Abstract
In-stent restenosis (ISR) remains the most common cause of stent failure after percutaneous coronary intervention (PCI). Recent data suggest that ISR-PCI accounts for 5-10% of all PCI procedures performed in current clinical practice. This State-of-the-Art review will primarily focus on the management of ISR but will begin by briefly discussing diagnosis and classification. We then move on to detail the evidence base underpinning the various therapeutic strategies for ISR before finishing with a proposed ISR management algorithm based on current scientific data.
Introduction
In-stent restenosis (ISR) is characterised by a significant reduction in the luminal diameter within the stented segment after a successful percutaneous coronary intervention (PCI) and remains the most common cause of stent failure12. While the relative incidence of ISR has reduced with newer drug-eluting stent (DES) technologies in comparison to the bare metal stent (BMS) era34, treatment of ISR still accounts for 5-10% of all PCI procedures performed in clinical practice56. In this State-of-the-Art review, we will comprehensively discuss current evidence-based strategies for the management of ISR in contemporary interventional practice.
In-stent restenosis
Traditionally, ISR has been defined as a reduction ≥50% of the luminal diameter within the previously stented segment or the vessel segments 5 mm proximal and distal to the stent (the “stent edges”), as assessed by coronary angiography7. Using intravascular imaging (IVI), which acquires data in three dimensions, ISR has been defined as a re-narrowing of ≥75% of the reference vessel area in cross-section8. This definition follows that of classical autopsy studies, which also usually define ISR as a pathological vessel re-narrowing ≥75% of the reference vessel area in cross-section910. However, ISR can also be considered as a pathophysiological continuum and can therefore also be reported using continuous parameters. This continuous pathophysiological approach may be better suited for comparisons of the relative anti-restenotic efficacy of stent- and balloon-based treatment modalities.
The term “clinical restenosis” is sometimes used to refer to ISR associated with symptoms or signs of ischaemia11. Given that not all ISR results in symptoms or signs of ischaemia (referred to as “silent restenosis”), rates of clinical restenosis are consequently lower than overall rates of ISR. Similarly to de novo coronary artery disease (CAD), percutaneous interventional treatment of ISR may be indicated for patients presenting with either acute coronary syndrome (ACS) or chronic coronary syndrome (CCS).
Relevance of ISR in modern interventional practice
ISR can be considered the “Achilles heel” of modern PCI. In the United States (US), recent data has suggested that treatment of ISR may account for up to 10% of all PCI procedures performed, whereas in Europe it has been reported to constitute ~5% of all PCI procedures56. ISR is recognised to be challenging to manage. Although ISR lesions can appear to be straightforward to treat percutaneously (and are nearly universally associated with angiographic success), they are associated with a high risk of recurrent clinical events after treatment, including recurrent target lesion revascularisation (TLR). ISR can also frequently present clinically as an ACS, with associated morbidity and mortality512. Therefore, the development of strategies to prevent and optimally manage ISR is an important challenge for interventional cardiologists, with the potential to improve patient outcomes and to reduce morbidity and mortality.
Angiographic classification
The most commonly used ISR classification system was proposed by Mehran et al13 according to angiographic patterns. This system classified patients with BMS-ISR into groups based on three characteristics: ISR length (≤10 mm: focal, >10 mm: diffuse), ISR location (within or beyond stent borders) and occlusion (yes or no). Application of this classification system results in four main groups: Type I: focal; Type II: diffuse, within stent; Type III: diffuse, within and beyond stent; and Type IV: occlusive13. In this study, late angiographic surveillance was not required but the different angiographic patterns were associated with prognostic value with regard to the subsequent need for TLR13. This classification was based on BMS-ISR and therefore may not be as relevant for DES-ISR, where a greater proportion of ISR is focal in nature14. In addition, this classification is based only on angiographic appearances and therefore provides no information about the underlying pathophysiological mechanisms of ISR. The types of interventional treatment used in this study (balloon angioplasty [BA], stent alone, rotational atherectomy [RA]±stent, and excimer laser coronary angioplasty [ELCA]±stent) varied widely between the four ISR types and it is important to consider that this may also have contributed to the differences observed in rates of subsequent TLR13.
Mechanisms of ISR
ISR is an umbrella term which can refer to a wide variety of underlying pathophysiological processes. Therefore, a key step in any ISR treatment algorithm is the determination of the underlying mechanisms15. Several contributory mechanisms may coexist simultaneously16 and all should be identified and addressed if possible during the ISR therapeutic procedure.
Potential mechanical or technical factors associated with ISR include stent undersizing, stent underexpansion, vessel calcification, stent fracture, and geographic miss1718. Biological mechanisms of ISR include neointimal hyperplasia and neoatherosclerosis19. Neointimal hyperplasia is defined as the accumulation of smooth muscle cells and extracellular matrix in the intima. Neoatherosclerosis is characterised by an accumulation of lipid-laden foamy macrophages, with or without necrotic core formation, and/or calcification within the neointima2021. Calcified neoatherosclerosis can be particularly challenging to manage and this finding may have relevance for decisions regarding percutaneous intervention2223.
ISR may be attributed to a variable degree of influence of 3 categories of factors in the stented coronary artery: extra-stent factors (i.e., which prevent adequate stent expansion like vessel calcification or multiple stent layers), stent-related factors (i.e., stent undersizing, stent fracture) and intra-stent factors (i.e., excessive tissue proliferation within the stent). These factors can coexist simultaneously and are summarised in Figure 1.
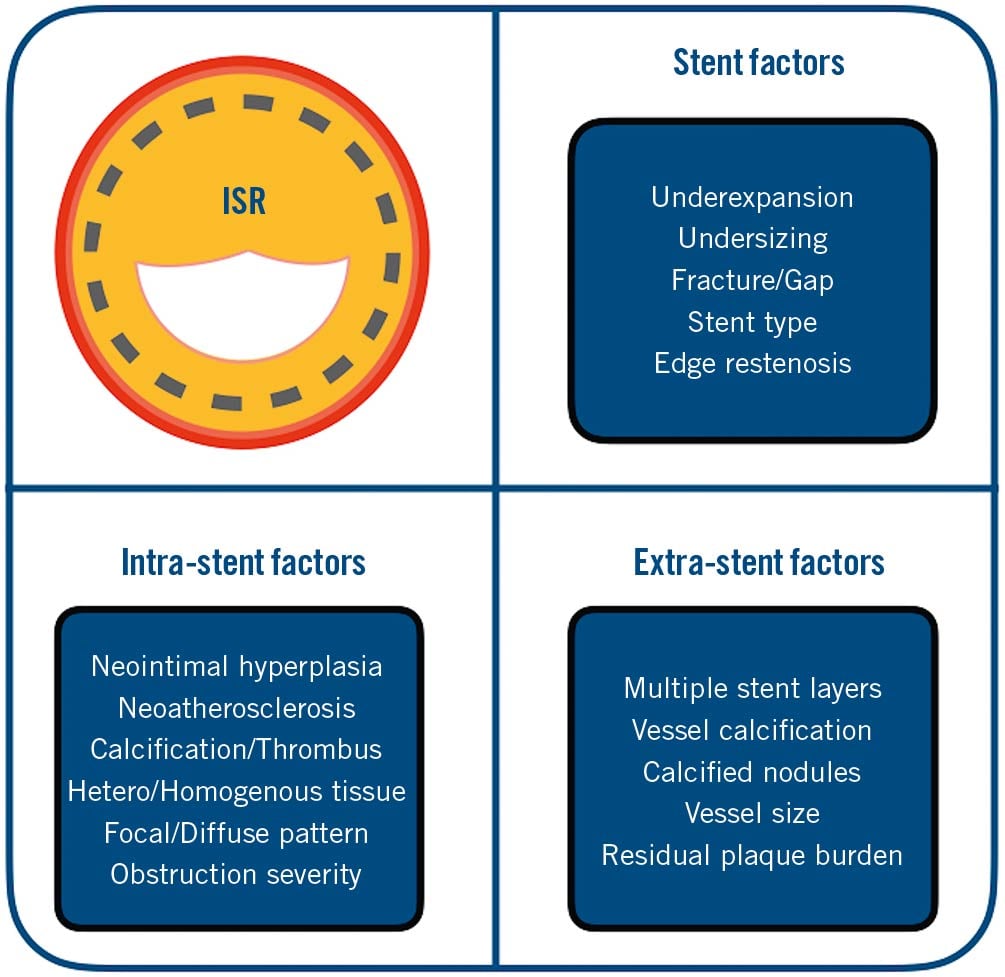
Figure 1. Factors to consider when performing ISR-PCI. ISR may be attributed to a variable degree of influence of 3 categories of factors in the stented coronary artery: extra-stent factors, stent-related factors and intra-stent factors. ISR: in-stent restenosis; PCI: percutaneous coronary intervention
Mechanisms of ISR: BMS-ISR vs DES-ISR
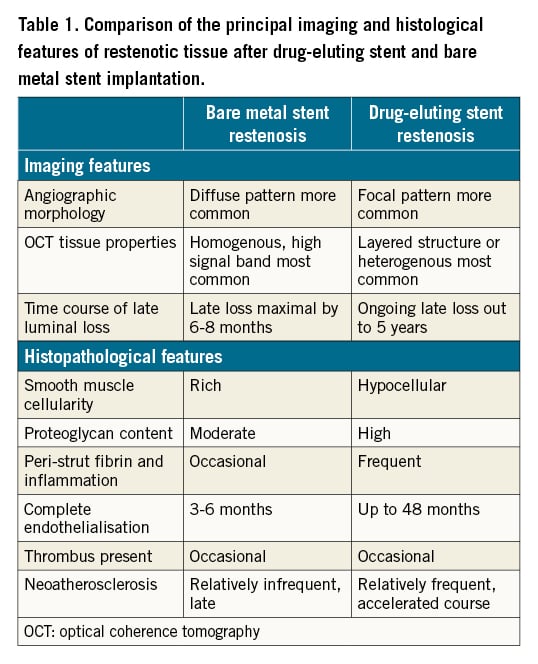
Despite the obvious superficial commonalities, current evidence suggests that BMS-ISR and DES-ISR could be considered as distinct pathological entities19. This paradigm may be supported by the recognised differences between the two conditions with regard to angiographic appearances, time course of late lumen loss (LLL), IVI morphology, histopathology, and clinical response to interventions10161920. These differences are summarised in Table 1. Therefore, knowing whether the originally implanted stent was a BMS or DES is very useful when managing ISR.
Traditionally, it has been reported that neointimal hyperplasia is encountered more frequently in BMS-ISR, whereas in DES-ISR, neoatherosclerosis is more common. However, this may be an oversimplification and it has been demonstrated that optical coherence tomography (OCT) findings suggestive of neoatherosclerosis are less common in DES-ISR within the first year post-PCI (early DES-ISR) compared to DES-ISR which develops after 1 year post-PCI (late DES-ISR)242526. In fact, a key factor in the difference in OCT findings observed between BMS-ISR and DES-ISR appears to be the timing of the ISR development associated with the two stent types, with BMS-ISR tending to develop earlier after PCI. Neo-atherosclerotic ISR appears to develop in tandem with native atherosclerotic disease progression, suggesting similar underlying mechanisms. However, neoatherosclerosis demonstrates an accelerated course in comparison to de novo atherosclerotic CAD27.
An intravascular ultrasound (IVUS)-based study reported that, of BMS-ISR lesions, 69% had dominant neointimal hyperplasia, 14% had dominant stent underexpansion, and 8% had co-dominant pathologies. In DES-ISR, 59% of lesions had dominant tissue proliferation, 18% had dominant stent underexpansion and 16% had co-dominant pathologies16. In that study, a cut-off value of <5 mm2 stent cross-sectional area (CSA) at the minimal lumen area (MLA) site was used to define stent underexpansion and a value of 50% percentage neointimal hyperplasia at the MLA site was used to define dominant intimal hyperplasia16. In the same study, 40% of second-generation DES-ISR lesions had a stent CSA of <5 mm2 and 66% had >50% neointimal hyperplasia, indicating both pathologies are commonly encountered in second-generation DES-ISR1628. However, a key limitation of this study was that it used greyscale IVUS and so could not evaluate the frequency of neoatherosclerosis.
Time course of ISR
The time course of ISR after stent implantation appears to be dependent on the underlying stent type and this may have relevance for the follow-up of patients post-coronary stent implantation. While BMS-ISR was recognised to peak within the first 6 months post-stent implantation, the incidence of DES-ISR appears to continue to increase steadily for several years after stent implantation as a result of different mechanisms, including accelerated neoatherosclerosis2029303132. It is important to consider that the incidence of ISR may also be dependent on the nature of the follow-up, with increased identification of “silent” ISR in patients who undergo routine angiographic surveillance.
Clinical presentation
ISR was initially considered to be a slowly progressive, relatively benign, pathological process. However, it is now increasingly recognised that ISR is not benign and can commonly present as an ACS3334. Many patients identified with ISR in clinical practice are found to have elevations in high-sensitivity troponin levels, fulfilling the current criteria for spontaneous myocardial infarction (MI)35. In patients with DES-ISR, IVI has demonstrated acute plaque rupture and stent thrombosis (ST), highlighting that these pathologies can be part of the spectrum of ISR presentations. Neoatherosclerosis may represent the link between ISR and these conditions, which were previously considered to represent distinct clinical entities. Notably, ACS presentation has also been reported to confer a higher risk of recurrent major adverse cardiovascular events (MACE) and angiographic restenosis after ISR-PCI36.
Imaging for ISR
IMAGING for ISR: stent enhancement
Enhanced fluoroscopic imaging techniques (i.e., StentBoost [Philips]), also known as “stent enhancement” techniques, have also been reported to be useful for the detection of inadequate stent expansion, demonstrating superior correlations for stent expansion measured by IVUS when compared with quantitative coronary angiography37. Stent enhancement is useful to both identify stent fracture and stent underexpansion and can be easily and quickly performed in the cath lab.
Imaging for ISR: intravascular imaging
IVI can overcome some of the diagnostic limitations of angiography and provide a wealth of further information to define and classify ISR. IVI enables not only a more accurate assessment of the degree of ISR but also an appropriate identification of the underlying pathophysiological mechanisms161738. Expert consensus and guidelines recommend (Class IIa, Level B) the use of IVI in order to assess ISR3940. Given that IVI allows for more precise determination of the underlying mechanisms and patterns of ISR, it is plausible to assume that this would enable more targeted treatment (through appropriate device and interventional strategy selection) with consequently improved outcomes41. However, it is important to acknowledge that there are currently no randomised controlled trials (RCTs) supporting the differential treatment of ISR based on IVI appearances. In fact, there is a paucity of data on long-term outcomes following IVI-guided treatment of ISR and the majority of RCTs on the management of ISR did not mandate the use of IVI424344. The two most common forms of IVI used in clinical practice are OCT and IVUS. Each has its own relative advantages and disadvantages in the management of ISR, which we will now briefly discuss.
Intravascular imaging: OCT
OCT, with a wavelength of 1.3 μm and axial resolution of 12-15 μm, provides 10 times the spatial resolution of IVUS39. For ISR, this allows uniquely detailed neointimal tissue characterisation and the identification of neoatherosclerosis1945. However, limitations of OCT include the need to use contrast media to ensure a blood-free field for image acquisition, which may be difficult to achieve in tight or ostial ISR lesions. Moreover, due to reduced tissue penetration with OCT compared to IVUS the external elastic lamina (EEL) cannot always be adequately identified, which can lead to difficulties with vessel sizing. This may be particularly challenging in patients with multiple metal stent layers. Although OCT requires the injection of higher volumes of contrast media, which may be proarrhythmogenic and increase the likelihood of post-procedural acute kidney injury, the occurrence of these complications is rare. As such, the advantages of OCT assessment are expected to outweigh the risk of complications in most patients with ISR.
Based on OCT appearances46, ISR can be classified into four groups:
– Homogeneous: uniform high signal intensity, low back-scatter, typical of areas of high smooth muscle cell content
– Heterogeneous: mixed signal intensity, may represent presence of proteoglycan-rich neointimal or early neoatherosclerotic plaque
– Attenuated: superficial high signal intensity, high back-scatter, likely indicative of lipid-core plaque
– Layered: most frequently presenting as superficial high signal intensity with deep low signal intensity often in peri-strut areas
Examples of OCT tissue patterns of ISR are shown in Figure 2. A homogeneous tissue pattern on OCT imaging is often considered typical of early-onset BMS-ISR46. The other three patterns (attenuated, layered and heterogeneous) may represent part of the neoatherosclerotic disease spectrum, which is more commonly seen in DES-ISR46. However, it is important to consider that all four patterns have been reported in both DES-ISR and BMS-ISR and their prevalence may differ with the timing of the ISR46. For example, while a homogenous tissue pattern may be considered typical of BMS-ISR, in very late BMS-ISR (>5 years post-stent implantation), a heterogenous tissue pattern has been reported to be more common47. Similarly, in DES-ISR, OCT findings corresponding to neoatherosclerosis become more common with increasing follow-up time post initial stent implantation2425. The tissue pattern may also vary along the length of the stent, with some segments showing a typical, healthy neointima and other segments displaying neoatherosclerosis, which may in some cases be complicated (i.e., with plaque rupture).
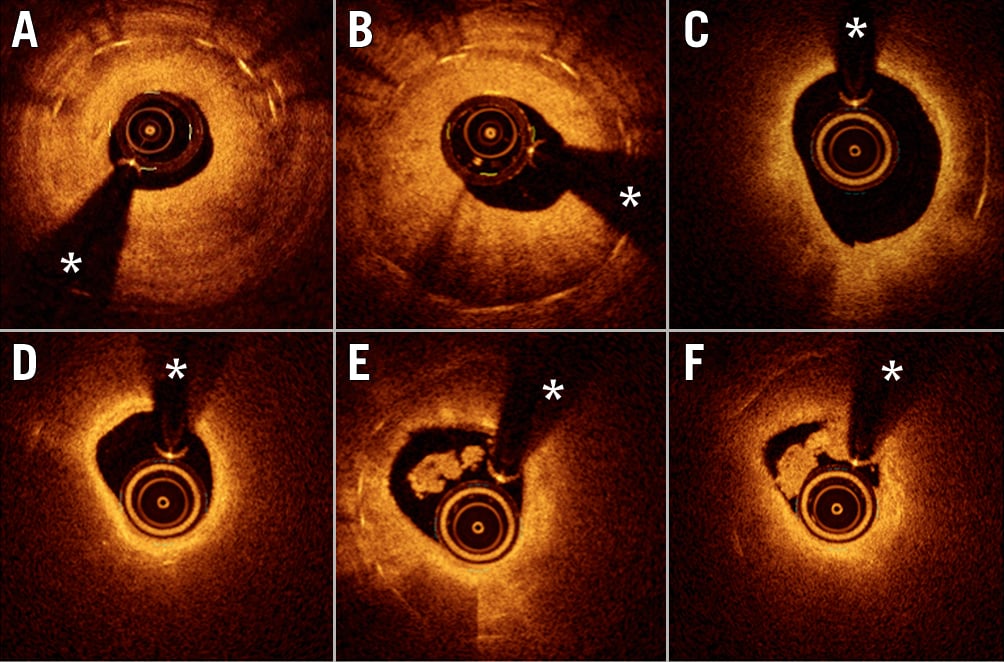
Figure 2. OCT images demonstrating distinct tissue patterns in patients with ISR. A - B) Demonstrate relatively homogeneous severe neointimal proliferation with some confined darker areas close to the underlying stent struts that are readily visualised (bright dots casting a dorsal shadow). C - F) Typical images of neoatherosclerosis. In C and D, a bright superficial intima and a large lipid plaque (dark area with diffuse borders) is demonstrated obscuring the underlying stent struts. A potential diagnosis of an intimal layer infiltrated by macrophages should be considered. In E and F, neoatherosclerosis with intracoronary thrombus is demonstrated. In E, the bright localised image with dorsal shadow (6-7 o’clock) is suggestive of clusters of macrophages. (* denotes wire artefact). ISR: in-stent restenosis; OCT: optical coherence tomography
Correlation between imaging and histology
The ISR IVI appearance is most likely representative of the underlying histopathology. A homogenous tissue appearance on OCT has been shown to correspond to neointima and fibrous connective tissue as a result of smooth muscle cell proliferation48. Conversely, heterogenous tissue patterns are associated with increased fibrin depositions and loose connective tissue48. In addition, large lipid pools have been detected in patients showing the typical lipidic pattern characteristic of neoatherosclerosis21. Knowledge of the likely underlying histopathology may be useful to inform treatment choices49. However, studies which assess whether selecting treatment modalities according to the underlying pathological appearance can lead to improved clinical outcomes are still required.
Intravascular imaging: IVUS
IVUS results in deeper tissue penetration, in comparison to OCT, and a blood-free field is not required. Given that the wavelength of IVUS is ~50 μm with an axial resolution of 150 μm, detailed tissue characterisation is not possible. However, IVUS can still demonstrate several findings relevant to ISR, including neointimal hyperplasia, mature neoatherosclerosis, stent underexpansion, stent undersizing, and vessel calcification1617. In addition, the EEL is usually well delineated, both at the reference segment and beyond the stent struts, allowing accurate vessel sizing. Virtual histology IVUS has also been used to demonstrate neoatherosclerosis in both BMS-ISR and DES-ISR, although this technology is not widely used in clinical practice at present50.
Intracoronary physiology
Symptoms or documented ischaemia associated with the ISR lesion should be demonstrated before proceeding with repeat revascularisation. In the clinical setting, the use of intracoronary physiology is sometimes advocated to aid decision-making for patients with ISR of moderate severity or in patients who are oligo-symptomatic. However, there are limited data on the use of fractional flow reserve (FFR) or instantaneous wave-free ratio (iFR) for the assessment of ISR51. Previously published data with one-year follow-up have suggested that conservative management of angiographically moderate (40-70%) ISR lesions with an FFR value of ≥0.75 is safe52. However, there are no randomised data supporting an FFR-guided ISR treatment strategy. In general, patients with ISR should go through the same diagnostic pathway as patients with de novo CAD.
Management of ISR
General principles
The general principles for the treatment of ISR do not differ significantly from those for the treatment of native coronary stenoses. However, the presence of an existing scaffold brings some additional considerations compared with de novo disease, and persistent issues leading to the original stent failure may need to be identified and addressed in order to avoid recurrence.
Angiographic performance, acute gain, and late luminal loss
Quantitative coronary angiography measures to assess and compare the relative anti-restenotic efficacy of stent- and balloon-based treatment modalities include minimum lumen diameter (MLD), percentage diameter stenosis (%DS), acute gain, and LLL. Acute gain is defined as the difference between the MLD preprocedure and the MLD immediately post-procedure. LLL is defined as the difference between the MLD immediately post-procedure and the MLD at follow-up angiography. When treating ISR, the aim is to maximise acute gain and minimise LLL. The temporal pattern of acute gain and LLL after PCI is shown in Figure 3. An important point is that drug-coated balloon (DCB) therapy is associated with reduced acute again and reduced late loss compared to DES PCI, which is generally associated with increased acute gain but also increased late loss. As such, the most important parameter when comparing the two modalities is net gain, as shown in Figure 3, although minimal lumen diameter or %DS at late follow-up may be also used.
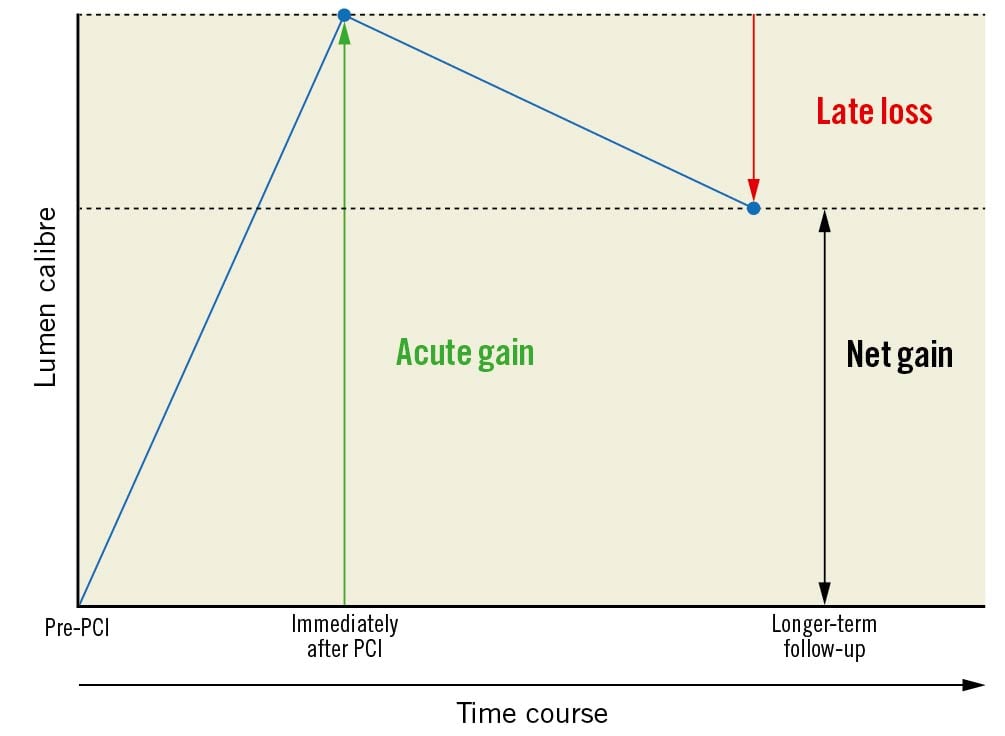
Figure 3. Acute gain and late luminal loss in ISR-PCI. When treating ISR, the aim is to maximise acute gain and minimise LLL. The most important parameter when comparing DESs and DCBs for the treatment of ISR is net gain. DES: drug-eluting stent; DCB: drug-coated balloon; ISR: in-stent restenosis; LLL: late lumen loss; PCI: percutaneous coronary intervention
It is important to note that in the setting of ISR, acute gain and LLL will be impacted by not only the choice of treatment modality but also by extrinsic mechanical factors. A significant proportion of ISR lesions are associated with stent underexpansion, which may itself be secondary to vessel calcification. In addition, calcified neo-atherosclerotic ISR can also result in specific challenges with respect to achieving maximal acute gain2223. Indeed, it is noteworthy that in ISR treated with DES, primary stent underexpansion (i.e., underexpansion of the re-stenosed stent) has been associated with an increased risk of secondary stent underexpansion (i.e., underexpansion of the second stent used to treat the re-stenosed stent). This in turn has been associated with an increased risk of MACE and ISR recurrence1718. Post-procedural stent underexpansion has also been shown to predict recurrence for ISR treated with DCBs53. These data could be interpreted as supporting the paradigm that failure to adequately address the root causes of ISR increases the future risk of recurrence. Indeed, suboptimal treatment of ISR can result in a vicious cycle, in which the risk of recurrence is increased and subsequent management becomes more and more challenging. This is of particular concern when patients are treated with an additional DES (the so-called “sandwich strategy”) leading to multiple stent layers or the “onion skin phenomenon”54. Aggressively tackling mechanical factors prior to dedicated ISR treatment may help avoid this problem, although high quality evidence in this regard is limited.
The evidence base for the treatment of ISR
In recent years, numerous RCTs have been performed to determine the optimal management of ISR and these trials are summarised in Supplementary Table 1, Supplementary Table 2, and Figure 4. A variety of treatment modalities have been compared directly in head-to-head studies and indirectly in network meta-analyses. When all of these treatment strategies have been compared using network meta-analysis, two modalities have consistently emerged as pre-eminent for the management of ISR: DES implantation and treatment with DCBs5556. These are the two therapeutic strategies recommended by current revascularisation guidelines40. Rather than providing an exhaustive historical review of all the different therapeutic strategies that have been studied over the past few decades, this review will initially focus on these two treatments for ISR before discussing adjunctive therapeutic modalities.
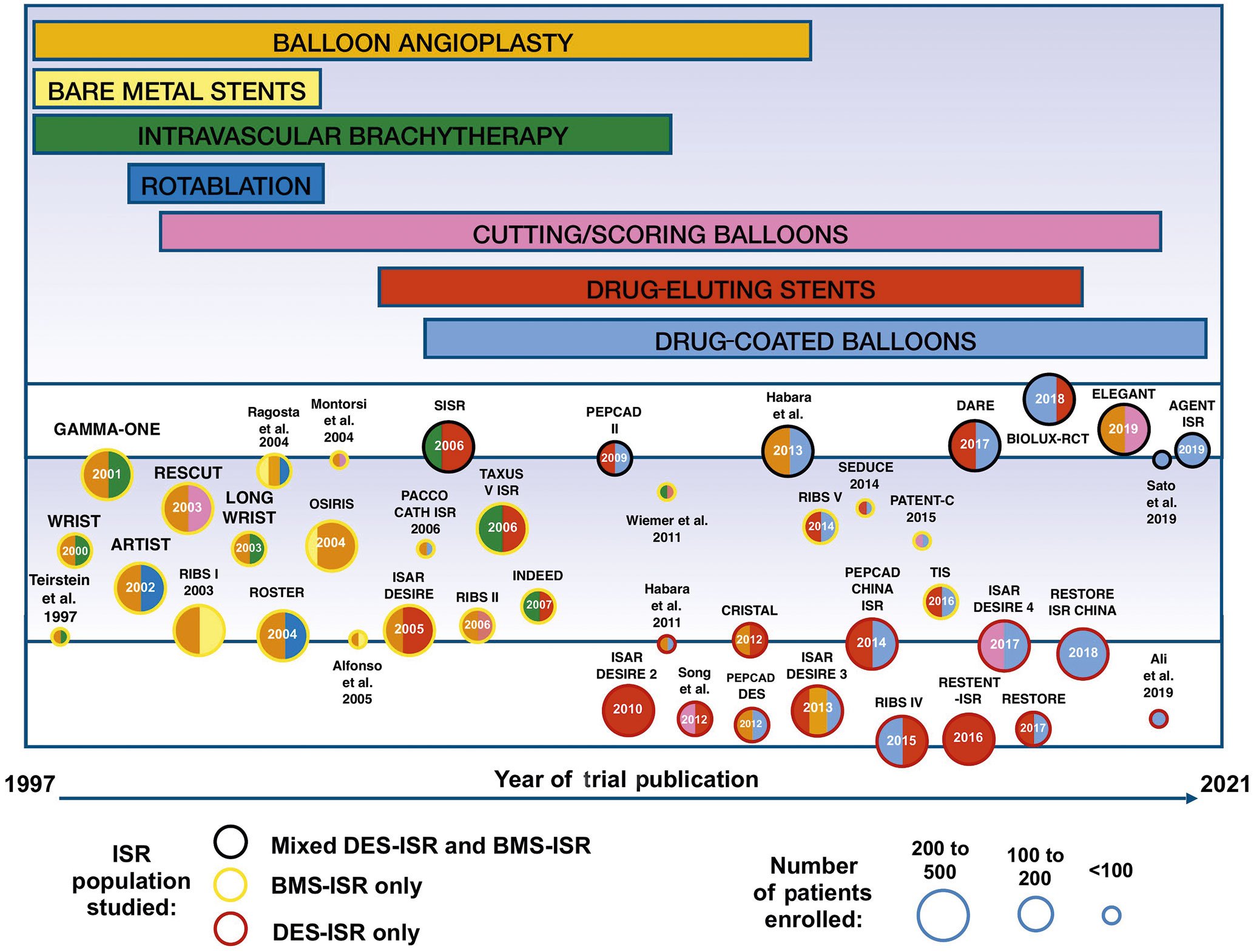
Figure 4. Trials assessing the treatment of ISR. The size of the circles corresponds to the size of the trial. The colour of the circles’ perimeter indicates the underlying stent type in the ISR population studied. The inner colour of the circle indicates the interventional strategies used in the trial, corresponding to the coloured bars at the top of the figure. The length of the coloured bars at the top of the figure indicates the time span during which each therapeutic modality has been studied for the treatment of ISR. Detailed descriptions of the studies and the randomised arms are provided in Supplementary Table 1. BMS: bare metal stent; DES: drug-eluting stent; ISR: in-stent restenosis
The results of several of the largest ISR trials (trials with >200 patients enrolled) are demonstrated in Figure 5. These are divided into 5 research themes: intravascular brachytherapy trials, rotational atherectomy trials, DES trials, DCB trials, and DES vs DCB trials. The outcome of interest highlighted in this figure is MACE.
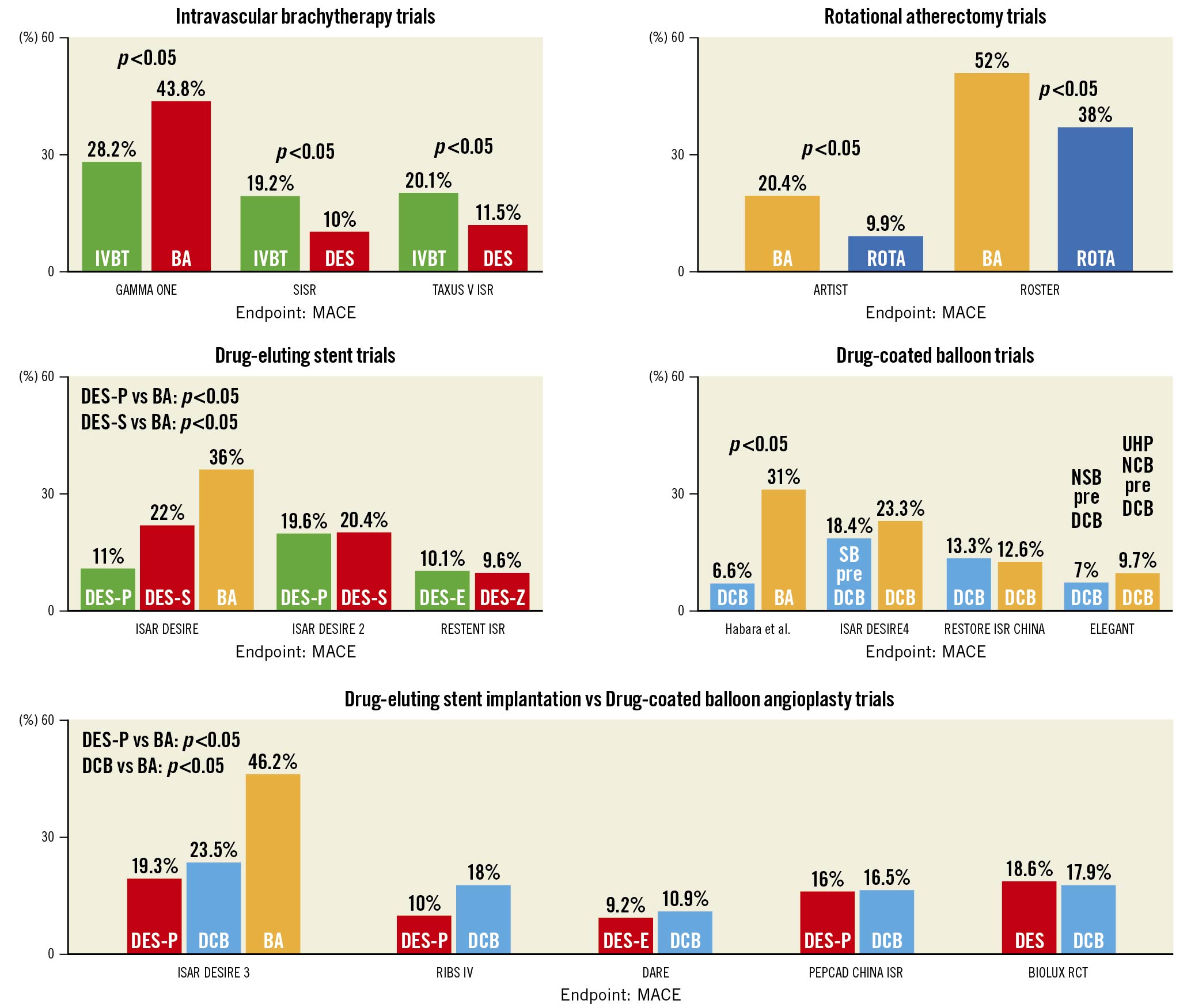
Figure 5. Clinical outcomes in major ISR trials. ISR trials with over 200 patients enrolled are divided into 5 research themes: intravascular brachytherapy trials, rotational atherectomy trials, DES trials, DCB trials, and DES vs DCB trials. MACE is the outcome of interest displayed for each trial. Three ISR trials with >200 patients enrolled are not shown in this figure: These are RIBS I (Bare Metal Stent vs Balloon Angioplasty), RESCUT (Balloon Angioplasty vs Cutting Balloon) and OSIRIS (Placebo vs Normal Dose Sirolimus vs High Dose Sirolimus). Details of all ISR trials identified in our search are provided in Supplementary Table 1 and Supplementary Table 2. BA: balloon angioplasty; DCB: drug-coated balloons; DES: drug-eluting stent; DES-E: everolimus-eluting stent; DES-P: paclitaxel-eluting stent; DES-S: sirolimus-eluting stent; DES-Z: zotarolimus-eluting stent; ISR: in-stent restenosis; IVBT: intravascular brachytherapy; MACE: major adverse cardiac events; NSB: non-slip balloon: SB:scoring balloon; UHPNCB: ultra-high-pressure non-compliant balloon
Systematic review methods
In order to identify trials comparing treatments for ISR, a literature search was performed in PubMed at the time of the writing of this document. The search strategy is reported in Supplementary Appendix 1. The reference lists from the identified papers and previous meta-analyses on ISR were also reviewed in order to identify any other related studies. Only RCTs comparing therapeutic modalities in patients with coronary ISR are included in Supplementary Table 1, Supplementary Table 2, and Figure 4.
Drug-eluting stents
DESs are recognised for their strong anti-proliferative properties34 and are therefore very attractive for the management of ISR. Indeed, DESs have superseded BMSs for the treatment of de novo CAD based on their lower restenosis rates34. Network meta-analyses have ranked DES implantation as the most effective treatment for ISR5556. In addition, head-to-head trials have demonstrated the superiority of DES implantation to several other therapeutic modalities for ISR, including BA425758, intravascular brachytherapy (IVBT)596061, and paclitaxel DCBs62. A potential drawback of DES implantation is that another layer of stent is implanted. This can lead to subsequent therapeutic challenges in the event of ISR recurrence. As such, care must be taken to ensure adequate lesion preparation has been achieved prior to implantation of a new DES for the treatment of ISR, with particular care taken to tackle any underexpansion of the original stent. Figure 6 and Figure 7 demonstrate the use of a DES to treat ISR with evidence of ruptured neoatherosclerosis in the LAD (left anterior descending coronary artery). In the case of stent fracture, re-stenting will be required in the majority of cases.
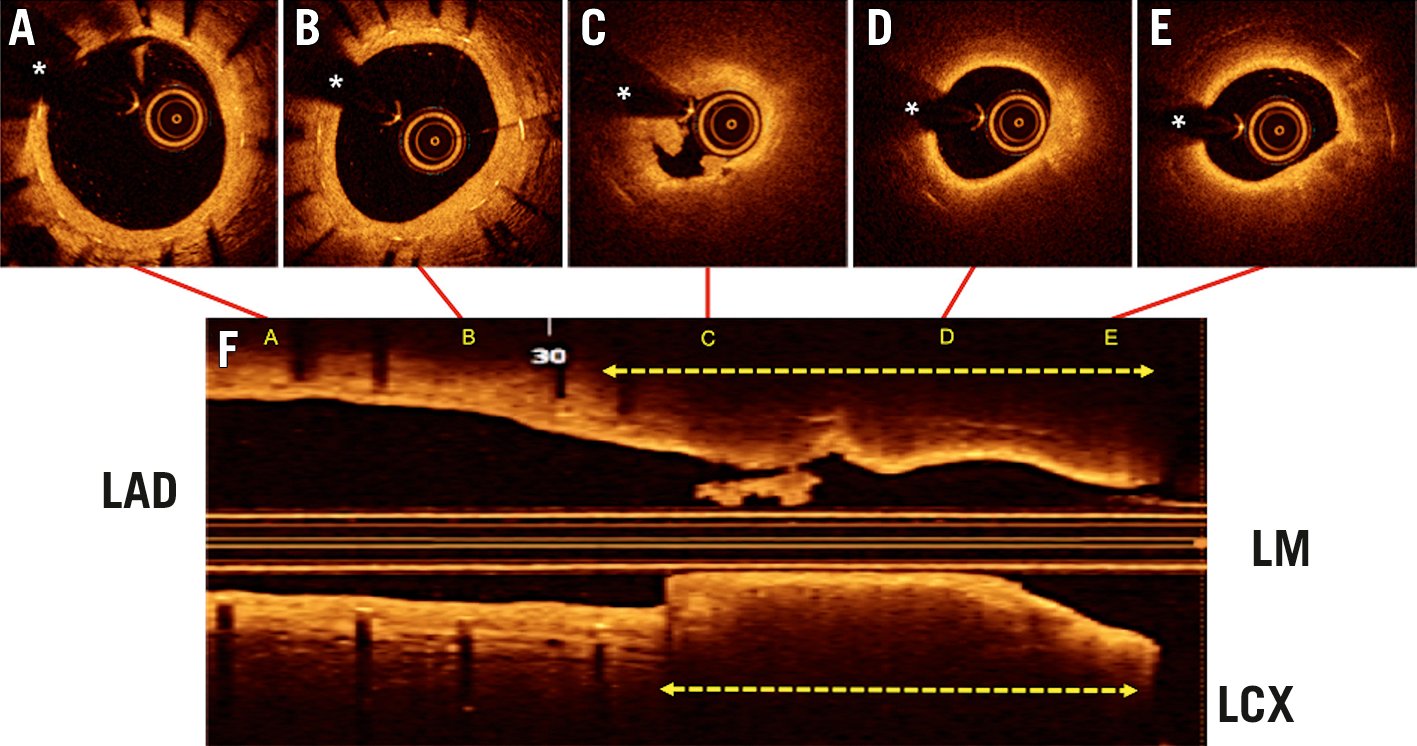
Figure 6. An 81-year-old man presented with prolonged chest pain (15 minutes) 12 years after the implantation of a DES in the proximal LAD. The ECG was normal but a rise in high-sensitivity troponins was detected. A-E) OCT images from the distal part of the stent (A) to the LAD ostium (E) are shown. F) The longitudinal OCT display depicting the location of the corresponding cross-sections. The distal part of the stent (A, B) showed a mild neointimal hyperplasia (with a homogeneous bright yellow colour) and an excellent residual coronary lumen (* denotes wire artefact). Alternatively, the proximal part of the stent (C-D) showed ruptured neoatherosclerosis with intracoronary thrombus (C, F). There is a ruptured cap with associated thrombus (C) and bright neointima (D,E) overlying a large lipid plaque (dark areas with undefined edges), which completely obscure (shadowing) most of the underlying stent struts. The broken arrows indicate the area which demonstrates obstructive neoatherosclerosis (F). DES: drug-eluting stent; ECG: electrocardiogram; ISR: in-stent restenosis; LAD: left anterior descending coronary artery; LCX: left circumflex coronary artery; LM: left main; OCT: optical coherence tomography
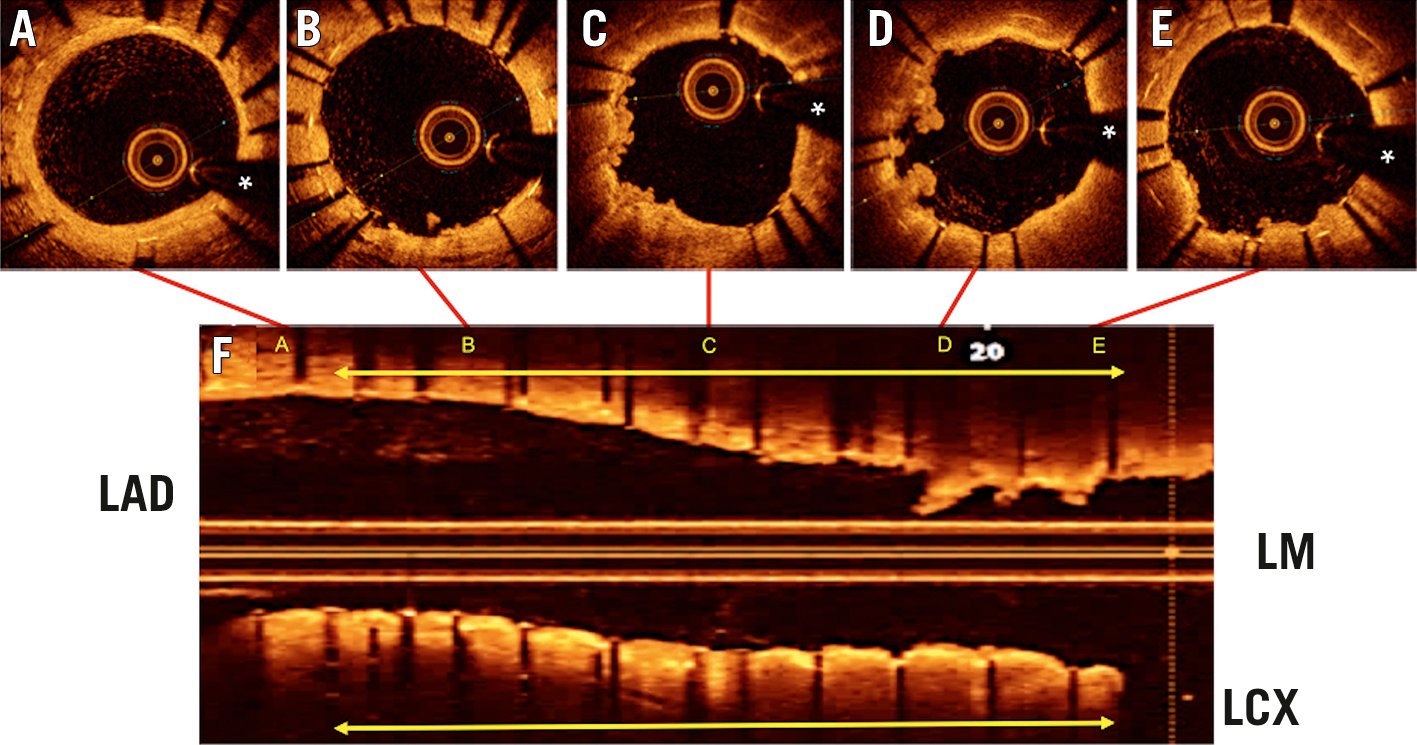
Figure 7. OCT findings (A-F) in the same patient as in Figure 6, after treatment of the ISR with repeat DES implantation. The location of the cross-section images (A-E, top) is illustrated in the longitudinal display (F, bottom). A) Distal untreated segment of the previous stent. B-E) Treated segment (yellow arrow) demonstrating the 2 stent layers. There is also a significant protrusion of soft irregular material into the coronary lumen. (* denotes wire artefact). DES: drug-eluting stent; ISR: in-stent restenosis; LAD: left anterior descending coronary artery; LCX: left circumflex coronary artery; LM: left main; OCT: optical coherence tomography
DES strategy: hetero-DES vs homo-DES
One specific subtopic of interest with regard to DES treatment for DES-ISR is the choice of anti-proliferative agent. It has been hypothesised that treating DES-ISR using a DES with an alternative anti-proliferative agent (the “hetero-DES” strategy) might provide superior outcomes to using a DES with the same anti-proliferative agent (the “homo-DES” strategy). This hypothesis is based on the concept that drug resistance may have played a role in the development of the initial DES-ISR. However, evidence in this regard has been mixed. The only randomised trial on this topic, ISAR DESIRE-2, did not show a benefit to the hetero-DES strategy for the treatment of sirolimus-eluting stent ISR43. Alternatively, the RIBS-III study had suggested a hetero-DES strategy may provide superior outcomes, although this study was not randomised and the alternative treatment decisions were at the discretion of local investigators63. A meta-analysis has suggested that there may be a benefit to a hetero-DES strategy but this included results from several observational analyses, limiting the validity of the findings64. Paclitaxel DESs are no longer available and large studies comparing the potential value of implanting a hetero-DES with a different -limus drug have not been performed.
Drug-coated balloons
DCB catheters are comprised of standard angioplasty balloons and a matrix coating that is applied to the surface of the balloon65. The balloon coating is typically comprised of two elements: a lipophilic active drug and a spacer or excipient which increases the solubility of the active drug and facilitates its transfer from the balloon surface to the vessel wall65.
DCBs provide anti-proliferative therapy without the requirement for an additional metallic scaffold. This technology is intuitively attractive for the management of ISR as it avoids multiple stent layers. DCBs may be particularly useful for clinical situations where the addition of another stent layer is undesirable (i.e., multiple previous stent layers, the presence of a major side branch) and may be particularly suited to clinical situations where the mechanism of ISR is stent maldeployment. In addition, patients treated with DCBs generally require a more abbreviated dual antiplatelet therapy (DAPT) regimen and therefore this strategy may be particularly useful in patients at high bleeding risk.
Paclitaxel DCBs
Initial commercially available DCBs eluted paclitaxel and the majority of clinical evidence relates to the use of paclitaxel-coated DCBs, particularly those based on iopromide666768. Paclitaxel was used in preference to other drugs in DCBs due to its lipophilic properties. RCTs have demonstrated the superiority of DCB therapy to several other treatment modalities for ISR, including BA and BMS implantation44697071. There is limited evidence comparing paclitaxel DCBs with different excipient coatings, although one small study suggested that outcomes are similar between iopromide and butyryl-tri-hexyl citrate-coated DCBs72.
Of note, concern was raised in a 2018 meta-analysis regarding excess mortality associated with paclitaxel-coated balloons and stents in femoropopliteal disease73. However, several subsequent studies have questioned these findings in peripheral arterial disease7475. Importantly, in the setting of CAD, the use of paclitaxel DCBs has never been linked with safety issues or excesses in mortality. Further reassuring information in this regard was recently provided by a large meta-analysis76.
Sirolimus DCBs
In recent years, sirolimus DCBs have also been developed and initial registry data has demonstrated encouraging results in both de novo CAD and ISR7778. Given that network meta-analysis has suggested that -limus-eluting DESs are associated with superior outcomes compared to paclitaxel-eluting DESs for the treatment of ISR56, it could be hypothesised that similar advantages might exist when comparing the two DCB technologies for the treatment of ISR. Until very recently, the technology to ensure adequate binding, persistence and transfer of -limus-based drugs from DCBs to the arterial wall did not exist. However, recent technological advances have enabled the development of -limus DCBs. Notwithstanding the potential attractiveness of -limus DCBs for the treatment of ISR, evidence on their safety and effectiveness in this scenario still remains limited. A small RCT comparing a novel sirolimus DCB balloon to a paclitaxel DCB reported similar 6-month angiographic and 12-month clinical outcomes in the two groups79. Of note, there are no published randomised data comparing sirolimus DCBs vs DESs for the treatment of ISR. In the future, larger studies will be useful to determine the relative efficacy of sirolimus DCBs compared to both paclitaxel DCBs and DESs. It is important to recognise that there is insufficient evidence currently to support the concept of a “class effect” for both limus- and paclitaxel-eluting DCBs and further research would be useful in this regard.
DCB: the importance of lesion preparation
Adequate lesion preparation is paramount when treating ISR, irrespective of the final proposed treatment modality. The efficacy of a DCB relies on rapid initial drug transfer of the anti-proliferative drug and subsequent tissue retention in the restenotic area. Therefore, the use of cutting or scoring balloons to impact on the ISR obstructive tissue prior to DCB treatment may help to improve the delivery of the anti-proliferative agent. The ISAR-DESIRE 4 trial demonstrated that neointimal modification with a scoring balloon improved the anti-restenotic efficacy of DCB therapy80. Interestingly, a drug-coated scoring balloon has been developed, combining both treatment modalities in one device. This has shown promising results in initial studies when compared to an uncoated scoring balloon alone81, though it should be acknowledged that the sequential use of the devices might be the most efficacious approach.
DES vs DCB for the treatment of ISR: direct evidence
Several studies have compared DESs to paclitaxel DCBs for ISR6869707172737475767778. Despite the theoretical advantages of DCBs in the management of ISR, a meta-analysis of randomised controlled data comparing DES versus paclitaxel-DCB angioplasty has demonstrated that repeat DES implantation for ISR is moderately more effective in reducing the rate of TLR at 3 years52. In this setting, repeat DES implantation provides better acute angiographic results than DCB treatment, including increases in the MLD and reductions in residual %DS. In most head-to-head RCTs, these superior acute angiographic results with DES as compared with DCB treatments are maintained at longer-term follow-up. The DAEDALUS study, a meta-analysis of patient-level data from 10 RCTs which included 1,976 patients, demonstrated that DESs reduced the need for subsequent TLR compared with paclitaxel DCBs at 3 years62. Of interest, it appears that the relative efficacy of these two treatments for ISR may depend on the underlying stent type8283. In patients with BMS-ISR, clinical efficacy and safety outcomes appear to be comparable with both DESs and DCBs83. Given that a DCB provides comparable efficacy without the need for an additional stent layer, this strategy may therefore be preferable in this setting. Conversely for the more challenging scenario of DES-ISR, repeat stenting with a DES is moderately more effective compared to treatment with a DCB regarding the need for TLR83. However, this increased efficacy must be weighed against the implantation of an additional stent layer. Importantly, irrespective of the selected treatment modality, the treatment of BMS-ISR is associated with superior late angiographic and clinical outcomes in comparison to the treatment of DES-ISR84.
Adjunctive therapeutic modalities for ISR
While current evidence supports DCBs and DESs as the optimal initial treatment modalities for ISR in the majority of cases, other treatment modalities may still have a complementary or adjunctive role. This may be particularly the case for recurrent ISR. In this section, we will discuss the evidence base and potential niche roles for these adjunctive ISR treatments. Given the varied and challenging nature of ISR disease, it may be useful for specialist centres performing complex ISR interventions to have several adjunctive treatment options in their armamentarium.
Balloon angioplasty
BA was the earliest treatment available for ISR but was subsequently shown to be inferior to multiple newer alternative treatment modalities424469. BA results in some acute gain due to tissue extrusion (both longitudinal and axial) in addition to stent expansion85. However, this acute gain is often short lived, with tissue re-intrusion occurring shortly after the last balloon inflation86. In addition, this strategy is plagued by recurrent severe tissue proliferation and has been virtually abandoned in Europe as a definitive therapy. In the US, where DCBs have still not been approved, isolated conventional BA is still used for cases with focal ISR where the risk of recurrence is deemed to be low. In the setting of an underdeployed stent, non-compliant or ultra-high-pressure non-compliant balloons (UHPNCBs) at high pressures should be used to improve stent expansion. However, based on current data, isolated BA is not routinely recommended for the treatment of ISR and this technique is best regarded as a method for lesion preparation prior to the use of other therapies or for final optimisation of DES implantation.
Cutting/scoring balloons
Cutting balloons are comprised of standard balloon catheters mounted with lateral metallic blades, which on inflation of the balloon incise into the treated stenotic plaque. Scoring balloons have a broadly similar mechanistic basis but employ low-profile nitinol wires (of the order of 125 μm) on the surface of the balloon catheter in a spiral formation.
The two main advantages to their use are that the incision of the blades into the stenotic plaque may favour subsequent tissue extrusion and the interaction of the blades with the plaque serves to anchor the balloon in the plaque. Both cutting and scoring balloons may play a valuable role in lesion preparation prior to DESs or DCBs in the treatment of ISR87. However, as a standalone treatment, both technologies are hindered by their inability to inhibit neointimal proliferation and suffer from similar limitations to BA8889. In the ISAR-DESIRE 4 trial, the use of a scoring balloon prior to a DCB has been shown to improve the anti-restenotic efficacy of the DCB80. Cutting/scoring balloons may also be useful to avoid the “watermelon seeding” phenomenon that can occur when dilating ISR lesions, particularly in the presence of severe or diffuse patterns of ISR90. “Watermelon seeding” is associated with prolonged procedure times, suboptimal acute angiographic results and inferior long-term outcomes. It can result in “geographic miss”, which can subsequently lead to recurrent edge-ISR. A non-slip element balloon has also shown similar efficacy to high-pressure non-compliant balloons in lesion preparation pre-DCB treatment for ISR91.
Intravascular brachytherapy
IVBT refers to the delivery of localised radiation within the stent. The aim of this treatment modality is to impede neointimal cell growth within the target area without damage to the surrounding tissue. The radiation achieves this effect via two primary methods: direct damage secondary to ionising emissions and injury secondary to free radical generation92. Several randomised clinical trials demonstrated that IVBT was superior to the mechanical alternatives available at the time939495. When DESs became available, they rapidly superseded IVBT due to both their greater simplicity and superior results in the setting of BMS-ISR596061. However, there are no randomised data on the use of IVBT in the setting of DES-ISR. Observational analysis has suggested that IVBT may have a role in recurrent ISR969798. This lack of evidence has prevented the common use of this technology in modern practice, where IVBT has been abandoned in most centres.
Ablative strategies
Commercially available ablative technologies for the management of ISR include RA and ELCA. Historically, these technologies were attractive for the management of ISR as they had the ability to ablate the restenotic tissue that obstructs the stent. While some of the early results in this regard were promising, when ablative strategies were compared to alternative treatment modalities, the results at late follow-up did not show any significant benefit compared to control. Therefore, their routine and systematic use for the management of ISR was abandoned. However, they may still play a niche role in the management of undilatable ISR lesions when other conventional strategies have failed, particularly when managing calcified neoatherosclerotic ISR.
Ablative strategies: excimer laser coronary atherectomy
ELCA is a debulking technique which uses ultraviolet spectrum wavelengths to ablate tissue99. It does this by generating heat and shockwaves100. Despite some early historical studies demonstrating the safety and feasibility of this technique101, there are limited recent data to support the systematic use of ELCA as the primary treatment for ISR and no randomised data are available on the use of ELCA for DES-ISR. However, in selected cases it may have an adjunctive role for lesion preparation102, particularly for recurrent ISR in the setting of severe calcification. For the rarely encountered patients with undilatable ISR as the result of severely underexpanded stents due to a heavily calcified arterial wall, this technique may facilitate stent expansion, particularly if contrast is injected to induce further barotrauma and microcavitation. Evidence in this regard stems only from small observational series but it nevertheless remains a useful option as a bailout strategy when other therapeutic strategies have failed.
Ablative strategies: rotational atherectomy
RA is another ablative technique which can be used to debulk ISR lesions and facilitate the application of subsequent treatments (as part of a combined strategy). There are historical RCTs comparing RA to BA in BMS-ISR103104. While the ROSTER trial (that mandated the use of IVUS during the intervention) suggested superior results with RA compared to BA, the much larger ARTIST trial reported inferior results with RA and a higher number of procedural-related complications103104. However, it should be noted that this may have been related to the trial protocol, which mandated lower balloon inflation pressures in the RA arm of this study, and to the lack of systematic IVUS use to rule out severe underexpansion. There are no randomised data for the use of RA in the treatment of DES-ISR. However, it may still have a role as an adjunctive technique for lesion preparation prior to DCB application or recurrent DES implantation22105106. It should be considered a high-risk procedure and particular care must be taken to avoid burr entrapment within the ISR lesion22106. The successful use of RA to ablate metal (“stentablation”/“rotastenting”) in the exceedingly rare cases of severely underexpanded and undilatable stents has also been reported. However, this procedure has obvious inherent potential risks (burr entrapment, vessel perforation), and indications for its use are likely to be extremely restricted, given that more attractive and safer strategies are now available to tackle this unique problem (see below).
Intravascular lithotripsy
Intravascular lithotripsy (IVL) is a relatively new technology which uses localised pulsatile sound waves to circumferentially modify vascular calcium107. IVL has been demonstrated to be safe and effective in de novo CAD108. The use of IVL to facilitate stent expansion in ISR has been described but there are limited data available on this technique and it is regarded as an off-label use109110111112. However, many observational reports have demonstrated that IVL may be used with success in patients with undilatable ISR resistant to conventional strategies, especially in the setting of stent underexpansion due to circumferential coronary artery calcification113. In a manner similar to ELCA, the energy produced modifies the compliance of the calcified plaque causing fractures beyond or within the stent. Compared with ELCA and RA, the use of IVL in patients with ISR is much more user friendly and less dependent on operator experience.
Bare metal stents
BMS implantation was used after BA for the treatment of BMS-ISR and showed some promise in terms of acute luminal gain compared to BA114. However, in the RIBS-I trial, BMS implantation failed to show an advantage at 6-month follow-up in comparison to BA for the treatment of BMS-ISR115. In that study, BMSs were superior to conventional BA only in the predefined subset of patients with large (≥3 mm) vessels. BMSs also proved to be superior to BA in patients presenting with edge-ISR116. There are no trials assessing the value of BMSs for the treatment of DES-ISR and as such, their role in the management of ISR is largely historical.
Bioresorbable vascular scaffolds
Bioresorbable vascular scaffolds (BVS) were considered potentially attractive for the treatment of ISR. However, the use of BVS for patients with ISR was associated with a higher TLR rate in comparison to results obtained with DESs in previous studies117118. Polymeric BVS are no longer commercially available119. The potential value of magnesium-based BVS (Mg-BVS) has been investigated in some early preliminary studies120121, but further research is required to determine whether they will play a future role in the treatment of patients with ISR.
Management of patients with BVS-ISR
The RIBS VII study prospectively registered the treatment of patients with BVS-ISR122. Overall and after adjusting for potential confounders, patients with BVS-ISR had similar clinical outcomes to patients with restenosis of metallic stents.
Coronary artery bypass grafting
Coronary artery bypass grafting (CABG) may be a reasonable treatment option in selected patients, but there are no RCTs comparing CABG to other treatment modalities for ISR. However, some observational analyses have reported that patients treated with CABG for ISR (85% of whom had multivessel disease) had superior outcomes compared to percutaneous therapies123. CABG may also be considered in patients with (1) ISR of the left main stem (LMS)124, (2) recalcitrant ISR in a major vessel, (3) associated multivessel disease, or (4) ISR located in the ostial LAD.
Adjunctive medical therapy
The use of several adjunctive anti-inflammatory or anti-proliferative medications has been suggested for patients with ISR, in particular for patients presenting with recurrent ISR. It was hypothesised that the use of adjunctive medical therapies would reduce the risk of ISR recurrence. In the OSIRIS study, oral sirolimus resulted in a significant improvement in 6-month angiographic parameters125. However, this early benefit was attenuated at longer follow-up and concern regarding potential side effects led to reduced interest in this therapy126. Currently there is no clear evidence supporting the use of adjunctive systemic anti-proliferative medical treatments in these patients.
Specific clinical scenarios
Some specific clinical scenarios with relevance for ISR include recurrent ISR, ISR in the setting of severe calcification, LMS ISR, ISR chronic total occlusion (CTO), and stent fracture.
Recurrent ISR
Recurrent ISR refers to ISR that has recurred after the initial treatment of ISR. If the ISR was initially treated with repeat implantation of a stent, this means that the recurrent ISR lesion will have two stent layers. Some ISR lesions may have more than two stent layers. These cases appear to be systematically associated with underexpansion of the initial stent. ISR-PCI with a DCB has been associated with worse outcomes for patients with three layers of stent than for those with one or two stent layers127. Therefore, it may be advisable to avoid a third layer of stent when treating recurrent ISR, although DES implantation has been reported to be safe and effective in recurrent ISR with two stent layers128. Another study reported that DES implantation was superior to BA in patients with recurrent ISR previously treated with a DES129. However, there are limited dedicated randomised data available to guide treatment decisions in this challenging lesion subset. IVBT has been reported to be a useful modality for recurrent ISR with multiple stent layers but a meta-analysis suggests that TVR still occurs in approximately 1 in 4 patients at 2 years in this setting9697. DCBs may also be useful in this challenging setting although the 1-year TLR incidence was reported as 14.5% in the 1-stent layer group, 14.9% in the 2-stent layer group and 41.2% in the 3-stent layer group in a study by Yabushita et al127.
When managing recurrent ISR, it is particularly important to determine if there are persistent mechanical issues which were not adequately addressed during the initial ISR therapeutic procedure, and which may have contributed to the ISR recurrence. Failure to deal with these persistent mechanical issues will likely increase the risk of future TLR. In most cases underexpanded stents appear to represent the trigger for recurrence, although localised stent fractures (i.e., at hinge points) may also contribute to recurrence in some cases130. Ensuring optimal final stent expansion is of paramount importance in this scenario. Whether the systematic use of IVL in these patients will help to improve clinical results in patients with recurrent ISR remains to be determined. Finally, as discussed earlier, CABG should be considered in patients with recurrent ISR affecting the LMS or the ostial LAD, and in patients with multivessel disease.
ISR in the setting of severe calcification and stent underexpansion
Coronary artery calcification is a recognised major risk factor for ISR. For ISR in the setting of calcification and stent underexpansion, calcium modification may be required. RA, ELCA, IVL109110111112 and UHPNCBs can all be used to modify calcium and facilitate stent expansion in this setting101105106131. Repeat IVI following calcium modification may be useful to ensure it has been successful, particularly if definitive therapy with DES implantation is planned. There are limited data comparing the various calcium modification strategies and the choice will likely depend on both local availability and skill set. If this cannot be performed locally, referral to a specialist centre may be mandated. Ideally, specialised ISR tertiary referral centres should have several calcium modification modalities available to ensure the widest array of ISR lesions can be treated.
Left main stem ISR
There are limited data on the management of LMS ISR132. The FAILS study reported that LMS ISR could be managed percutaneously in the majority of cases133. A retrospective analysis has also suggested that DES and DCB treatments can provide similar outcomes in this setting134. However, a high mortality after TLR for LMS stent failure has been reported135, and CABG should be considered in suitable patients.
ISR-CTO
ISR-CTO was suggested to represent its own distinct class of ISR in the Mehran classification13. ISR-CTO PCI has been associated with a higher risk of complications and adverse events during follow-up in comparison to CTO-PCI in some reports136137. However, other studies have reported comparable results to de novo CTO-PCI138139140.
Visualisation of the stent in different projections during the ISR-CTO PCI procedure provides a roadmap that may facilitate advancement of special wires within the true lumen. Procedural outcomes may be impacted by operator experience and ISR-CTO PCI should be regarded as a highly specialised, high-risk procedure.
Stent fracture
Stent fracture may be identified in association with ISR141142143 and is generally defined as complete or incomplete separation of the stent strut on angiography and/or the absence of a stent strut on at least one slice on IVUS141142. For ISR lesions secondary to stent fracture, repeat stenting will be required in the majority of cases, although it must be acknowledged that there is an absence of high-quality, randomised data to support this recommendation.
ISR treatment algorithm
Based on the data discussed in this review, we present a proposed ISR treatment algorithm. It should be noted that this algorithm is intended for managing restenosis within the stent, rather than edge-restenosis, which will require repeat stenting in the majority of cases.
When we consider the scientific evidence summarised in this review, it appears that two of the most important factors to consider when deciding how to treat ISR are the stent type implanted during the index procedure (BMS or DES) and the presence or absence of mechanical issues preventing adequate stent expansion. This is because the optimal treatment for ISR (DES or DCB) appears to be impacted by the underlying stent type, and failure to adequately address mechanical issues appears to increase the risk of future ISR recurrence1783.
Mechanical issues are best identified using IVI and, consistent with current guidelines, we would recommend the use of IVI for all ISR cases3940144. The use of enhanced fluoroscopic imaging techniques (i.e., StentBoost) may also be useful in particular to identify mechanical issues like stent fracture and stent underexpansion.
Based on the IVI findings, the operator can then decide how best to address mechanical issues (if present) and prepare the ISR lesion. Adequate lesion preparation is an important determinant of procedural success and should be dictated by both the presumed underlying pathology and the local skill set. IVI can also be repeated following lesion preparation to ensure mechanical issues have been addressed prior to more definitive therapy. If IVI is not available, operators should focus on adequately predilating the ISR lesion and optimising stent expansion. This may include the use of non-compliant balloons and UHPNCBs. Cutting/scoring balloons can also be useful and should be used prior to DCB therapy where possible in order to optimise DCB efficacy.
The operator can then decide how to treat the lesion. Given the available scientific evidence, DCBs or DESs should be used to treat ISR in the majority of cases. In some cases, suboptimal results (i.e., significant dissections or persistent residual stenosis >40%) are observed despite multiple attempts at predilatation. In these cases, the use of DCBs as definitive treatment may yield similar results to the initial balloon predilatation and therefore DESs should be used preferentially145. Repeat DES implantation is moderately more effective than DCBs for DES-ISR and so could be considered the favoured treatment modality in this setting. Similarly, if the underlying stent type is not known, operators should favour DESs given that they have been shown to be moderately more effective than DCB therapy overall for the management of ISR. However, operators need to balance this moderate efficacy benefit against the implantation of another stent layer. As such, in some cases initial treatment with DCBs could be preferable, for instance if there are already two layers of stent present. Given that DCB and DES implantation have comparable outcomes in BMS-ISR, DCB may be preferable in the first instance. Other factors may also impact on clinical decision-making in this regard (i.e., presence of a significant side branch). In patients with high bleeding risk, the use of DCBs may also facilitate a more abbreviated duration of DAPT.
Repeat IVI post-treatment should usually be performed to determine if an acceptable procedural result has been achieved. If not, further measures may be required to optimise the result and this process can be repeated until a satisfactory result is achieved. An algorithm summarising these recommendations is demonstrated in Figure 8.
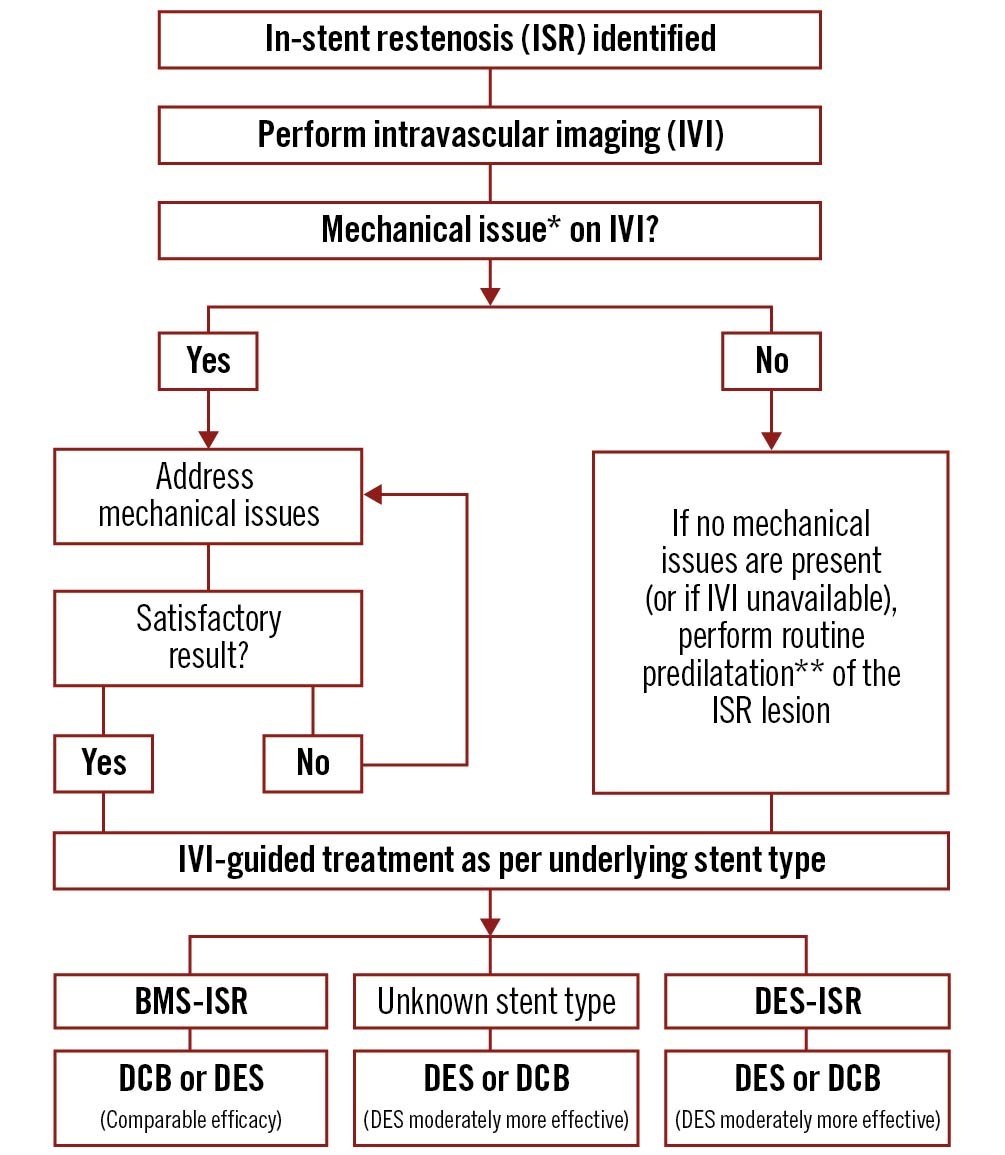
Figure 8. Algorithm for the management of in-stent restenosis. *Mechanical issues can include stent undersizing, stent underexpansion, vessel calcification, stent fracture, and geographic miss. Mechanical issues can be addressed via a variety of methods dependent on the identified pathology and local skill set. These methods can include BA, RA, ELCA, IVL, UHPNCB, cutting, and scoring balloons. **Predilatation can be performed with BA, UHPNCB, cutting, or scoring balloons. Cutting and scoring balloons may be particularly useful prior to planned DCB therapy. BA: balloon angioplasty; BMS: bare metal stent; DCB: drug-coated balloon; DES: drug-eluting stent; ELCA: excimer laser coronary atherectomy; ISR: in-stent restenosis; IVI: intravascular imaging; IVL: intravascular lithotripsy; RA: rotational atherectomy; UHPNCB: ultra-high-pressure non-compliant balloons
For recurrent ISR, previously attempted interventions should be reviewed (if possible) prior to managing the recurrence. This should include determining if mechanical factors were adequately identified and treated during the previous ISR treatment procedure.
Future interventional paradigms
In our proposed treatment algorithm for ISR, we suggest that the decision regarding the optimal treatment modality can be guided by the underlying restenotic stent type (BMS or DES). This interventional paradigm is based on currently available randomised data. However, it has also been speculated that the tissue causing the ISR may play a role in predicting the response to different therapeutic strategies. OCT in particular provides unique insights, not only to identify mechanical factors predisposing to ISR, but also to gain further knowledge of the tissue (ISR substrate) within the stent. Guidance of ISR treatment according to the underlying ISR tissue type would also be appealing within a personalised medicine paradigm. However, there are limited data to support this hypothesis at present. A recent observational analysis has suggested that the ISR tissue pattern (heterogenous or homogenous) observed on OCT could also potentially be useful to guide treatment146. This preliminary study suggested that in patients with heterogenous ISR on OCT, DESs showed an advantage over DCBs. No such advantage was seen in patients with a homogenous pattern on OCT. Replication of these results in prospective RCTs may lead to a future ISR interventional paradigm in which treatment decisions can be algorithmically guided based on ISR tissue patterns on OCT. Indeed, this hypothesis will be examined by the upcoming ISAR-DESIRE 5 trial, an RCT with a factorial design randomising patients with homogenous and heterogenous ISR tissue patterns on OCT to either DCB or DES treatment. Until then, the treatment of patients with ISR should be based on current scientific data, as outlined in Figure 8.
Optimal follow-up and treatment after ISR-PCI
Follow-up
ISR is recognised as being challenging to treat, with high rates of recurrence at medium- to long-term follow-up. Despite this, there are limited data to guide the optimal follow-up of patients after ISR-PCI. Routine control angiography after ISR-PCI is one potential strategy to identify recurrent restenosis, although this approach has not been assessed in RCTs and might lead to an increase in the number of repeated revascularisations without clinical indication. Routine control angiography has the potential benefit of enabling the identification of “silent restenosis” and has been shown to provide prognostic information after index PCI procedures34. However, in the absence of RCT evidence, follow-up after ISR-PCI should primarily consist of clinical follow-up aimed at identifying signs or symptoms of recurrent ischaemia, which could be suggestive of ISR recurrence. Given that patients undergoing ISR-PCI have been shown to have a significant event rate at late follow-up, higher than that associated with de novo lesion PCI, clinical surveillance should be continued beyond one year after ISR-PCI147148. In addition, evidence-based secondary prevention measures should be implemented, with aggressive management of cardiovascular risk factors.
DAPT duration after ISR-PCI
Currently, there is insufficient evidence to suggest that patients undergoing PCI for ISR require a different DAPT composition and duration to patients undergoing PCI for de novo lesions. In general, DAPT composition (i.e., the use of conventional or more potent P2Y12 inhibitors) and duration should be guided by the clinical presentation of patients with ISR. Of note, patients treated with DCB will, in general, require a shorter duration of DAPT in comparison to patients treated with DES. However, it is also important to recognise that currently there is insufficient evidence that DAPT composition and duration should be guided by the type of PCI (DCB or DES) for ISR.
A subanalysis of the PRODIGY trial has suggested that a prolonged DAPT regimen (24 months) may be beneficial for patients undergoing ISR-PCI compared to an abbreviated DAPT regimen (6 months), although this requires confirmation in larger, dedicated trials149. A final important point is that, although not specifically evaluated for patients with PCI for ISR, evidence generated by recent antiplatelet therapy de-escalation trials is likely to also be valid for patients undergoing ISR-PCI.
In Figure 9, we present a summary of the "do’s and don’ts” of ISR-PCI. These heuristic guidelines may provide operators with some useful “rules of thumb” to follow when performing ISR-PCI. While each ISR procedure will have its own unique considerations, these principles will be broadly applicable to the vast majority of cases encountered in clinical practice. The major principles of managing ISR are highlighted in the Central illustration.
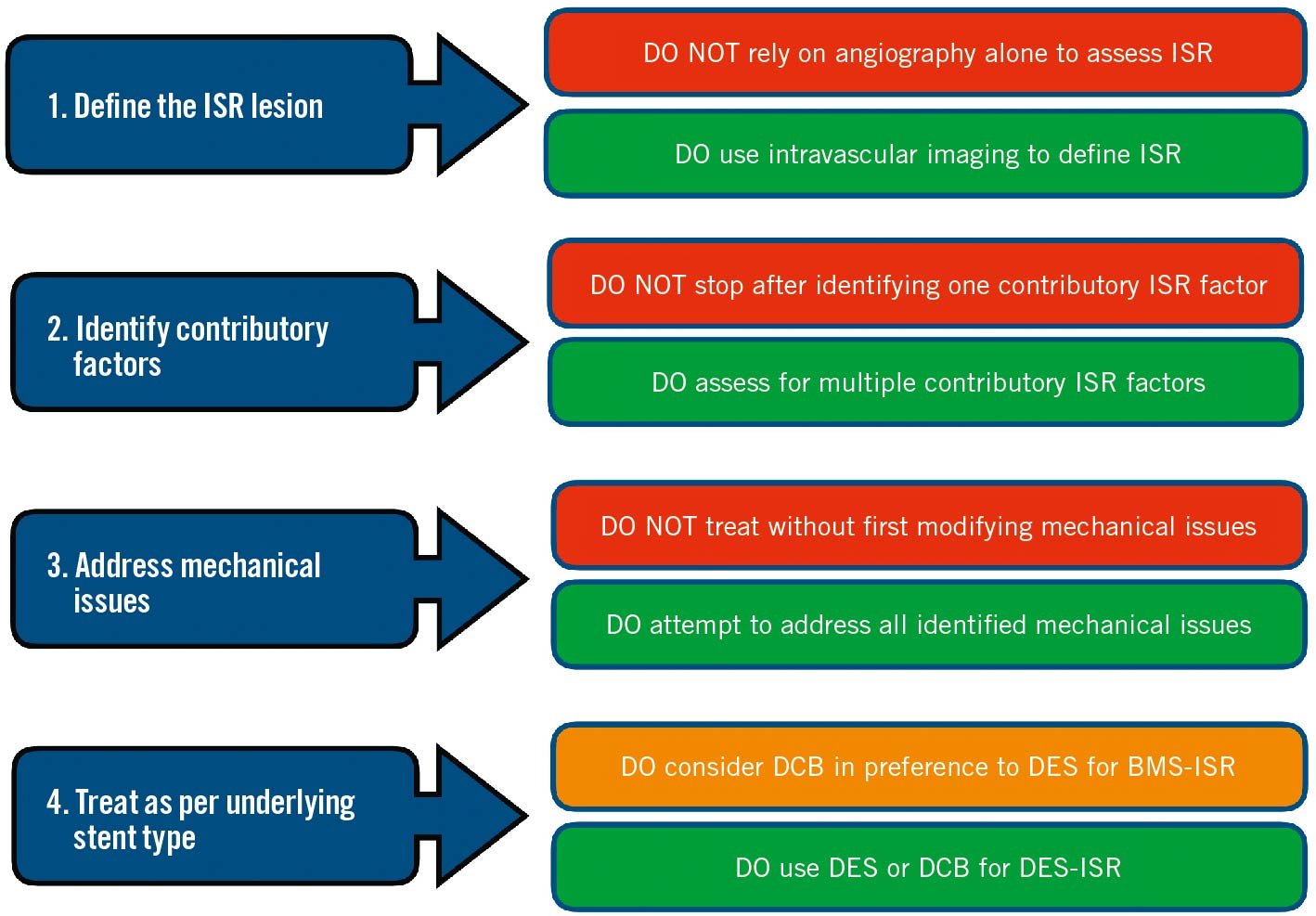
Figure 9. Do’s and Don’ts of ISR-PCI. BMS: bare metal stent; DCB: drug-coated balloon; DES: drug-eluting stent; ISR: in-stent restenosis; PCI: percutaneous coronary intervention
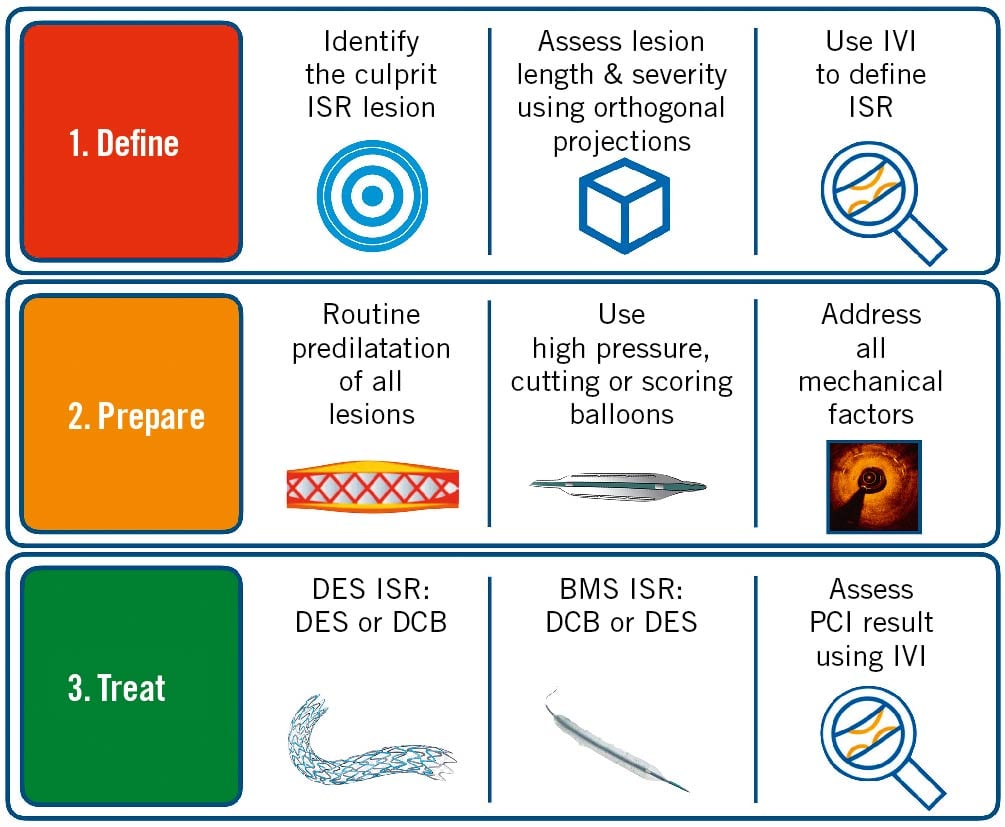
Central illustration. A summary of the major principles of managing ISR. First, the ISR lesion should be identified and defined using IVI. The lesion should then be prepared using balloon dilatation. High-pressure, cutting or scoring balloons can be used as required. Mechanical factors contributing to ISR should be addressed. This may require adjunctive modalities, including RA, ELCA, and IVL, depending on local expertise. Once the lesion has been adequately prepared, treatment with a DES or DCB should be used for the majority of cases. DESs are moderately more effective than DCBs in DES-ISR. For BMS-ISR, DCBs and DESs are comparatively effective and so DCBs should be considered preferentially. After PCI, IVI should be considered to confirm a satisfactory result has been achieved. BMS: bare metal stent; DCB: drug-coated balloon; DES: drug-eluting stent; ELCA: excimer laser coronary atherectomy; ISR: in-stent restenosis; IVI: intravascular imaging; IVL: intravascular lithotripsy; PCI: percutaneous coronary intervention; RA: rotational atherectomy
Conclusions
Despite recent advances in PCI, ISR remains a significant issue and the most common cause of stent failure, accounting for 5-10% of all PCI procedures in modern clinical practice.
While the relative rate of ISR has been reduced with newer-generation DESs in comparison to the BMS era, increasing procedural volume and complexity has resulted in a higher absolute number of ISR-PCI procedures being performed in modern practice.
Current evidence suggests that DESs or DCBs are the optimal treatment modalities for the majority of ISR cases.
IVI can provide useful information to guide treatment decisions in ISR-PCI, and future ISR interventional paradigms may be guided by IVI ISR tissue patterns.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

