Abstract
Aims: In drug-eluting stent (DES) restenosis, the contribution of drug hyporesponsiveness is poorly defined. We sought to evaluate if, in the setting of treatment for in-stent restenosis, the relative efficacy of sirolimus-eluting stents (SES) and of paclitaxel-eluting stents (PES) depends on the underlying substrate in which the stents are implanted, i.e., on whether the restenosis occurs within bare metal stents or within SES.
Methods and results: We pooled data from the ISAR-DESIRE and ISAR-DESIRE 2 randomised trials and analysed outcomes in SES-treated and PES-treated patients. In all, 650 patients were included. Angiographic follow-up was available for 87% of patients. In SES-treated patients, both late loss (LL) and percentage diameter stenosis (%DS) were lower in patients treated for bare metal stent restenosis compared with SES restenosis (0.21±0.59 mm versus 0.41±0.66 mm, p=0.007; 27.6±19.4% versus 34.0±20.9%, p=0.015, respectively). In PES-treated patients, LL and %DS were similar in patients treated for bare metal stent restenosis compared with SES restenosis (0.48±0.59 mm versus 0.39±0.71, p=0.47; 33.5±22.2% versus 32.7±18.6%, p=0.75, respectively). Similarly, in terms of overall clinical efficacy, in SES-treated patients clinical outcomes were better in patients with bare metal stent restenosis compared with SES restenosis while in PES-treated patients outcomes were similar in both groups. At multivariate analyses the use of SES to treat restenosis within SES was predictive of both higher LL and %DS.
Conclusions: The efficacy of sirolimus-eluting but not paclitaxel-eluting stents is significantly reduced when used for treatment of SES restenosis as compared to bare metal stent restenosis. The lower antirestenotic efficacy following SES implantation in patients with SES restenosis may support a role for drug resistance in restenosis within these stents.
Introduction
Drug-eluting stents (DES) are highly successful in preventing coronary restenosis and have been widely adopted into clinical routine worldwide1. As a consequence of this, in spite of their high efficacy, when a clinician is faced with in-stent restenosis in practice, it is usually restenosis within DES2. However, despite a decade of clinical experience with these devices, the specific mechanisms underlying DES restenosis remains an issue of some controversy. In particular, although it is hypothesised that the aetiology of restenosis within DES is multifactorial comprising biological, mechanical and technical factors, the contribution of drug hyporesponsiveness to this pathophysiological process is poorly defined3,4.
To investigate this question, we pooled data from the Intracoronary Stenting and Angiographic Results: Drug Eluting Stents for In-Stent Restenosis (ISAR-DESIRE)5 and ISAR-DESIRE 26 randomised trials, which investigated the optimal treatment of patients with restenosis within bare metal and drug-eluting stents, respectively. In the current analysis we sought to evaluate if, in the setting of treatment for in-stent restenosis, the relative efficacy of sirolimus-eluting stents (SES) and of paclitaxel-eluting stents (PES) depends on the underlying substrate in which the stents are implanted, i.e., on whether the restenosis occurs within bare metal stents or within SES.
Methods
The methods of the ISAR-DESIRE and ISAR-DESIRE 2 clinical trials have been reported in detail previously5,6.
In brief, the ISAR-DESIRE trial enrolled patients with restenosis occurring within bare metal stents. Patients were randomly assigned to treatment with plain balloon angioplasty, SES (CYPHER®; Cordis, Warren, NJ, USA) or PES (TAXUS™; Boston Scientific, Natick, MA, USA). The primary endpoint was binary angiographic restenosis at six to eight-month follow-up angiography.
In ISAR-DESIRE 2, patients with restenosis occurring within sirolimus-eluting DES were randomly assigned to receive either SES (CYPHER) or PES (TAXUS). Patients with restenosis occurring in either the CYPHER SES or the sirolimus-eluting ISAR stent (Individualizable drug-eluting Stent system to Abrogate Restenosis; based on Yukon [Translumina, Hechingen, Germany] backbone)7 were enrolled in the study. The primary endpoint was in-stent late lumen loss.
Inclusion criteria were comparable in both studies: patients older than age 18 with ischaemic symptoms or evidence of myocardial ischaemia (inducible or spontaneous) in the presence of a restenosis ≥50% located in the native vessel segment were considered eligible, provided that written, informed consent by the patient or her/his legally-authorised representative for participation in the study was obtained. Patients with a target lesion located in the left main stem, acute myocardial infarction within the preceding 48 hours, cardiogenic shock, malignancies or other comorbid conditions with life expectancy less than 12 months, known allergy to the study medications (sirolimus, paclitaxel) or pregnancy (present, suspected or planned) were considered ineligible for the study.
Study protocols
Treatment protocols were comparable in both studies. An oral loading dose of 600 mg clopidogrel was administered to all patients prior to the intervention, regardless of whether the patient was taking clopidogrel prior to admission. During the procedure, patients were given intravenous aspirin, heparin or bivalirudin; glycoprotein IIb/IIIa inhibitor usage was at the discretion of the operators. After the intervention all patients, irrespective of treatment allocation, were prescribed 200 mg/day aspirin indefinitely, clopidogrel 150 mg for the first three days (or until discharge) followed by 75 mg/day for at least six months and other cardiac medications according to the judgement of the patient’s physician (e.g., ß-blockers, ACE-inhibitors, statins, etc.). After enrolment, patients remained in hospital for at least 48 hours. Blood samples were drawn every eight hours for the first 24 hours after randomisation and daily afterwards for the determination of cardiac markers (CK, CK-MB, Troponin T). Daily recording of ECG was also performed until discharge. All patients were evaluated at one and twelve months by phone or office visit. Repeat coronary angiography was scheduled for all patients at six to eight months.
Data management, endpoints, and definitions
In both studies data were collected and entered into a computer database by specialised personnel of the Clinical Data Management Centre. All events were adjudicated and classified by an events adjudication committee blinded to the treatment groups. Baseline, postprocedural, and follow-up coronary angiograms were digitally recorded and assessed off-line in the quantitative angiographic (QCA) core laboratory (ISARESEARCH Centre, Munich, Germany) with an automated edge-detection system (Medis Medical Imaging Systems, Leiden, The Netherlands) by experienced operators unaware of the treatment allocation. Measurements were performed on cineangiograms recorded after the intracoronary administration of nitroglycerine. Baseline QCA measurements were performed using the single worst view projection for the index lesion; the same view projection was used for the measurements after stent implantation. In the follow-up angiogram, the QCA measurements were performed using the single worst view projection at that time point. The contrast-filled non-tapered catheter tip was used for calibration. Quantitative analysis was performed on both the “in-stent” and “in-segment” areas (including the stented segment, as well as both 5 mm margins proximal and distal to the stent). Restenosis morphology was adjudicated according to criteria modified from Mehran et al8.
In the present analysis the primary endpoints of interest were in-stent late loss (defined as the difference between the minimal luminal diameter at the end of the procedure and the minimal luminal diameter at follow-up angiography) and in-segment percentage diameter stenosis at follow-up angiography. Recurrent binary angiographic restenosis was defined as diameter stenosis ≥50 % in the in-segment area at follow-up angiography. The secondary endpoint of interest was the composite of death, myocardial infarction or target lesion revascularisation (TLR) at 12 months. Details of definition of the individual endpoint components are as described previously5,6.
Statistical analysis
Patients were analysed in two groups according to the type of drug-eluting stent treatment received, i.e., SES-treated and PES-treated. The objective of the study was to compare outcomes in: (i) SES-treated patients who were treated for restenosis within bare metal stents versus restenosis within SES; and (ii) PES-treated patients who were treated for restenosis within bare metal stents versus restenosis within SES. Continuous data are presented as mean (SD). Categorical data are presented as counts (%). Clinical outcome data are presented as counts (% by survival analysis). Differences between groups were checked for significance using Student’s t-test or Kruskal-Wallis rank sum test in case of skewed distribution for continuous data and chi-squared test (or Fisher’s exact test where the expected cell value was <5) for categorical variables. Two separate multivariate analyses (linear regression model –ANOVA) were used to identify the independent predictors of LL and in-segment %DS. All variables in Table 1 reporting a p-value <0.10 were entered into the models. In addition, further variables (the presence of restenosis within SES, the use of SES to treat in-stent restenosis, and the interaction of SES use within SES restenosis) were entered into the model. Survival was assessed using the methods of Kaplan-Meier and compared using the log-rank test. For all calculations a p-value <0.05 was considered statistically significant. Statistical software S-PLUS, version 4.5 (S-PLUS, Insightful Corp, Seattle, WA, USA) was used for analysis.
Results
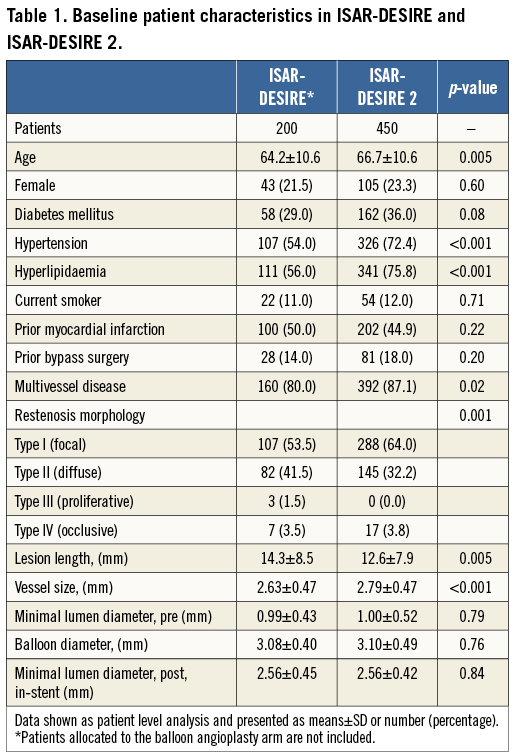
In all, 650 patients were included, comprising 200 patients from ISAR-DESIRE who were randomised to receive either SES (n=100) or PES (n=100), as well as 450 patients from ISAR-DESIRE 2 who were randomised to receive either SES (n=225) or PES (n=225). Baseline characteristics were well matched across the study arms of each trial. Comparison of baseline patient, lesion and procedural characteristics between patients enrolled in ISAR-DESIRE and ISAR-DESIRE 2 are presented in Table 1. Patients in ISAR-DESIRE had significantly smaller vessels, longer lesion length and more often diffuse pattern restenosis. Patients enrolled in ISAR-DESIRE 2 were significantly older, and more likely to have hypertension, hyperlipidaemia and multivessel disease. Angiographic follow-up was available for 87% of patients; the results in SES-treated (n=281) and PES-treated (n=283) patients are presented in Table 2.
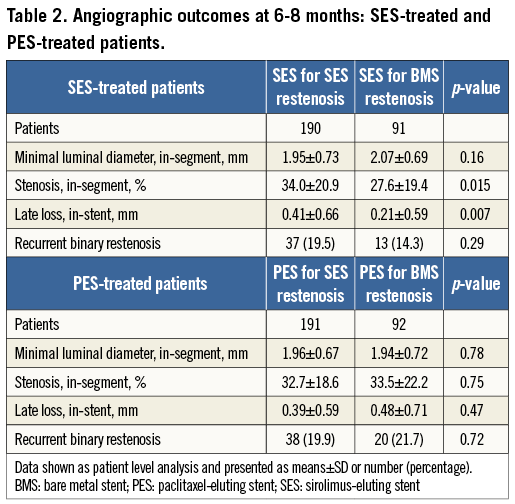
In SES-treated patients, both late loss and percentage diameter stenosis were lower in patients treated for bare metal stent restenosis compared with SES restenosis (0.21±0.59 mm versus 0.41±0.66 mm, p=0.007, Figure 1A; 27.6±19.4% versus 34.0±20.9%, p=0.015, respectively).
Against this, in PES-treated patients, late loss and percentage diameter stenosis were similar in patients treated for bare metal stent restenosis compared with SES restenosis (0.48±0.59 mm versus 0.39±0.71 mm, p=0.47, Figure 1B; 33.5±22.2% versus 32.7±18.6%, p=0.75, respectively).
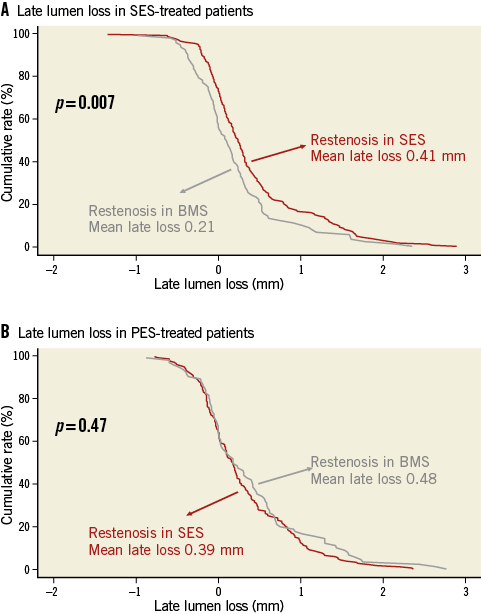
Figure 1. A) SES-treated patients: cumulative frequency distribution curves for in-stent late loss according to index stent type. B) PES-treated patients: cumulative frequency distribution curves for in-stent late loss according to index stent type. BMS: bare metal stent; PES: paclitaxel-eluting stent; SES: sirolimus-eluting stent
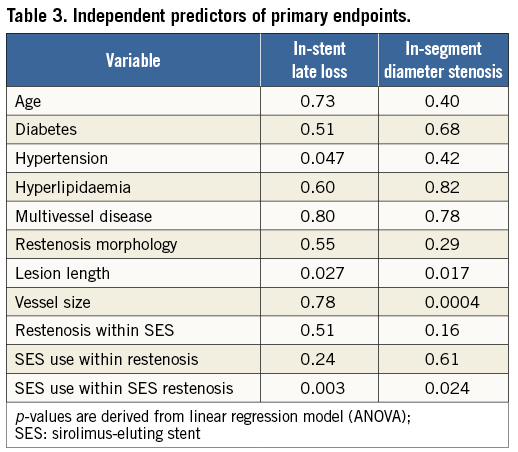
At multivariate analyses (Table 3), hypertension, longer lesions and the use of SES to treat restenosis within SES were predictive of LL, whilst longer lesions, smaller vessels and the use of SES to treat restenosis within SES were predictive of %DS.
Details of clinical follow-up in SES-treated and PES-treated patients are presented in Table 4. In terms of overall clinical efficacy, in SES-treated patients the composite of death, myocardial infarction or TLR was lower in patients treated for bare metal stent restenosis compared with SES restenosis (11[11.1%] versus 44[20.4%], p=0.05, Figure 2A). On the other hand, in PES-treated patients the composite of death, myocardial infarction or TLR was similar in both groups (22[22.4%] versus 41[19.6%], p=0.45, Figure 2B).
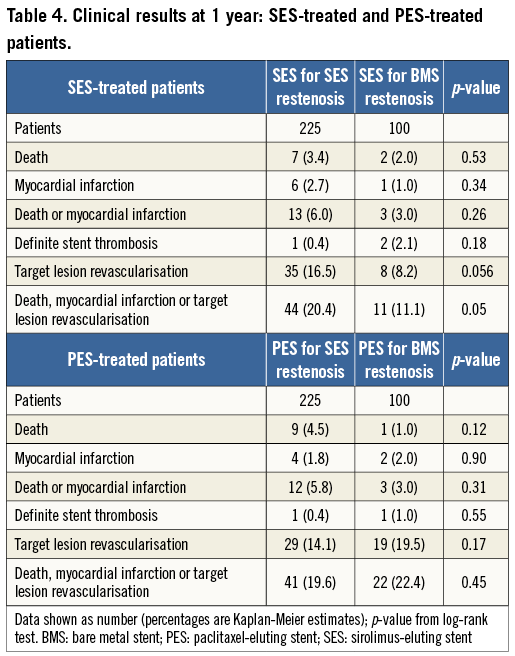
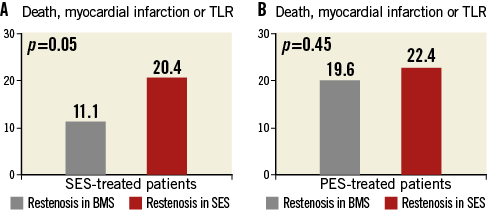
Figure 2. A) SES-treated patients: death, myocardial infarction or TLR at 12 months according to index stent type. B) PES-treated patients: death, myocardial infarction or TLR at 12 months according to index stent type. BMS: bare metal stent; PES: paclitaxel-eluting stent; SES: sirolimus-eluting stent; TLR: target lesion revascularisation
Discussion
The results of the current analysis demonstrate that the efficacy of sirolimus-eluting but not paclitaxel-eluting stents is significantly reduced when used for treatment of SES restenosis as compared to bare metal stent restenosis. In particular, lower absolute levels of neointimal suppression (measured as angiographic late loss or percentage diameter stenosis) were observed following SES implantation in patients presenting with SES restenosis as compared with bare metal stent restenosis. This difference was not observed following PES implantation. At multivariate analysis, SES implantation in patients presenting with SES restenosis was independently associated with in-stent late loss and percentage of in-segment diameter stenosis. These findings lend some support to the aetiological relevance of drug hyporesponsiveness in restenosis occurring within SES. Although the present study lacks sufficient power to address definitively the clinical impact of the observed lower antirestenotic efficacy of SES implantation in patients presenting with SES restenosis, the correlation of angiographic performance with reduced overall clinical efficacy supports the clinical relevance of these observations.
Although these findings may be regarded as novel, some caution is required in the interpretation of the results and the data should be regarded as hypothesis-generating in nature. Two limitations in particular should be considered in further detail. First of all, the analysis is of pooled individual data from two randomised trials enrolling different patient populations (those with bare metal stent restenosis in ISAR-DESIRE and those with DES restenosis in ISAR-DESIRE 2) at different historical time points. Indeed, comparison of differences in the overall study populations (Table 1) is in itself not without merit. Patients with restenosis within DES enrolled in ISAR-DESIRE 2 were older and more likely to have hypertension, hyperlipidaemia and multivessel disease. This highlights the uptake of catheter intervention in increasingly complex patient groups following the introduction of DES therapy. Against this, patients with restenosis within bare metal stents enrolled in the ISAR-DESIRE trial were notable for longer lesion length and smaller vessel size (both subgroups where bare metal stent efficacy was most significantly compromised) as well as more diffuse pattern restenosis (a well-recognised feature of bare metal stent restenosis). On the other hand, the internal validity of the current analysis is supported by common inclusion criteria and recruitment centres in the individual studies, the equal contribution of both trials to the SES-treated and PES-treated groups and the lack of baseline differences between the treatment groups in the individual randomised trials.
Secondly, it is widely appreciated that drug-eluting stent restenosis is a multifactorial clinicopathological entity3,4. As such, it must be considered that the observed decrement in efficacy when the CYPHER SES was implanted for SES restenosis might conceivably be due to other device-specific factors, including polymer hypersensitivity or loss of structural integrity (stent fracture). In this respect, however, by comparing the relative efficacy of the SES and PES in bare metal stent versus SES restenosis, we attempted to isolate the potential contribution of drug hyporesponsiveness to neointimal inhibition and clinical efficacy, using the performance of both DES in bare metal stent restenosis as a benchmark.
Recent experience from other clinical trials provides conflicting evidence on the impact of drug hyporesponsiveness in DES restenosis in terms of defining the optimal treatment of these patients. In the RIBS-III study, Alfonso and colleagues reported that a “hetero-DES” strategy –namely the switch to a different DES to manage DES restenosis– is superior to a “homo-DES” strategy –the use of the same DES for DES restenosis– in terms of both angiographic and clinical efficacy9. On the other hand, however, an earlier angiographic study by Cosgrove et al showed similar results with a same or switch strategy (albeit with an angiographic follow-up rate of only 69%)10 and Garg et al also found no differences in clinical events in a small observational cohort11.
At a mechanistic level, the resistance or hyporesponsiveness to sirolimus and its analogues is well described in the oncological literature12,13. Sirolimus and related limus compounds (rapamycins) exert their cellular actions by complexing with FKBP12 before inhibiting the mammalian target of rapamycin (mTOR), a central integrator of cellular signal transduction. At a vascular smooth muscle level, these drugs inhibit the injury-induced growth-factor-mediated cellular proliferation that is a hallmark of vascular restenosis and this forms the cornerstone of effective DES therapy. The mechanisms of resistance to sirolimus are multiple and include decreased drug binding to FKBP12 and impaired interaction of the FKBP12-drug complex at the mTOR receptor, usually due to mutation in either of these proteins. In addition, mutations or defects in principal downstream effector molecules such as S6K1, 4E-BP1 and p27Kip1 result in resistance to the antimitogenic effect of sirolimus. The prevalence of such mutations in coronary disease patients and their impact on DES restenosis remain to be delineated. Potentially, however, genetic testing might conceivably be used to identify a priori the patients whom we encounter in clinical practice who appear to exhibit resistance to this class of drugs. Such data might in turn provide an evidence base for tailoring stent choice in selected patient groups.
It should be acknowledged that the results relating to potential drug hyporesponsiveness in the present analysis do not relate to patients with restenosis within PES. However, in this respect we previously published an analysis of patients with angiographic follow-up after SES implantation for PES restenosis14. These patients had an in-stent late loss of 0.32 mm and an in-segment percentage diameter stenosis 36.5% – findings not dissimilar to the SES restenosis group treated with SES in the current analysis. These relatively high percentage diameter stenosis rates in particular serve to emphasise that outcomes for DES restenosis patients in general remain suboptimal2,15 – a condition that might be termed patient (not just drug) hyporesponsiveness. Finally, in keeping with the design of both included studies, the current analysis focuses primarily on angiographic endpoints; this analysis is underpowered for comparisons in relation to clinical events.
In conclusion, the findings from the present analysis of the ISAR-DESIRE and ISAR-DESIRE 2 trials show that the efficacy of sirolimus-eluting but not paclitaxel-eluting stents is significantly reduced when used for treatment of SES restenosis as compared to bare metal stent restenosis. The lower antirestenotic efficacy following SES implantation in patients with SES restenosis lends support to the hypothesis that drug resistance plays a role in restenosis within these stents.
Funding
There was no extramural funding used for this work.
Conflict of interest statement
The authors have no conflicts of interest to declare.

