Treatment of patients with in-stent restenosis (ISR) remains a technical challenge and continues to represent a major clinical problem1. Although bare metal stents (BMS) are still widely used they suffer from a relatively high restenosis rate, especially in complex clinical and anatomic scenarios1,2. Drug-eluting stents (DES) have drastically reduced the appearance of severe neointimal proliferation and the clinical need for repeat revascularisation. However, some patients treated with DES still develop ISR1,2. Timing, morphological patterns, underlying substrate and response to treatment differ slightly in patients with DES-ISR and those with BMS-ISR1. Indeed, DES-ISR may present relatively late after the initial procedure, tends to show a “focal” angiographic pattern (frequently involving the stent edges), and is frequently related to underlying mechanical problems including severe underexpansion, geographic miss or even strut fractures1. Moreover, neoatherosclerosis occurs not only more frequently but also earlier after DES than after BMS3. Finally, from a therapeutic standpoint, treatment of DES-ISR is associated with a higher rate of clinical recurrences as compared with interventions for BMS-ISR4,5.
Currently, the therapy of choice for patients presenting with “recurrent” DES-ISR remains largely unsettled. This subset of patients is considered to be at a high risk for additional recurrences and available therapies might be perceived as hopeless in some patients with “recalcitrant” ISR. In this regard, the study published in this issue of the journal by Kubo et al6 comparing DES with conventional balloon angioplasty (BA) in patients with recurrences after DES for ISR, is of major interest.
Previous studies
Many studies have demonstrated that in patients with ISR the use of DES is superior to alternative therapeutic modalities, including BA, BMS or even brachytherapy1,7. Most of this information, however, was obtained in patients with BMS-ISR. Less information is available on the relative merits of currently available strategies for patients with DES-ISR1. Nevertheless, the “sandwich” DES strategy has been widely embraced as the default therapy for patients with DES-ISR1. The ISAR-DESIRE 2 study demonstrated that in patients with “Limus” DES-ISR the use of paclitaxel DES or sirolimus DES provided similar long-term results8. More recently, the ISAR-DESIRE 3 trial9 suggested that in patients with “Limus” DES-ISR the use of paclitaxel DES and paclitaxel drug-coated balloons (DCB) yielded very similar clinical and angiographic results which were significantly superior to those seen with BA alone (control arm).
However, “recurrent” DES-ISR represents a major therapeutic dilemma1. Lemos et al10 were the first to suggest that “Limus” DES were more effective in patients with de novo DES-ISR (recurrent restenosis rate 18%) than in patients with recurrent DES-ISR (recurrent restenosis rate 29%). In lesions with a double metal layer developing recurrent ISR the use of a new DES has been suggested to be associated with good results but, until now, data in this regard has remained very limited. In a previous study11 we analysed the results of a third stent implantation in 21 patients with recurrent ISR. In most cases stent underexpansion was considered the underlying substrate likely triggering the recurrences11. A resistant residual waist in the balloon was frequently noticed during reinterventions despite the use of high pressures (mean 20 atmospheres) and non-compliant balloons. On intravascular ultrasound, stent underexpansion was the rule in these patients who also showed severe calcification at the vessel wall. After reinterventions, most stents remained underexpanded (mean stent expansion 67%). At late angiography the in-segment late loss was 0.4 mm. The one-year event-free survival was 90%. Although clinical events were rare, they were relevant: one patient died after coronary surgery for recurrent ISR and another patient died following a large myocardial infarction (probable stent thrombosis). In this preliminary study only time-to-ISR and intravascular ultrasound-detected stent underexpansion were associated with target vessel failure11. Figure 1 and Figure 2 illustrate the value of intravascular ultrasound and optical coherence tomography to unravel the underlying substrate in patients presenting with recurrent DES-ISR.
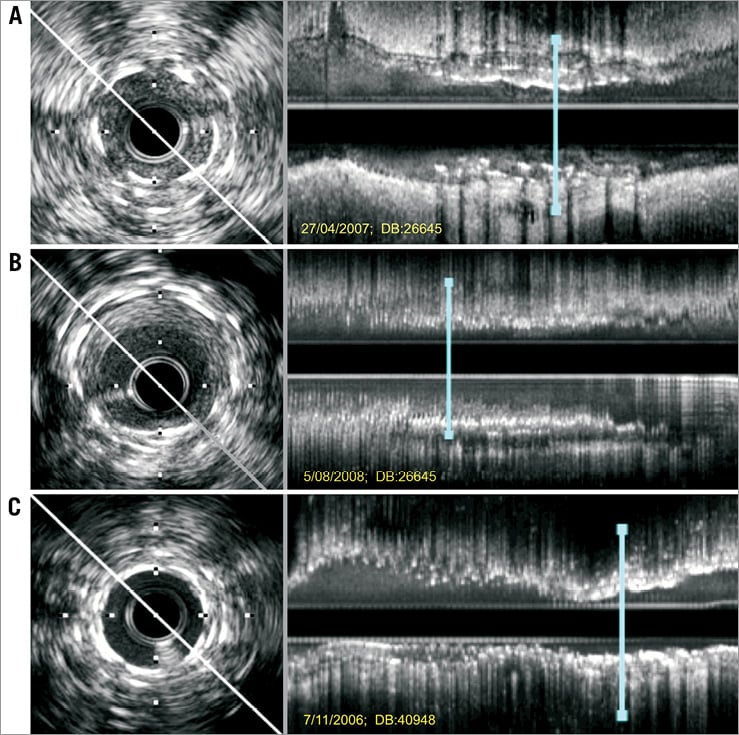
Figure 1. Intravascular ultrasound studies of patients with recurrent restenosis after stent implantation for in-stent restenosis (ISR). Left: cross-sectional images. Right: corresponding longitudinal views (the blue line indicates the location of the short-axis views). A) Severe recurrent ISR. The cross-sectional image readily reveals the circumferential distribution of two metal layers and the imaging catheter wedged into occlusive neointimal tissue. The longitudinal display discloses significant stent underexpansion. A third drug-eluting stent was implanted at very high pressures with excellent angiographic result. B) The same patient at late follow-up. The three stent layers were recognised, this time showing adequate expansion. Despite moderate neointimal proliferation the residual lumen was largely preserved (2.2×2.5 mm). C) Another patient with recurrent ISR in whom a third drug-eluting stent was implanted at high pressure. After the intervention the two superficial layers of stent appeared closely superimposed, but residual tissue was recognised between the first and second stent (onion skin). The longitudinal view disclosed persistent focal and severe stent underexpansion in a vessel already covered with stents along its entire length (full metal jacket).
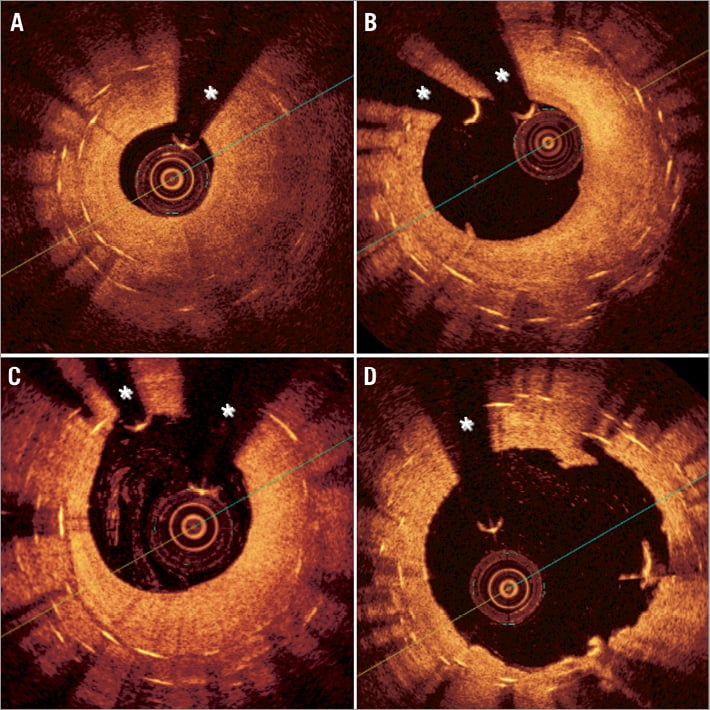
Figure 2. Optical coherence tomography images of a patient with recurrent in-stent restenosis (ISR). Notice the superb resolution of the lumen-neointima interface but also the poorer tissue penetration as compared with intravascular ultrasound. (A) Homogeneous neointima causing severe ISR. In some segments only one or two stent layers were detected whereas in other areas (B-C) three layers of metal were readily recognised (onion skin appearance). In this patient a drug-coated balloon was used after high-pressure balloon predilation with a good final result (D). A large lumen with some minor residual intra-stent neointimal disruptions was readily visualised after the procedure (D). *wire artefact
Present study
In this issue of the journal, Kubo et al6 report the results of a very large series of patients requiring reinterventions for recurrent ISR after DES implantation for ISR. From 1,101 patients treated for DES-ISR in a single centre during a seven-year time period, 142 with recurrent ISR (148 lesions) were retrospectively analysed. Of these, 70 patients (72 lesions) were treated with BA alone whereas 72 patients (76 lesions) were treated with a new DES implantation. These therapeutic strategies were selected according to the operator´s criteria. Of note, some important baseline characteristics were unbalanced in the two groups and, therefore, a selection bias cannot be ruled out. Patients in the BA group more frequently had a total occlusion at the time of the initial procedure (one-third of patients), had a shorter time to recurrent ISR (400 vs. 600 days), more frequently had renal failure (one-third of patients) and a longer underlying DES, as compared with patients treated with DES implantation. In addition, intravascular ultrasound guidance was less frequently used in the BA group. Lesion severity and vessel size were comparable and, eventually, final balloon size and pressure were also similar. All the potential confounders were nicely addressed by a conventional multivariate analysis that indicated that the use of a BA strategy was the most important independent predictor of adverse events during follow-up6.
However, considering the study design the potential influence of other unmeasured confounders remains an issue of potential concern. First, it appears that the BA alone strategy was only accepted when satisfactory results were achieved. It is therefore likely that patients with suboptimal results or complications after BA were eventually treated with an additional DES. This would favour the BA alone group. Second, it is also likely that in patients with a resistant waist during balloon predilation (undilatable lesions), the use of a third stent layer (by definition condemned to severe underexpansion) might have been avoided by the operator whenever possible. This, in turn, might have negatively affected the results of the BA group. Finally, one may further speculate that in patients with adverse edge-ISR (i.e., extending over a significant segment of the adjacent vessel), the operator might have preferred the implantation of a new DES12. All these issues, however, remain speculative –as the required details were not provided in the study– but might have influenced the study results. Surprisingly, stent fractures were not only very frequent (22%), but also remained equally distributed in the two arms. In previous studies the incidence of underlying stent fracture has been much lower and, actually, this diagnosis may be challenging in patients with multiple stent layers. Anyway, most investigators would advocate the implantation of another DES when this substrate is documented.
From a technical standpoint, it is important to emphasise that uniquely high pressures (mean 22 atmospheres) were required. These pressures were likely required to tackle underlying underexpanded stents. In addition, poorer late results were found in the BA group when the immediate results were not optimal (minimal lumen diameter <2.5 mm). Again, this underscores the need to optimise final results, especially when a BA alone strategy is selected for these patients. Importantly, in the present study the BA strategy was the most important independent predictor of adverse events during follow-up. Also of major interest, intravascular ultrasound guidance was identified as an independent predictor of freedom from target lesion revascularisation. Although criteria for intravascular ultrasound guidance were not described, it appears reasonable to conclude that this technique provides a valuable tool to tackle underexpansion and to optimise final results which, eventually, will translate into improved clinical outcome6.
Notably, this study provides unique information on the largest series of patients treated with a third intervention for ISR after DES for ISR6. Moreover, a late angiographic assessment was obtained in virtually all patients (95% of cases) and, reassuringly, also a uniquely long clinical follow-up (median 4.5 years) was reported. These should be highlighted as important methodological strengths of the study, quite rare in comparable observational studies. Initial angiographic results were significantly better after repeat DES implantation. Considering that late loss was eventually similar with both strategies, these superior initial results would appear critical in explaining the superior late angiographic findings obtained with DES. The clinical advantage of DES over BA emerged early after the procedure and, after the first year, a landmark analysis showed a low event rate with cumulative curves running flat and parallel in the two arms6.
Although repeat DES implantation clearly provided better outcomes than BA alone, the overall results of this strategy remained unsatisfactory6. Indeed, at late follow-up, late loss after DES was high (0.8 mm) and 1/4 patients developed recurrent angiographic ISR. More importantly, half of these patients suffered adverse clinical events. Reassuringly, however, no episodes of stent thrombosis occurred in the DES group. However, we should keep in mind that most DES used in the study were first-generation DES, and therefore it remains possible that newer-generation DES might provide superior results. On the other hand, a hetero-DES strategy was used in 57% of patients but with results similar to those seen with a homo-DES strategy.
Finally, with such a high rate of clinical recurrences it is quite surprising that coronary artery bypass surgery was so rarely used during follow-up (only in four patients, 2.8%). Actually, baseline characteristics of this selected patient cohort were highly unfavourable and it remains possible that many of these patients were deemed inoperable to start with. This might help to explain the relatively high mortality rate at late follow-up, considering that 2/3 of these deaths were classified as non-cardiac.
Final remarks
Treatment of “recurrent” DES-ISR remains a major challenge. In this setting the use of a new DES appears to be associated with better results than BA alone6. However, in patients with ISR and unexpandable stents, the use of additional stenting should not be routinely advocated. In this uniquely adverse anatomic scenario merely minor degrees of neointimal growth might trigger recurrences. Therefore, DES implantation, coupled with aggressive attempts to expand the underlying stents, might be considered as a last resort option, at least until alternative strategies become available11. Common sense, however, would suggest that keeping implanting additional metal layers (“onion skin full metal jacket” strategy), even despite the advent of novel, highly attractive and effective DES, may not be the right way forward. For patients with recalcitrant DES-ISR in a major vessel coronary surgery should always be considered, especially if significant disease is present in other vessels. Bioabsorbable scaffolds hold “theoretical promise” in this scenario13, but currently there is no information on their real value in patients with ISR and current designs would limit their use to relatively large vessels.
Multiple studies, however, have unequivocally demonstrated the efficacy of DCB in patients with both BMS-ISR and DES-ISR1,14-19. In this setting, DCB provide superior clinical and angiographic results as compared with BA and are at least as effective as first-generation DES14-19. Unfortunately, the results of a sizeable cohort of patients treated with DCB in the present study (30 patients, 33 lesions) were not reported6. The RIBS-4 and RIBS-5 randomised studies will determine the value of DCB versus second-generation DES in patients with DES-ISR and BMS-ISR, respectively. Nevertheless, further studies are clearly warranted to determine the value of DCB in patients with recurrent DES-ISR, especially in the presence of multiple metal layers.
Conflict of interest statement
B. Scheller is the co-inventor on several patent applications for restenosis inhibition, including drug-coated balloons. F. Alfonso has no conflicts of interest to declare.

