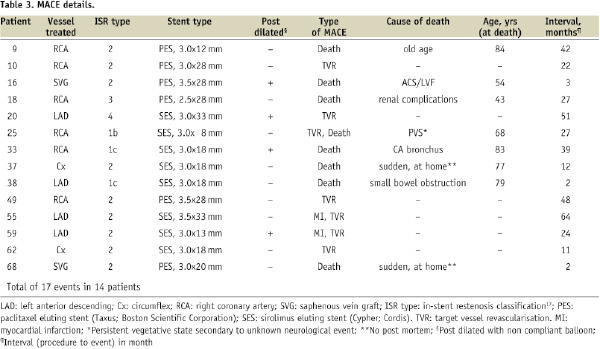
A total of 17 MACE events occurred in 14 patients (first event rate, 20.3%). Excluding non-cardiac death, 12 MACE events occurred in 10 patients (first event rate, 14.5%). MACE events occurred between two months and 64 months post-procedure (mean 26.7 months±19.7).
After a mean follow-up of 4.8 years, eight patients (11.5%) had died. Five of the deaths were non-cardiac. Of the three probable cardiac deaths, one patient presented three months post-procedure with an acute coronary syndrome and cardiogenic shock, and died before further angiography could be undertaken. This patient had undergone previous CABG, and DES treatment for ISR of a SVG. The remaining two deaths occurred suddenly, at home, at two months and 12 months post-procedure. One of these patients had also had previous CABG, with DES for ISR within a SVG. Two patients presented to hospital with myocardial infarctions (2.9%), at 24 and 64 months post-procedure. Both had single vessel disease and both underwent target vessel (but not target lesion) revascularisation with further DES. In total, including the two cases presenting as MI, 10 patients required further revascularisation: four required PCI to the target vessel, three underwent PCI for de novo disease in other vessels, and CABG was performed in three for multivessel disease (all involving ISR). Fifty-five patients (79.7%) were free of MACE at long term follow-up (Figure 1a).
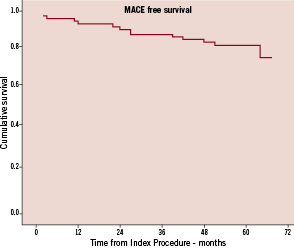
Figure 1a. Kaplan-Meier curve representing MACE free survival over the follow-up period.
The probability of a patient having MACE did not differ between those patients receiving CYPHER stents and those in whom TAXUS stents were used (p=0.66) (Figure 1b).
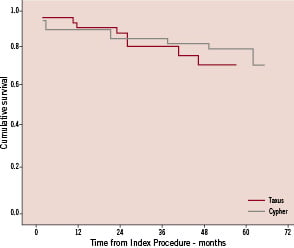
Figure 1b. Kaplan-Meier curve representing MACE free survival in patients treated with Cypher versus Taxus stents (p=0.66).
Of the fourteen patients in whom MACE occurred, eight received CYPHER stents and six received TAXUS.
Clinically driven angiography was performed in 24 patients (34.7%). Thirteen patients had no angiographic evidence of ISR and no further action was taken. Significant ISR was evident in six patients (8.6%): one patient was treated medically because of diffuse ISR, and in five patients further revascularisation was required. The remaining patients had no evidence of target vessel ISR but had progressive coronary artery disease in other arteries requiring further revascularisation by CABG or PCI (as described above). Stent type did not determine angina status or the need for clinically driven angiography.
Among the 55 patients without MACE events, there was clear symptomatic improvement at long-term follow-up: 45 of the surviving 61 patients reported that they were free of angina (73.7%). Mean CCS class was 1.2±0.4 (median CCS class 1, compared to a pre-procedure mean CCS class of 2.5, median 2).
By univariate or multivariate analysis, no single characteristic such as age, gender, diabetes, hypertension, hypercholesterolaemia, smoking, previous CABG, bifurcation disease, target vessel, and stent or lesion parameters, was found to be a predictor of MACE or restenosis in this cohort.
Discussion
To the best of our knowledge, this study reports the longest clinical follow-up of patients treated with DES for BMS-ISR. We have demonstrated that over a mean period of nearly five years, DES implantation is safe and effective in the treatment BMS-ISR. Procedural success was 100%, and no in-hospital adverse events occurred. Subsequent MACE rates were low, comparing favourably with published data for both balloon angioplasty11 and vascular brachytherapy4 in the treatment of BMS-ISR. There was a clear symptomatic benefit with more than two thirds of surviving patients being angina free at follow up. In those who underwent clinically driven angiography - including patients with diabetes, small vessels, long lesions, bifurcations, multivessel disease and saphenous vein grafts - the rate of recurrence of ISR was only 8.6%. Although angiography was not protocol driven and was instead determined by clinical status, the low rate of target lesion revascularisation is encouraging evidence of the effectiveness of DES in our population and compares favourably with published trials12,13.
We were unable to determine any predictors of MACE or restenosis by univariate analysis. This may reflect the small sample size and relatively low event rates. Diabetes, female gender, stent length and minimum luminal diameter (MLD) at baseline have long been recognised as predictors of ISR after balloon angioplasty14-16. Factors known to increase the risk of BMS-ISR are similar and include smaller MLD, prior restenosis, length of stented vessel, and diabetes17.
No difference in clinical outcome was noted between those patients receiving sirolimus-eluting stents (SES) versus paclitaxel-eluting stents (PES). A retrospective comparison of patients treated with SES versus PES for diffuse ISR revealed an overall angiographic restenosis rate of 22% in the SES treated group versus 25% in the PES treated group at 8.2 months (p=0.55)18. MACE rates too showed no significant difference (19% SES, 24% PES, p=0.56). In contrast, the ISAR-Desire study although not designed to compare the efficacy of SES and PES, found that SES had a reduced risk of TVR (0.42, 95% CI=0.19-0.92). More recently a study compared six month angiographic data in SES versus PES treated ISR patients and demonstrated that despite different patterns of restenosis (diffuse ISR more common in PES and focal ISR in SES), there was no significant difference in subsequent TLR rate19. To date neither stent has an unlimited worldwide license for use in ISR despite the practice being widespread.
Stent thrombosis (ST) is infrequent but its occurrence can result in sudden death or myocardial infarction20. Employing the ARC definitions, three patients in this cohort had possible late ST (4.3%), all occurring within one year of implantation. Two patients died at two and three months post-procedure and were still on dual antiplatelet therapy. The remaining patient died suddenly at 12 months post-procedure and was on aspirin only. Without angiography and/or post mortem data, it is difficult to be certain of direct causation, and only one patient reported ill health prior to death, which occurred in hospital. The remaining patients died at home aged 75 and 77.
Our study had several limitations. It was a non-randomised, observational study and all data collection was retrospective. In addition, the cohort was small which limited the ability to perform detailed statistical analysis. Lastly, follow-up included clinically driven angiography rather than routine re-catheterisation so data on angiographic restenosis was not available. This study was however, designed to describe clinical outcomes in standard clinical practice at a single interventional centre. As such, it represents an unselected population rarely addressed by randomised controlled trials and is the longest reported follow up of this patient group. In conclusion, this observational study supports the long-term safety and efficacy of DES as a strategy for recurrent restenosis within bare metal stents.
Acknowledgement
The authors wish to thank Brian Walker, Clinical Lead Radiographer at Manchester Royal Infirmary for his assistance in the data collection.

