Abstract
Treatment of patients with in-stent restenosis (ISR) remains a technical challenge. This problem has been reduced since the advent of drug-eluting stents (DES), but continues to represent a significant burden during daily practice in interventional cardiology. Treatment of ISR after bare-metal stent implantation has evolved and currently DES constitute the intervention of choice. However, DES may also develop ISR. The best therapeutic alternative for patients suffering from ISR after DES implantation remains to be elucidated. This review will focus on treatment of patients with ISR emphasising currently available alternatives, technical issues, limitations and future perspectives.
Scope of the problem: prevalence of ISR
Treatment of patients presenting with in-stent restenosis (ISR) remains a technical challenge and constitutes a significant clinical problem1,2. The advent of drug-eluting stents (DES) has reduced the number of patients suffering from ISR after these interventions. However, DES are not immune to ISR, especially when used in clinical and anatomic scenarios of ever increasing complexity. The optimism generated by DES in the interventional cardiology community opened new therapeutic venues in patients with complex anatomy and induced a more aggressive approach in the clinical decision making process involved in the appropriateness of coronary revascularisation3. This change was associated with increasing rates of ISR, far away from the single digit figures initially obtained with DES in early series of favourable patients. In addition, bare metal stents (BMS) are still been used in a large number of patients undergoing percutaneous coronary interventions (PCI)3. BMS are indicated in patients unable to comply with a long-term dual antiplatelet regimen or whenever this therapy is associated with an increased bleeding risk. Fears of late stent thrombosis, particularly in off-label indications, still remain a major caveat which limits a wider use of DES4. Finally, economic reasons still play a role in many countries.
The increasing global numbers of patients undergoing PCI, together with the use of DES in more complex clinical and anatomic scenarios, explains why the clinical problem of ISR persists3. In Spain, ISR represented a 6.3% of the total PCI activity in the year 2003 (with 20% of DES penetration) whereas it was 5.5% in 2007 (with 58% of DES penetration)3. Interestingly, these figures translated into an absolute “increment” in the number of patients requiring repeated PCI for ISR (from 2,409 to 3,277 procedures). Likewise, the number of patients presenting with DES ISR may approach 200,000 per annum in the United States alone5.
Clinical presentation: should all patients with ISR be treated?
Patients developing ISR usually present with clinical recurrences1,2. In most cases, symptoms may be considered as relatively stable. A careful clinical history will be able to unravel the presence of recurrent symptoms that, in general, resemble those present before the initial PCI. However, in cases with multivessel disease or incomplete revascularisation, symptomatic status is a less reliable method to identify ISR. Furthermore, classically, ISR has been associated with a benign clinical presentation1,2. Recent reports, however, indicate that some patients with ISR present with unstable symptoms or even with a myocardial infarction6. In our experience, ISR presenting as a large (Q-wave or non-Q-wave) acute myocardial infarction is very rare1,2. The adoption of more sensitive definitions of myocardial infarction (mainly based on a troponin rise) may explain these findings whereas stent thrombosis, rather than ISR, should be suspected in patients with larger myocardial infarctions.
ISR is an angiographic definition. ISR is usually defined as a recurrent percent diameter stenosis >50% in the previously stented segment or at its edges (including the adjacent 5 mm coronary segments at both sides)1,2. However, treatment of ISR should be based on clinical judgement and whenever possible the “oculo-stenotic reflex” should be avoided. Many patients with ISR are asymptomatic and in this setting the benefit of repeated revascularisation is not well established. In our experience7, patients with asymptomatic restenosis tend to have less severe lesions, smaller vessels or restenosis in infarct-related vessels7. Many patients with asymptomatic ISR were asymptomatic at the time of the initial PCI, had diabetes or totally occluded vessels before the initial procedure. Different studies have demonstrated that the clinical outcome of patients with asymptomatic restenosis is favourable7-9. This is particularly true in patients with negative results for ischaemia on non-invasive techniques (exercise test, stress echocardiography or nuclear perfusion studies). On the other hand, we should keep in mind that ISR is largely caused by neointimal proliferation obstructing the stent lumen. This neointimal growth represents a dynamic healing phenomenon with evolving changes that may resemble those seen in scar tissues. This phenomenon has been implicated in explaining the occasional presence of spontaneous regression of ISR severity with “improvement” in minimal lumen diameter at very long-term follow-up8-9. Although these changes are small and only have been demonstrated in asymptomatic patients without severe ISR, they constitute a clear argument for conservative management in these patients. All these reasons emphasise the importance to demonstrate myocardial ischaemia before a new PCI is considered. Non-invasive tests are of especial valuable in patients undergoing “routine” late angiographic studies for either clinical or research purposes. In asymptomatic patients with severe ISR in large vessels, perfusing normally contracting myocardium PCI may be considered. However, in these cases it is prudent to use additional intracoronary diagnostic techniques to help in this decision. Intravascular ultrasound (IVUS) provides valuable anatomic information in these patients2,10,11 and it is very useful to measure minimal lumen area which has been associated with objective demonstration of ischaemia. Furthermore, IVUS gives further insights on the exact mechanisms of ISR and can be used to guide subsequent interventions2,10,11. Alternatively, measuring the fractional flow reserve constitutes an elegant functional assessment of ISR severity which may be readily used as a surrogate for myocardial ischaemia in the catheterisation laboratory12.
Patterns of ISR
ISR is almost exclusively attributable to neointimal hyperplasia. Angiography is not only able to determine the presence of ISR and its severity but also reveals different morphologic patterns. Early studies13-15 demonstrated that patients with “diffuse” ISR (lesion length >10 mm) have a poorer clinical and angiographic outcome after repeated interventions as compared with patients with “focal” ISR (<10 mm in length). Mehran et al14, proposed a dedicated classification of ISR based on angiographic patterns that became widely used. Pattern I, consisted of focal ISR (IA articulation, IB margin, IC body, ID multifocal); Pattern II, was reserved for diffuse ISR within the stent; Pattern III, was used for “proliferative” ISR where the angiographic narrowing extended beyond the stent margins; finally, Pattern IV was used for ISR presenting as an occluded vessel (Figure 1). The Mehran classification14 had major clinical implications and the requirement for target lesion revascularisation at follow-up was 19%, 35%, 50% and 83% for Types I, II, III and IV, respectively.
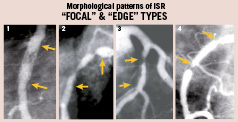
Figure 1. Different patterns of “focal” in-stent restenosis (ISR). 1) Body, 2) Multiple, 3) Edge, 4) Edge with a classical “candy wrapper” morphology. (arrows indicate the edges of the underlying stent).
In our experience15, the ACC/AHA angiographic lesion classification is also of value in these patients. B2-C ISR lesions are not only more frequently associated with suboptimal immediate angiographic results but also with a higher restenosis rate and a poorer long-term clinical outcome.
Although conventional angiography is of clear value in clinical decision making, quantitative coronary angiography is better suited for research purposes, namely the assessment of results obtained with different interventions in these patients1,2. However, the extent of neointimal proliferation is better detected by IVUS. IVUS is of great value to unravel the mechanisms of ISR (neointimal proliferation, stent under-expansion or both), to accurately determine the location of the stent in relation to the stenosis (edge ISR), and to guide reinterventions2,10,11. Accurate mechanistic comparisons of minor degrees of neointimal hyperplasia among different DES are ideally performed with IVUS and, more recently, using optical coherence tomography due to its higher spatial resolution16,17. With optical coherence tomography, some studies have speculated that images suggestive of necrotic core could be visualised within some DES experiencing ISR.
Balloon angioplasty
Balloon angioplasty (BA) was the initial strategy for patients with ISR13. The use of BA in this setting proved to be user friendly, consistently associated with good initial results and associated with a very low incidence of complications. IVUS studies18 demonstrated that the mechanism of lumen enlargement in patients with ISR was twofold: further expansion of the underlying stent and tissue extrusion with longitudinal redistribution along the stent length and its edges (56% and 44% of lumen gain respectively). Although satisfactory initial clinical and angiographic results are systematically obtained with BA, the long-term outcome of this treatment is shadowed by a high restenosis rate, specially in patients with diffuse ISR13-15. Indeed, relatively high clinical and angiographic restenosis rates (31% to 54%) have been found after BA for ISR in different studies19,20. Other studies, however, reported more favourable clinical21 and angiographic22 outcomes. Reimers et al21 found a cumulative event-free survival of 81% at two years. In the study of Bauters et al22 with a 85% of late angiographic follow-up, the restenosis rate was 22%, and only 17% of patients required revascularisation. These data would support the use of BA as the primary treatment in patients with “focal” ISR. Determinants of a good clinical outcome after BA for ISR are well established13,19-22. In our experience, clinical factors are key prognostic markers. We found13 that patients with diabetes and those with a short (<4 months) time interval from stent implantation to repeat intervention had a three-fold risk increase for adverse cardiac events during follow-up. Other studies suggested that angiographic variables –ISR lesions severity and length, ejection fraction, multivessel disease, saphenous vein graft location– are also major determinants of the long-term outcome19-22.
The technique of BA for ISR consists of optimising final results using adequate balloon/artery ratios and high pressures. Careful analysis of the technique used during the previous PCI and analysis of the initial results are of help. However, whether a more aggressive dilation is associated with improved outcomes has not been determined. In fact, aggressive dilations can be associated with edge dissections that jeopardise results. BA for ISR is more frequently associated with the phenomenon of balloon slippage (“watermelon seeding”) than BA in de novo lesions. In our experience23, this tends to occur in more severe and diffuse ISR lesions and with the use of oversized balloons. Balloon slippage may induce edge-dissections and it is associated with cumbersome procedures, suboptimal results and adverse clinical and angiographic outcomes23. Besides, management and fate of side-branches emerging from ISR lesions remain of interest24. Large, jailed, side-branches should be adequately protected (particularly if their ostium is compromised) and –if required– treated with BA. Other smaller side-branches may experience transient flow deterioration during PCI but this is rarely associated with adverse events. These side-branches systematically reappear at late angiography24.
Cutting balloon
The cutting balloon (CB) is a special balloon catheter with three or four micro-surgical blades bonded longitudinally to its surface. It appears well suited to incise neointimal material and to facilitate its redistribution and extrusion. Conventional balloons, especially short balloons, when positioned within ISR lesions, tend to move forward or backward into the adjacent larger segments during inflation. Conversely the blades of CB anchor the balloon to the lesions during inflation, reducing the risk of displacement and dissection at the stent margins.
Observational studies25-26 suggested that CB might be superior to conventional BA in the treatment of ISR. In a large matched study26 648 ISR lesions were divided into four groups according to the treatment strategy: CB, rotational atherectomy, additional stenting and BA. Patients treated with CB had a lower late loss and a reduced restenosis rate at angiographic follow-up. On multivariate analysis, CB was an independent predictor of the absence of target lesion revascularisation26.
RESCUT was a multicentre, randomised, European trial comparing CB with BA in 428 patients with all types of ISR27. Balloon slippage was less frequently seen in the CB group (6.5 vs 25%). This group also required fewer balloons and had a trend toward a lower need for additional stenting. However, at late follow-up, the binary restenosis rate (29.8% vs 31.4%) and clinical events were equivalent in both groups. In conclusion, both strategies are equally effective to prevent recurrences of ISR although CB may be of particular benefit when avoiding the damage of the adjacent vessel wall appears particularly important24-28.
Debulking techniques
BA treatment of intrastent neointimal hyperplasia is challenging and frequently is associated with suboptimal results. This is due to the persistence of residual neointimal material within the stent. Therefore, alternative approaches, namely debulking techniques, have been advocated as an attractive means of removing intrastent tissue, improve angiographic results and decrease recurrences. Nevertheless, these procedures are more expensive and technically demanding than BA. The strategy was to debulk most of the obstructive material followed by gentle balloon dilation29-34. The idea was to optimise final results while minimising vessel trauma to avoid triggering recurrent neointimal proliferation. Initial studies with excimer laser were promising. In one study30, the use of laser+BA, resulted in greater lumen gain, more ablation/extrusion of neointima, larger final lumen areas, and a tendency for less frequent need for subsequent target vessel revascularisation, as compared with BA alone. Nevertheless, the results of these sophisticated procedures were soon shadowed by the demonstration of high recurrence rates. A study using excimer laser angioplasty with systematic angiographic follow-up indicated a restenosis rate of 54%29. Some IVUS studies revealed that excimer laser only produced a modest ablation of the tissue causing ISR30. Whether these results were the result of technical issues or the limited ablation capability of the fibres used was not fully elucidated but, eventually, the technique was abandoned and a proper randomised clinical trial was never performed.
Directional atherectomy had more efficient debulking proprieties in de novo lesions and it was also tested in patients with ISR. Results of different observational series were favourable32. However, this device was too bulky to be readily advanced in distal vessels and could not be used in many cases of ISR. In addition, the limited length of the cutter, prevented the treatment of patients with diffuse ISR.
Rotational atherectomy was considered of high interest in this setting. Early studies demonstrated the feasibility and safety of rotational atherectomy in patients with ISR and its effectiveness in debulking neointima. This technique allowed a more effective debulking of tissue fulfilling the stent especially when large burrs were used. Volumetric IVUS analysis of rotational atherectomy procedures demonstrated a greater reduction in intimal hyperplasia volume as compared with excimer laser31. The ARTIST study33 was a large multicentre, randomised European trial that included 298 patients with ISR treated with rotational atherectomy+BA or BA alone. Rotablation was performed by using a stepped-burr approach followed by adjunctive BA with low inflation pressure. Unexpectedly, at follow-up angiography, the net angiographic gain was significantly larger (0.67 vs 0.45 mm) and the binary restenosis rate significantly lower (51 vs 65%) in the BA arm. Furthermore, despite being performed in experienced centres, procedural complications were more frequent detected in patients assigned to the rotational atherectomy group. Eventually, the 6-month event-free survival (91 vs 79%) was significantly better after BA33. To explain these findings, it was speculated that the strategy of low-pressure adjunctive BA could had been unable to properly expand some poorly expanded stents in the rotational atherectomy arm. Conversely, in the smaller, single centre, ROSTER trial32 results of rotational atherectomy were superior to those seen in the BA arm. In this study, however, IVUS was systematically used to exclude patients with under-expanded stents (up to 1/3 of patients). However, the poor clinical and angiographic results obtained in the larger ARTIST trial tempered the enthusiasm for the use of ablative techniques in these patients.
Repeat stenting
As compared with BA, BMS reduce the risk of restenosis in de novo lesions. This fuelled the interest to investigate their role in patients with ISR. The larger acute angiographic gain obtained by repeat stenting was considered a potential major advantage in this setting. Initially, however, only cases experiencing complications (dissections) after a prior BA attempt were considered for BMS implantation35. Subsequently, patients with suboptimal results after BA or debulking techniques were treated with repeat stenting (sandwich stenting)36. In all these cases with poor initial results, BMS demonstrated their ability to guarantee excellent immediate angiographic results. Early series also suggested that “elective” BMS implantation was of particular value in these patients36. As a matter of fact, the clinical and angiographic outcome of BMS was superior in elective patients as compared with those in whom repeat stenting was used to correct complications or suboptimal results following another technique36. Both angiographic and IVUS studies demonstrated significant residual lumen stenosis after BA, as compared with the excellent results obtained with repeated stenting37. Indeed, as compared with all available interventional alternatives, repeat stenting was the technique providing a larger final minimal lumen area in patients with ISR37. In addition, detailed mechanistic studies demonstrated that an “early lumen loss phenomenon” may further jeopardise the results of BA or ablation techniques38. This finding, caused by early tissue re-intrusion back into the stent lumen, may also be implicated in some recurrences. In a dedicated, randomised, mechanistic volumetric IVUS study39 we demonstrated that this phenomenon was virtually abolished by repeated stenting whereas it was readily identifiable (within one hour) after BA. (Figure 2) Finally, another theoretical advantage of repeat stenting is that it prevents balloon slippage during optimisation efforts.
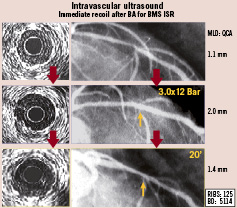
Figure 2. Early lumen loss phenomenon. Left: intravascular ultrasound findings. Right: angiographic findings. Top panel: severe in-stent restenosis (ISR) in the left anterior descending coronary artery. Middle panel: good angiographic and intravascular ultrasound results after balloon angioplasty (BA). Bottom panel: early (after 20 minutes) deterioration of minimal lumen diameter. Notice tissue re-intrusion within the stent on intravascular ultrasound. MLD: Minimal lumen diameter. QCA: Quantitative coronary angiography.
The RIBS-I randomised trial1 (24 sites from Spain and Portugal), allocated 450 patients with ISR to either BA or BMS. Initial angiographic results (minimal lumen diameter and acute gain) were significantly better after BMS implantation. However patients in the stent arm experienced a larger late lumen loss and, eventually, the minimal lumen diameter at six months was similar in both groups (Figure 3). This translated into similar rates of recurrent restenosis (38 vs 39%) and a comparable requirement of target vessel revascularisation1. However, BMS was clearly superior to BA in the pre-specified subgroup of patients with large vessels (> 3 mm in diameter)1. Additional subgroup analyses suggested that that BMS were also superior to BA in patients presenting with edge-ISR40.
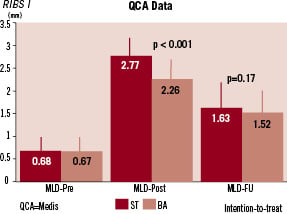
Figure 3. Angiographic data in the RIBS I Study. QCA: quantitative coronary angiography. MLD: minimal lumen diameter. ST: stent. BA: balloon therapy.
Therefore, BMS provides an attractive option for patients not suitable for long-term dual antiplatelet therapy and in cases with suboptimal results or complications after another technique. Further, patients with edge-ISR and those with large vessels obtain a satisfactory clinical and angiographic outcome with repeat BMS implantation1,40.
Brachytherapy
Brachytherapy proved to be a highly effective technique to suppress neointimal proliferation after coronary wall injury41-46 and this strategy has been used, with enormous success, in patients with ISR. In fact, considering evidence-based medicine, few areas in interventional cardiology have been so exhaustively investigated and with results so consistent. Multiple randomised trials (using beta and gamma sources) have clearly established the superiority of brachytherapy over alternative strategies (mainly BA or debulking techniques). All these clinical trials consistently demonstrated the benefit of brachytherapy (in terms of angiographic restenosis and repeat revascularisation) in patients with ISR41-46. The effectiveness of this therapy was so impressive that major efforts were made to overcome the logistics constrains required to develop and maintain this sophisticated technique. A close collaboration with radiotherapists was required. During these procedures it was important to carefully control the boundaries of the injured segment. Many investigators favoured the use of CB to safely predilate these lesions. Otherwise, edge-ISR as the result of the “geographic miss phenomenon” could occur43. The effectively irradiated area should include the lesion, the adjacent damaged wall and its boundaries. However, the first important shadow was cast by the report of patients suffering from late thrombosis after these procedures41-44. It was suggested that the profound ability of brachytherapy to inhibit smooth muscle cells was intimately associated with a delayed endothelisation process which, in turn, could be a trigger for late vessel thrombosis. The presence of occasional striking patterns of late positive vessel remodelling was also nicely visualised by IVUS43,44. Whenever possible, stent implantation in this context was avoided and prolonged dual antiplatelet therapy was recommended. Finally, a late catch-up phenomenon was demonstrated after brachytherapy accounting for a significant reduction in efficacy overtime45.
Two pivotal randomised studies compared DES with vascular brachytherapy in patients with BMS ISR41,42. The SISR trial41 included 384 patients assigned to brachytherapy or sirolimus-eluting stenting. Rates of target vessel failure at nine months were 21.6% in the brachytherapy group compared with 12.4% in the DES group. The TAXUS-V trial42 studied 396 patients with BMS ISR assigned to vascular brachytherapy or paclitaxel-eluting stent implantation. At nine months, target-vessel revascularisation rates were 17.5% in the brachytherapy group versus 10.5% in the DES group.
For all these reasons, together with a reduced commercial interest in the technique and, above all, the advent DES as the preferred therapeutic modality, brachytherapy was virtually abandoned and currently is not longer widely available to treat patients with ISR41-46.
Drug-eluting stenting
DES have demonstrated a dramatic ability to suppress neointimal proliferation and to reduce the need for target vessel revascularisation. Therefore, the use of DES in challenging lesions subsets, such as ISR, was soon advocated. Early observational studies demonstrated that DES are user friendly and highly effective in patients with ISR although results tended to be less favourable than those found in simpler lesions. In this setting, both sirolimus-eluting stents and paclitaxel-eluting stents were able to provide excellent clinical and angiographic results2,47. In patients with ISR it is important to avoid damaging adjacent normal coronary segments during BA predilation since this could trigger edge-ISR after DES implantation. Accordingly, predilation with short and undersized balloons appears indicated. In addition, the presence of under-expanded stents should be evaluated with IVUS and, when detected, aggressively managed. This is important, because under-expanded stents have been proposed as a frequent underlying problem in patients experiencing recurrences after DES for ISR2,48,49.
In the ISAR-DESIRE trial, Kastrati et al47 demonstrated that DES were superior to BA in reducing the need for new revascularisations at 12 months. They found that the incidence of restenosis was significantly reduced after sirolimus-eluting stent (14.3%) and paclitaxel-eluting stent implantation (21.7%) as compared with conventional BA (44.6%). This translated into a lower requirement of target vessel revascularisation in the two DES arms. Likewise, the RIBS II randomised study2 also demonstrated the superiority of DES over BA in patients with ISR. Patients treated with sirolimus-eluting stents had a lower restenosis rate (11%) and a better 1-year event-free survival, mainly as the result of a reduction in target vessel revascularisation as compared with those treated with BA (Figure 4). This benefit was consistent across 10 pre-specified clinical and angiographic variables. Furthermore, in RIBS-II a detailed, volumetric, IVUS sub-study confirmed the striking antiproliferative efficacy of DES in these patients whereas a significant late neointimal proliferation was detected in the BA arm2. Subsequently, a pooled analysis demonstrated that, as compared with BMS, DES improve clinical and angiographic outcome in patients with ISR50.
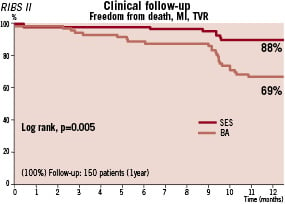
Figure 4. 1-year event-free survival in the RIBS II study. MI: myocardial infarction. TVR: target vessel revascularisation. SES: sirolimus-eluting stents. BA: Balloon angioplasty.
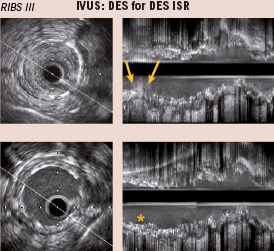
Figure 5. Cross-sectional (left) and longitudinal (right) reconstruction of intravascular ultrasound (IVUS) images in a patient with focal in-stent restenosis (ISR) of a drug-eluting stent (DES) (arrows) (Top). Bottom: results after treatment with a second DES implantation (*).
Finally, it has been suggested that DES may be associated with an increased risk of late thrombosis mainly when used with “off-label” indications in “real world” clinical practice. Daemen et al4 reported in routine clinical practice a late DES thrombosis rate of 0.6% per year. Patients treated for ISR might be at higher risk for thrombosis. However, a very long-term follow-up (three to four years) of patients included in the RIBS-II trial confirmed that an excellent long-term prognosis was maintained in patients treated with sirolimus-eluting stents51. These findings dissipated potential concerns of a late “catch-up” phenomenon –similar to that occurring after brachytherapy– that could diminish their long-term clinical efficacy. On multivariate analysis, DES implantation, time to ISR, the ACC/AHA angiographic classification and the Mehran classification were identified as independent predictors of adverse clinical events at late follow-up51. Although not routinely recommended, the potential value of extended use of clopidogrel beyond 12 months in complex patients such as those with ISR remains to be defined.
Management of patients with restenosis after drug-eluting stents
DES are very effective to suppress neointimal proliferation but, especially in complex patient/lesion subsets, ISR has not been eradicated. ISR after DES tend to occur more frequently in diabetics, long lesions, small vessels, ISR and patients with suboptimal results52. ISR after DES has distinct angiographic patterns. DES ISR is usually rather focal and frequently locates at the edges52. Outcome appears to be better for focal than for diffuse DES ISR. Edge problems are frequent in cases where DES were not implanted to cover all the coronary segment traumatised during predilation. In some cases, DES ISR is associated with frank DES under-expansion and in more rare circumstances occurs at sites of stent fracture. IVUS appears well suited to rule-out or, alternatively, correct areas of DES under-expansion and to optimise final results49. The best treatment of patients with DES ISR remains yet to be elucidated. Observational series suggested that results of repeat interventions were poorer in DES ISR than in BMS ISR5,52-57. In patients with DES ISR Lemos et al53 found a binary restenosis rate of 42%, although recent reports provide more favourable results54-57. Results obtained with BA appear to be worse than those achieved with DES53. Some reports suggest that brachytherapy may still be considered in these cases58. In a recent study, 119 patients treated with DES for DES ISR were compared with a matched group of 119 patients undergoing DES implantation for BMS ISR57. Target vessel revascularisation was more frequently required (22.2% vs 10.3%, p<0.01) in patients with DES ISR. This study illustrates that DES ISR constitutes a particularly challenging scenario. Theoretically speaking, DES ISR may be the result of suboptimal deployment, drug failure or both. Once DES under-expansion has been ruled out (or aggressively managed) many investigators prefer to use a different DES (switch strategy) during the repeated interventions52-57. However, to date only a limited number of studies, including small patient cohorts, have assessed the value of this strategy of cross-over as compared with using the same DES and currently results are mixed and inconclusive52-57. The use of balloons coated with paclitaxel, appear to be highly effective in patients with BMS ISR59, but its value in patients with DES ISR remains undefined. Future studies are warranted to determine the intervention of choice for patients with DES ISR.
Finally, we should keep in mind that coronary surgery should always be considered in cases with recurrent or recalcitrant ISR, especially if located in the proximal left anterior descending coronary artery or if additional vessels can benefit from this time-honoured form of revascularisation.

