Abstract
Aims: Recent trials with different designs indicated that drug-eluting stents may be superior to vascular brachytherapy (VBT) for the treatment of in-stent restenosis (ISR). We performed a randomised, double-centre, clinical, quantitative coronary angiographic (QCA) and intravascular ultrasound (IVUS) acute and 3-years comparison of 90Sr/90Y-VBT and sirolimus-eluting stent implantation (SES) for ISR.
Methods and results: Ninety-one (91) consecutive patients were included. By QCA, SES led to a higher acute gain (2.08±0.41 mm vs. 1.54±0.70 mm, p<0.0001), higher postprocedural minimum lumen diameter (2.76±0.39 mm vs. 2.39±0.52 mm; p<0.0001), lower late lumen loss at follow-up (0.09±0.29 vs. 0.39±0.79 mm, p=0.042), and a higher net lumen gain of the target lesion (2.05±0.51 vs 1.18±1.08 mm, p<0.0001). By IVUS, the smaller acute gain following VBT was the result of residual intima hyperplasia, the intima hyperplasia formation following SES was extremely low, and the edge-effect was virtually absent after SES, respectively. At 6-month follow-up, both the angiographic restenosis rate (4.7 vs. 22.7%; p<0.0001) and target lesion revascularisation rate (2.3 vs. 10.4%; p=0.025) were lower in SES. Importantly, SES showed a stable clinical course at 3-year follow-up while VBT was associated with a sustained incidence of target vessel failure (11.6 vs. 46.7%; p<0.0001).
Conclusions: SES for ISR is associated with superior QCA, IVUS and clinical results at 6-month and 3-year of follow-up when compared with VBT.
Introduction
Despite significant progress in prevention and treatment, in-stent restenosis (ISR) remains a challenge. Unfortunately, various interventional techniques yielded, despite favourable acute results, unsatisfactory long-term results1-5. The largest evidence of medical effectiveness for the treatment of ISR still exists for vascular brachytherapy (VBT), with a proven reduction of the incidence of repeat restenosis and major adverse cardiovascular events by approximately 50%, as compared to angioplasty alone6-11. Yet, VBT is time-consuming, requires an interdisciplinary approach, and its clinical efficacy is hampered by the edge-effect, late thrombotic occlusion and late catch-up. The use of drug-eluting stents might overcome most of these limitations. Easy to use, their potential to reduce neointima formation in de novo lesions has been demonstrated in several randomised clinical trials12-15. When used for ISR, data from recent observational and randomised studies with varying trial designs and endpoints indicate similarly favourable results for both paclitaxel- (PES) and sirolimus-eluting stents (SES)16-26. The respective radiation arms of two randomised trials published recently were heterogeneous in a way that either beta- and gamma-radiation or 90Sr/90Y and 32P nuclides were used23,24 or exclusive beta-VBT with 90Sr/90Y was compared with paclitaxel-eluting stents only25,26. Furthermore, no systematic intravascular ultrasound analysis was performed in these trials. Long-term data are available only from the SISR and TAXUS-V trials and inherit the above mentioned limitations27,28. Thus, we conducted a prospective randomised, double-centre, clinical, quantitative coronary angiographic (QCA) and intravascular ultrasound (IVUS) trial comparing adjunctive beta-VBT using 90Sr/90Y exclusively and SES exclusively for the treatment of ISR, with complete clinical and morphometric follow-up at six months and clinical follow-up at three years. The reported study is the first European trial of this kind, and had already been initiated when the short-term results of the two American randomised trials were published.
Methods
Study design and population
To determine the necessary sample size, a power calculation was performed based on the following assumptions: 30% reduction of intima hyperplasia formation with SES compared to VBT, a power of 80%, a level of significance of <0.05 and a drop-out rate of 15%. We thus calculated a necessary study population of 100 patients. Inclusion criteria were objective signs of exercise-induced myocardial ischaemia, reference vessel diameter ≥2.5 mm and angiographic diameter stenosis ≥50%. The ISR pattern had to be diffuse and limited to the stent body. Exclusion criteria were acute myocardial infarction within the previous four weeks, intolerance of antiplatelet therapy, history of gastrointestinal bleeding, pregnancy, left main coronary artery disease, significant lesion calcification, a target lesion in arterial or venous bypass-grafts, and oral anticoagulation. Myocardial infarction was defined as chest pain lasting longer than 30 minutes and associated with either electrocardiographic elevation or depression of the ST-segment and/or elevation of the creatine phosphokinase for at least twice the upper limit. Stent thrombosis was assumed only when coronary angiography revealed the typical respective pattern.
After patients had been given signed informed consent they were randomised to the respective stratum before the initiation of the index procedure. Data were analysed on an intention-to-treat principle.
Angioplasty and brachytherapy procedure
Angioplasty was performed via the transfemoral approach in all patients. Heparin was administered intravenously to achieve an activated clotting time >250 sec. Preprocedural coronary angiography was performed after intracoronary administration of 0.25 mg nitroglycerine. Preprocedural IVUS was performed using a solid state electronic phased array system (Invision Gold™; Jomed, Uppsala, Sweden). In the VBT cases, dilatations, using plain balloons or a cutting-balloon, were restricted to the stent body and repeated until a visually estimated residual stenosis smaller than 20% was obtained. Irradiation was applied using a monorail-type 5 Fr delivery catheter (Novoste™; Novoste Corporation, Norcross, GA, USA) to hydraulically deliver a source train of 90Sr/90Y seeds with a length of 60 mm. For SES implantation the direct stent technique was attempted, but pre-dilatation was allowed if necessary. Stent length was selected in order to entirely cover the injured vessel segment by implantation of a single SES (Cypher™; Cordis, Warren, NJ, USA). Post dilatation was performed only if the primary angiographic result was not satisfactory. Postprocedural coronary angiography and IVUS completed the procedure. Clopidogrel was administered for one year in VBT patients, and for six months in SES patients.
Angiographic measurements
Coronary angiography was performed in two orthogonal projections using identical projection angles and table height throughout the entire procedure. After intracoronary injection of 0.25 mg nitroglycerine reference lumen diameter, minimum lumen diameter, percent diameter stenosis and lesion length were calculated (CAAS II; Pie Medical, Maastricht, The Netherlands) off-line by experienced investigators not involved in the index procedure. Derived variables comprised: acute gain= postprocedural minimum lumen diameter – preprocedural minimum lumen diameter; late loss=postprocedural minimum lumen diameter – follow-up minimum lumen diameter; net gain= follow-up minimum lumen diameter – preprocedural minimum lumen diameter. Angiographic success was defined as residual stenosis <20%, TIMI grade III flow and absence of significant dissection or intraluminal filling defect.
Intravascular ultrasound analysis
Images were acquired by standardised motorised pullback at a speed of 0.5 mm/sec. Data were stored digitally. Two experienced investigators, blinded to the actual angioplasty procedure, performed quantitative analysis using a commercially available dedicated software package (Marvin™; Tim Becker, Kiel, Germany). Results were averaged. The analysed vessel segment comprised the stented segment and the proximal and distal reference segment (5 mm each). Cross-sectional area of the lumen, the stent and the external elastic membrane were manually traced in steps of 1 mm. The individual image sections of the respective vessel segments were averaged per patient and subsequently processed. Calculated parameters were: reference segments: mean plaque + media cross-sectional area=mean external elastic membrane cross-sectional area – mean lumen cross-sectional area; stented segment: mean peristent plaque cross-sectional area=mean external elastic membrane cross-sectional area – mean stent cross-sectional area; mean intimal hyperplasia cross-sectional area=mean stent cross-sectional area – mean lumen cross-sectional area.
Data collection and statistical analysis
Clinical, procedural, QCA and IVUS data were collected in a computerised database (Filemaker Pro 8.5™; FileMaker, Santa Clara, CA, USA). Statistical analysis was performed by a dedicated software package (SPSS for Windows 15.0; SPSS Inc., Chicago, IL, USA). Categorical variables were expressed as percent frequencies and continuous variables as mean value±standard deviation. χ2-test was used to compare categorical variables; unpaired t-test was performed for comparison of continuous variables. Estimates of survival were calculated by the Kaplan-Meier method. A p-value <0.05 was considered significant.
Results
Clinical and angiographic baseline characteristics
The clinical data of the 91 study patients are given in Table 1.
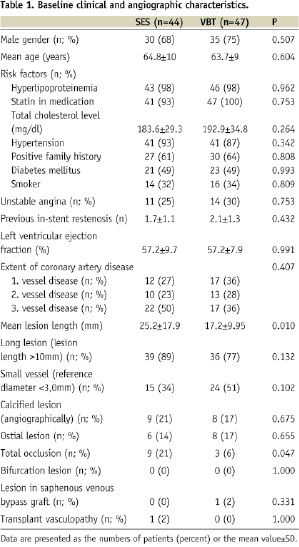
At baseline there were no significant differences between groups besides a higher prevalence of total occlusions (20.5 vs. 6.4%; p=0.047) and a greater lesion length (25.2 vs. 17.2 mm; p=0.010) in SES. Compared to the extent of coronary artery disease, patients were relatively young, but yielded a high prevalence of cardiovascular risk factors. Approximately 40% of the patients had three-vessel coronary artery disease. The prevalence of long lesions (>10 mm) and of small vessel reference diameter (<3.0 mm) was very high, and complex lesion morphologies were frequent.
Procedural and quantitative angiographic data
Eighty-six (86) patients (95%, SES 43, VBT 43) had an angiographic follow up after six months. Procedural and quantitative angiographic parameters at baseline and at 6-month follow-up are provided in Table 2.
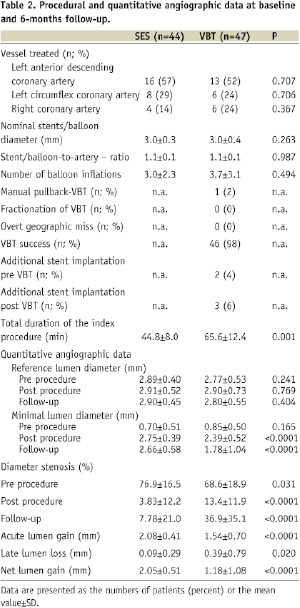
The index procedure was successful in all cases. Angioplasty devices were moderately oversized, with no differences between groups. In 12 patients (26%) of the VBT group additional implantation of one single stent was necessary. The radiation dose, prescribed at 2 mm from the longitudinal axis of the source train, varied according to the angiographically determined reference diameter (2.7 to 3.35 mm: 18.4 Gy; 3.35 to 4.0 mm: 23.0 Gy; >4.0 mm: 25.3 Gy). Fractionation of irradiation was not necessary in any case, overt geographic miss could be avoided in all patients, and additional stent implantation following VBT was infrequent. The time required for the index procedure was significantly shorter in SES when compared with VBT (SES 44.8±8.0 min vs. 95.6±12.4 min; p=0.001). All patients received dual antiplatelet therapy for six months. By QCA, the acute gain measured in SES was significantly higher than in VBT (SES 2.08±0.41 mm vs. VBT 1.54±0.70 mm, p<0.0001), leading to a higher postprocedural minimum lumen diameter in SES (SES 2.75±0.39 mm vs. VBT 2.39±0.52 mm; p<0.0001). The late loss was very low in SES and significantly lower than in VBT (SES 0.09±0.29 mm vs. VBT 0.36±0.79 mm, p=0.020), resulting in a significantly higher minimal lumen diameter at follow-up (SES 2.66±0.58 mm vs. 1.78±1.04 mm; p<0.0001) and net gain (2.05±0.51 mm vs. 1.18±1.08 mm; p<0.0001) in SES, respectively.
Intravascular ultrasound findings
A total of 8,190 cross-sectional images were analysed. Eighty-six percent (7,034) of the images were suitable for quantitative analysis. The relevant parameters are summarised by Figures 1 and 2.
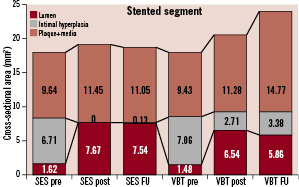
Figure 1. Cross-sectional analyses before and after the index procedure and at follow-up of the stented vessel segment. Treatment of in-stent restenosis by implantation of a sirolimus-eluting stent (SES) is compared with angioplasty and adjunctive vascular brachytherapy (VBT).
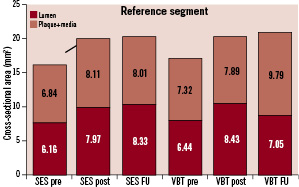
Figure 2. Cross-sectional analyses before and after the index procedure and at follow-up of the reference segment after implantation of a sirolimus-eluting stent (SES) or angioplasty and adjunctive vascular brachytherapy (VBT) for in-stent restenosis.
Baseline parameters did not differ between groups. The preprocedural stent cross-sectional area (SES 8.33±1.2 mm2 vs. VBT 8.54±1.6 mm2, p=0.236) is indicative of a good expansion of the originally implanted stents. A higher increase of the lumen cross-sectional area in SES (SES 6.05±1.4 mm2 vs. VBT 5.06±1.5 mm2, p<0.0001) led to a higher postprocedural lumen cross-sectional area (SES 7.67±1.5 mm2 vs. VBT 6.54±1.7 mm2, p<0.0001). Stent malapposition was not observed. After SES implantation, the final lumen cross-sectional area measured 92.8% of the preprocedural stent cross-sectional area (VBT 76.5%). At follow-up, obtained in 86 patients (95%, SES 43, VBT 43), a lower intima hyperplasia formation was observed in SES (SES 0.13±0.5 mm2 vs. VBT 0.67±0.8 mm2, p<0.0001), resulting in a higher lumen cross-sectional area (SES 7.54±2.0 mm2 vs. VBT 5.86±2.1 mm2, p<0.0001) and net gain at follow-up. Of note, a higher external elastic membrane cross-sectional area could be demonstrated in VBT (SES 18.65±7.8 mm2 vs. VBT 24.01±11.1 mm2, p=0.001), indicating positive remodelling at the target lesion group following VBT.
At the reference segment, there were no differences between groups before and after the index procedure. In contrast, the lumen cross-sectional area at 6-months follow-up was smaller in VBT both at the proximal (SES 8.73±3.4 mm2 vs. VBT 7.62±3.3 mm2, p=0.002) and the distal (SES 7.94±3.0 mm2 vs. VBT 6.75±3.0 mm2, p=0.006) reference segment. This was the result of a more pronounced edge-effect caused by an increase of the plaque + media cross-sectional area resulting in a higher plaque + media cross-sectional area at follow-up.
Clinical follow-up
The clinical long-term outcome is delineated in Table 3 and Figures 3 and 4, respectively.
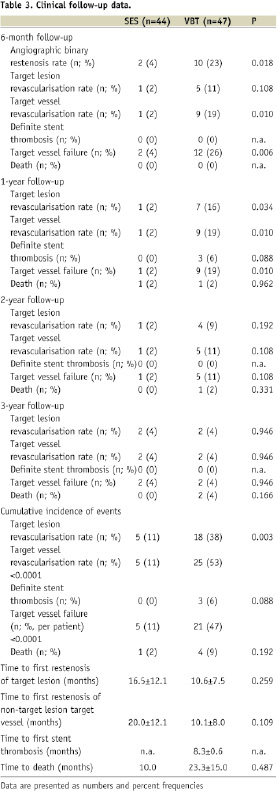
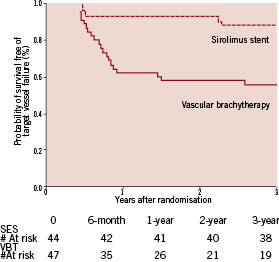
Figure 3. Kaplan-Meier estimates of the probability of freedom of target vessel failure following vascular brachytherapy or sirolimus-eluting stent implantation over three years of follow-up.
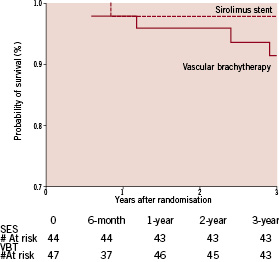
Figure 4. Kaplan-Meier estimates of the probability of survival over three years of follow-up.
The incidence of target lesion revascularisation (TLR) was higher in VBT throughout the first two years after the index procedure, yet only moderately significant or by trend. The cumulative incidence of TLR at 3-year follow-up, in contrast, was higher in VBT (SES 11% vs. VBT 38%; p=0.003), reflecting the sustained restenosis process in VBT. Target vessel revascularisation (TVR) was more frequent in VBT predominantly during the second half of the first year after the index procedure, indicating the development of edge-effect within this time-frame. No single edge-effect had been observed in the SES group. Thus the overall TVR rate showed a highly significant difference between groups (SES 11% vs. VBT 53%; p<0.0001). Similarly, the total incidence of target vessel failure per patient (Figure 3), including angiographic restenosis (target lesion revascularisation), target vessel revascularisation and thrombotic occlusion, was greater in VBT (SES 11% vs. VBT 47%; p<0.0001). All revascularisation procedures were driven by clinical or objective signs of ischaemia. According to the ARC-definitions29 there was one possible (sudden cardiac death) but no definite stent thrombosis observed in SES throughout the entire observation period while there were three definite stent thromboses in VBT, all of them within the first year after the index procedure. In all cases of definite stent thrombosis the patients had received an additional stent at the index procedure and had discontinued dual antiplatelet therapy. Death (Figure 4) occurred in one patient of either group within the first year (SES sudden cardiac death, VBT chronic heart failure), in one patient in VBT within the second year (end-stage renal failure) and in two patients in VBT within the third year (cardiogenic shock, pulmonary embolism), respectively.
Discussion
Before drug-eluting stents had been introduced in clinical medicine, VBT had been regarded the gold-standard in the treatment of ISR, with a large evidence of superiority over all previous interventional techniques1-10. Yet, VBT constantly has been criticised for potential drawbacks like late thrombotic occlusions, edge-effect and late catch-up. Moreover, the implantation of drug-eluting stents was reported to be superior to VBT at 9-month follow-up20-26. Yet, data on longer follow-up intervals in randomised trials are still scarce, but nevertheless clinically important regarding the ongoing safety discussion of new anti-restenotic treatment strategies. To date, only the 2-year follow-up of the TAXUS-V and the SISR trials have been published, indicating relatively stable results after the implantation of paclitaxel- or sirolimus-eluting stents27,28. The other published trials were either not randomised or used Rhenium as beta-radiation source in the VBT group30,31.
In a dual-centre, prospective and randomised study with a clinical follow-up of up to three years, we analysed and compared the underlying mechanisms of SES, using the Cypher™ stent, and beta-VBT, with the Novoste™ 90Sr/90Y beta-irradiation, in the treatment of ISR. The investigation included a QCA and, in contrast to all other published trials, a complete IVUS analysis. The study results revealed that SES, in contrast to VBT, were associated with a larger acute lumen gain and a less pronounced late lumen loss, respectively, resulting in a larger lumen diameter at follow-up. Furthermore, VBT was associated with a considerably higher prevalence of edge-effect. Clinically, the incidence of target vessel failure was sustained in the VBT group while stable in SES.
Procedures were all performed successfully, and there were no major clinical complications including distal embolisation, stent thrombosis leading to acute myocardial infarction, or death. This has to be regarded at in the light of the morbidity of the study population and the complexity of the lesion morphology.
As a determinant of long-term success after angioplasty of ISR, the postprocedural final lumen diameter has been identified as the single most important procedural predictor of restenosis32. Yet, the realisation of a sufficient acute lumen gain when treating ISR is difficult. It has been shown that a postprocedural lumen cross sectional area equal to the original stent cross-sectional area can not be achieved.33-35 In our report, the percent post-interventional lumen cross-sectional area after SES (92.8%) was more favourable than after VBT (76.5%), but nevertheless did not reach original stent dimensions. Regarding SES, our data indicate the need for high balloon pressures to achieve sufficient acute lumen gain underlying the notion that implantation of a second stent in ISR had been associated with severe stent underexpansion21,35. Mechanistically the low vessel compliance as a result of pre-existing plaque burden, the originally implanted stent, the pre-existing non-compressible intimal hyperplasia and the additional stent may serve as an explanation for this critical issue.
Another major determinant of long-term success following angioplasty – independent of the therapeutic modality – is late lumen loss. In VBT studies where serial IVUS was performed the intima hyperplasia formation averaged 0.5 mm2 (range 0.1 mm2 to 0.9 mm2)7-10. In our study, the amount of intimal hyperplasia cross-sectional area in the VBT group measured 0.67 mm2, thus comparing well with previous data. When SES were implanted to treat ISR, the extent of intima hyperplasia showed to be approximately twice as compared with de novo lesions (intima hyperplasia cross sectional area of 0.19±0.36 mm2 after four or 12 months, respectively, and 0.2±0.5 mm2 after six months), which yet yielded to be less than half of what had been reported for VBT16,18,19,21,22. The intimal hyperplasia cross-sectional area data of our study measured 0.13±0.5 mm2, thus compared favourably with previous results and confirmed the notion that SES for the treatment of ISR is more effective than VBT when used in ISR. To appraise the results of our trial in relation to the two largest randomised trials, QCA data have to be compared since IVUS was not performed in these studies. In the SISR (Sirolimus-Eluting Stents vs. Vascular Brachytherapy for In-Stent Restenosis Within Bare-Metal Stents) the late loss at 6-month follow-up was not significantly different between groups, possibly due to a relatively high late loss in the SES group and a low late loss in the VBT group (SES 0.27±0.55 mm vs. VBT 0.33±0.54 mm; p=0.17, analysis segment)24. In the TAXUS V trial the median late loss was lower in the PES arm by trend (PES 0.13 mm vs. VBT 0.22 mm; p=0.08, analysis segment)25. Of note, the absolute data have to be appreciated as very favourable being widely equal to most trials on de novo lesions. Of note, the findings are divergent from the results of the ISAR-DESIRE study (intracoronary stenting and angiographic results – drug-eluting stents for in-stent restenosis) where SES and PES were compared head-to-head (SES 0.32 mm, interquartile range 0.03 to 0.74 mm, vs. PES 0.54 mm, interquartile range 0.23 to 0.90 mm)36.
The accumulation of plaque burden without adaptive positive remodelling at the reference segment “edge-effect” is another important confounder of long-term outcome following angioplasty6-10,37. Indeed, “edge-effect” following VBT was prominent in our study. For SES, initial studies also reported the existence of relevant “edge effect”, thought to be due to incomplete coverage of the entire injured vessel segment with the SES14. In subsequent trials where complete coverage was achieved, this problem seemed to be resolved. In our study, “edge-effect” was not present in SES.
Our data provide good evidence that the restenosis process following VBT is sustained over longer observational intervals. This is in accordance to data published for VBT using both gamma and beta-irradiation28,38-40. More favourably, the clinical course of SES implantation for ISR seemed to be very stable after three years of follow-up, with cumulatively only five TVR procedures, distributed homogenously along the observation period. Thus the clinical efficacy of SES for ISR may be well comparable to the long-term results obtained from studies on SES for the treatment of de novo lesions. The results may also be appreciated in the light of complete absence of thrombotic occlusions following SES implantation.
The reported data inherit strengths and limitations. Due to the fact that the study design is distinctly different from the other two randomised trials, with a more complex patient population, with a comparison of beta-VBT and sirolimus-eluting stent implantation not investigated before, with complete intravascular analysis and a comparably long follow-up period, our study may well add some noteworthy details to the existing evidence on the restenosis process following treatment of in-stent restenosis.
Several limitations of the study deserve attention. The number of patients is limited. Nevertheless, our study could demonstrate not only clear-cut differences of surrogate, but also of clinical endpoints. The clinical and angiographic complexity of the SES group may be regarded as slightly higher. Despite this the reported results were clearly favourable in the SES group and might be regarded as the expected minimum. The lesions included in the study were highly selected. They were restricted to diffuse and non-proliferative types of in-stent restenosis and significant calcification was an exclusion criterion. Thus the analysed population is not completely representative.
Conclusion
SES for ISR is associated with superior QCA, IVUS and clinical results at six months and three years of follow-up when compared with VBT.

