Abstract
Intracoronary brachytherapy (ICB) was developed as an attempt to prevent restenosis after percutaneous coronary interventions. Early clinical experiences showed impressive results especially in the subset of patients with in-stent restenosis. This led to the design of large multicentre trials that demonstrated the efficacy of ICB as adjunctive therapy in patients with in-stent restenosis as compared to conventional treatment. Despite these outstanding initial results, several limitations arose such as late thrombosis, edge effect or late catch-up phenomenon. These, together with the difficult logistic process to implement the ICB in the cath lab and the development of the drug-eluting stent shelved definitely the technique. This review describes the potentials and limitations of this therapy, as well as the current status in the drug-eluting stent era.
Rationale and history of intracoronary brachytherapy
Intracoronary brachytherapy (ICB) was developed as an attempt to prevent restenosis after percutaneous coronary interventions in the early and mid 90’s. The rationale behind it was the fact that radiotherapy had proven to be effective in treating the exuberant fibroblastic activity of keloid scar formation and other non-malignant processes such as ocular pterygia1,2. As in-stent restenosis was mainly induced by an excess of neointimal proliferation, it was assumed that this adjunctive therapy would also inhibit this process. The first experimental study in this field was carried out in 1964 by Friedman et al through the use of Iridium 192 (192Ir) in the cholesterol-fed rabbit3. In 1992, in Frankfurt, Liermann and colleagues performed the first four cases of brachytherapy in patients who had undergone a femoral percutaneous angioplasty4. A second wave of experimental work was carried out in the United States by Wiedermann and Weinberger in New York5, Waksman and Crocker in Atlanta6 and Mazur and Raizner in Houston7. In parallel, Verin and Popowski in Geneva conducted experimental studies with the pure ß-emitter 90Yttrium (90Y) in carotid and iliac arteries of rabbits8. The first clinical experience in coronary arteries in humans was performed by Condado et al using a hand-delivered 192Ir wire into a non-centred, closed-end lumen catheter9 and by Verin et al using a ß-source and a centred device10. Both studies demonstrated that the delivery of radiation in the coronary artery is feasible and safe, although the restenosis rate remained relatively high. The positive results of the first randomised trial aimed to determine the effectiveness of γ-radiation for the treatment of restenotic lesions11 encouraged the investigators to design an extraordinary number of studies that will quickly shed light on the utility of brachytherapy for the prevention of restenosis.
Radiation therapy could be delivered to the coronary arteries by external radiation or by brachytherapy methods either using catheter-based systems or radioactive stents12. Catheter-based systems could handle either ß- or γ-emitters which delivered the prescribed dose in either high or low-dose rate, manually or automatically. Radioactive stents utilised mainly pure ß-emitters in a very low-dose rate.
Efficacy of intracoronary brachytherapy in the pre-drug eluting stent era
Overall, ICB demonstrated to be highly efficacious for the treatment of in-stent restenosis by the use of catheter-based radiation systems (with either γ- or ß-radiation). Teirstein et al11 designed the first randomised trial with γ-radiation for the treatment of restenotic lesions. Fifty-five patients with restenosis, either after balloon angioplasty (n=20) or after stent implantation (n=35), who were scheduled for new stent implantation were enrolled in this trial. Angiographic indices of restenosis were markedly different in the irradiated arm as compared to the placebo group: late loss was 0.38±1.06 in the 192Ir group as compared to 1.03±0.97 in the placebo group (p=0.009); restenosis rate (including the stent and the border) was 16.7% in the irradiated group and 53.6% in the placebo arm (p=0.025). The global beneficial effect in the 192Ir group was maintained at 3-year follow-up13: target lesion revascularisation 15.4% in the 192Ir group and 48.3% in the placebo group (p<0.01); restenosis rate 33% in the irradiated group versus 64% in the non-irradiated group (p<0.05). The main results in terms of restenosis prevention of the most important randomised controlled trials12,14-19 comparing ICB and conventional treatment for patients with in-stent restenosis are expressed in the Figure 1. The reported, splendid results, led this technique to receive the class recommendation I level of evidence A for the treatment of in-stent restenosis in native coronary arteries and class recommendation I, level of evidence B for the treatment of in-stent restenosis in saphenous vein graft20.
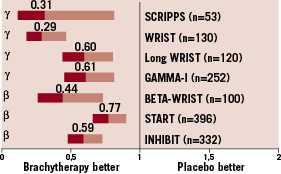
Figure 1. Summary of benefit of ICB vs. conventional treatment for restenosis prevention in main randomised controlled trials12,14-19.
The benefit of this therapy for the treatment of patients with in-stent restenosis was not repeated for the treatment of de novo coronary lesions. This setting was evaluated by several randomised controlled trials21,22. Although beta-radiation therapy did reduce the degree of neointimal proliferation within the stent, the occurrence of edge effect and late stent thrombosis clinically counteracted the initial angiographic benefit.
Finally, radioactive stents were overall unsuccessful by the occurrence of edge effects (Figure 2)23.
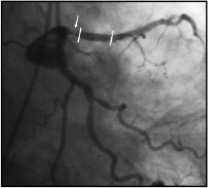
Figure 2. Edge restenosis (arrow) at proximal end of a radioactive stent (between lines) implanted in proximal left anterior descending artery.
Patterns of recurrence of restenosis after ICB
Edge effect: As mentioned above, a potential limitation of intracoronary brachytherapy is the development of new stenotic lesion at both edges of the irradiated segments. This so-called edge effect or «candy wrapper» effect was originally described after high activity (>3 µCi) radioactive stent implantation23 (Figure 2). However, this phenomenon is not exclusive to radioactive stent, but may also affect coronary segments treated by means of catheter-based system24. Vessel wall injury, concomitantly to low-dose radiation at the edge of the irradiated segment, may be involved in the pathophysiology of this phenomenon. To integrate both components, we proposed the concept of the «geographic miss»25. This concept is translated from a term in radio-oncology defining a cause of treatment failure due to low dose. In such cases, a small part of the treatment zone has either escaped radiation or been inadequately irradiated because the total volume of the tumour was not appreciated and hence an insufficient margin was taken. Typically, this phenomenon occurs by injuring the edges of the irradiated segment where, by definition, the dose received is rather low. In those «geographic miss» edges, a quantitative coronary analysis demonstrated a significantly higher late loss (0.84±0.6) as compared to both the irradiated segment (0.15±0.4) and the uninjured edges (0.09±0.4; p<0.0001). Similarly, binary restenosis was significantly higher in the geographic miss edges25.
Conceptually, after radioactive stent implantation, the incidence of «geographic miss» is 100%, since the length of the balloon used to deliver the stent is always longer than the radioactive stent. Thus, both edges are always injured and receive low-dose radiation.
Volumetric intravascular ultrasound studies, demonstrated that lack of positive remodelling or vessel shrinkage, together with plaque increase, were the contributors to the lumen shrinkage at both edges of the irradiated segment either after catheter-based brachytherapy24,26 or after radioactive stent implantation23. To demonstrate the fact that both low dose radiation and injury should coexist to induce the development of the «edge effect», Kozuma et al27, studied, by means of three-dimensional intravascular ultrasound (IVUS) and volumetric analysis, the geometric changes between non-injured edges of irradiated and placebo segments. Both groups showed comparable degrees of plaque volume increase, total vessel volume changes and luminal volume decrease during the follow-up period. Thus, the outcome of those edges, without macroscopic signs of injury, was not negatively influenced by the low-dose radiation received during the brachytherapy treatment. This IVUS analysis confirmed previous angiographic evidences of development of higher-than-expected restenosis rates at injured edges as compared to both irradiated segments and uninjured edges25. To minimise this harmful edge effect the use of longer sources to allow enough margins to fully cover the injured segment has been advocated when catheter-based brachytherapy is applied.
This «candy-wrapper» effect has been the Achilles heel of the use of radioactive stents23,26. Again, IVUS analysis demonstrates that at stent edge, negative remodelling and plaque growth contributed to luminal narrowing. Further attempts, either to negate the impact of negative remodelling at the edges of the stent with the «cold ends» radioactive stent or to decrease plaque growth with the use of «hot ends» radioactive stent, were unable to avoid the occurrence of the problem. The former induced a shift in the location of the IVUS-assessed neointimal hyperplasia towards the transition between the active and the inactive part of the «cold ends» stent28. The latter provoked an excess of tissue growth mainly located at the proximal hot edge. Finally, the use of a square-shouldered balloon to deliver the radioactive stent and thus, to minimise edge injury, was unsuccessful as well. As a result, the radioactive stent was never used in clinical practice beyond studies.
Late catch-up phenomenon: From the theoretical point of view, ICB may induce a delay of the restenotic process rather than a permanent inhibition of the restenosis29. Considering that a single acute dose of 12 or 16 Gy would result in a depopulation of smooth muscle cells about 10–3 to 10–6 (about 1 cell in 1000 to 1 million would survive), the number of doublings of the surviving cells to produce enough progeny to block the artery would be between 12 to 20, which would take between 12 to 24 months. Although smooth muscle cells are not malignant, and therefore do not have the capacity for indefinite proliferation, at least theoretically, one cannot assume that the restenosis process ends after six months29. This concept has been observed in clinical trials. In the Scripps trial, mean minimal luminal diameter in those patients not treated by the 6-month angiography decreased from 2.49±0.81 at six months to 2.12±0.73 at three years in the 192Ir group, whereas it had not significant change in the placebo group13. Similarly, in the WRIST trial, target lesion revascularisation rate was more frequent in the brachytherapy arm (17% vs. 2%, p=0.002) from six to three year-follow-up30. Recently, another randomised trial31 observed this delayed restenotic process after ICB up to five years follow-up.
Safety concerns after ICB
Late thrombotic occlusion (Figure 3): The occurrence of coronary thrombosis beyond one month after angioplasty or stent under aspirin and ticlopidine or clopidogrel (for 15 days to one month) regimen was anecdotal in the bare metal stent era32. However, this undesirable phenomenon became apparent in the first series of patients treated with brachytherapy world-wide. In the first 92 patients treated with intracoronary brachytherapy at the Thoraxcenter, a higher-than-expected incidence (6.6%) of thrombotic clinical events two to 15 months after treatment was observed33. This finding was confirmed in the American series of patients treated with γ-radiation. Data pooled from the WRIST, Long-WRIST, SVG-WRIST, GAMMA-1 and BETA-WRIST trials demonstrated an incidence of late thrombotic occlusion of 9.1% as compared to 1.2% in the placebo groups at 5.4±3 months after the procedure34. The implantation of a conventional stent within the irradiated segment has been considered a main contributor to this phenomenon. In this regard, considering only the cohort of stented patients, the rate of late thrombosis increases to 8.8% in the Rotterdam series and 14.6% in the American series. The delay in the stent re-endothelialisation has been considered as a trigger mechanism of this event35. Besides, the possibility that brachytherapy may induce late stent malapposition by not being able to follow the vessel enlargement promoted by radiotherapy, has been advocated in this process36. Several reports also indicated the possibility that ICB would induce true aneurysm formation9,24, although this phenomenon could not be directly associated with the occurrence of late thrombotic events. Finally, in patients treated only with balloon angioplasty followed by intracoronary brachytherapy, the presence of unhealed dissections at 6-month follow-up is a common phenomenon37. For all the above-mentioned reasons, dual antiplatelet regimen with aspirin and clopidogrel for at least 12 months was advocated after ICB treatment20,38.
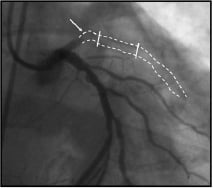
Figure 3. Late stent (between lines) thrombosis in a coronary irradiated segment (arrow).
ICB in the drug-eluting stent (DES) era
The burst of DES onto the scene drastically changed the utilisation of ICB. First, the overall number of patients with restenosis decreased as the penetration of DES increased. Second, large companies decided not to invest in ICB technology in light of the outstanding results offered by the use of DES. Finally, randomised controlled trials39,40 that compared ICB and DES for the treatment of in-stent restenosis demonstrated a clear superiority of DES (sirolimus-eluting stent and paclitaxel eluting stent) in this setting. In the TAXUS V-ISR trial, 396 patients with bare metal stent ISR referred for percutaneous coronary intervention were prospectively randomised to either paclitaxel-eluting stent or a beta source ICB. At 24-month follow-up, ischaemia-driven target lesion revascularisation was significantly reduced, with PES compared with ICB (10.1 vs. 21.6%, P<0.003), as was ischaemia-driven target vessel revascularisation (18.1 vs. 27.5%, P=0.03). There were no significant differences between the two groups with regard to death, myocardial infarction, or target vessel thrombosis cumulative to 24 months. The SISR trial randomised 384 patients to sirolimus-eluting stent or ICB. At 9-months, the rate of target vessel failure was 21.6% (27/125) with ICB and 12.4% (32/259) with the sirolimus-eluting stent (relative risk [RR], 1.7; 95% confidence interval [CI], 1.1-2.8; P=.02). Both trials were included in a recent meta-analysis41 demonstrating the benefit of DES as compared to ICB for the treatment of bare-metal in-stent restenosis.
The last remaining niche for ICB in the DES era might be the treatment of DES restenosis. In this setting, the only report exploring the usefulness of ICB was the Radiation for Eluting Stents in Coronary FailUrE (RESCUE) Registry42. It was an international, Internet-based registry of 61 patients who presented with ISR of a DES and were assigned to ICB therapy with commercially available systems after PCI. Outcomes of these patients were compared with those of a consecutive series of 50 patients who presented with ISR of a DES and were assigned to repeat DES (r-DES) treatment. Baseline clinical and angiographic characteristics were similar between groups, except for more Cypher stents as the initial DES that restenosed in the r-DES group than in the intravascular radiation therapy group (88.5% vs. 69%, p <0.01). At eight months, there were fewer overall major adverse cardiac events in the ICB therapy group compared with the r-DES group (9.8% vs 24%, p<0.044). The need for target vessel and target lesion revascularisations was similar in the two groups at eight months. There has been no report of subacute thrombosis in either group.
Conclusions
ICB was the first technique that could demonstrate that it reduced the need for repeat revascularisation as co-adjuvant therapy during percutaneous coronary intervention for bare-metal in-stent restenosis. However, important limitations (namely edge restenosis and late thrombosis) were encountered with first experiences in this field, leading physicians to improve the technique (avoidance of «geographic miss» with complete coverage of the injured segment) and to prolong dual antiplatelet therapy (up to 12 months) in order to avoid late thrombotic occlusion. The treatment of in-stent restenosis by a DES demonstrated itself to be more efficacious and displaced ICB from the current armamentarium of the interventionalist. However, the contribution of ICB in the pathophysiological understanding of the risks of delivery of antiproliferative agents into a coronary artery should be kept in mind, and serve for designing future developments in interventional cardiology (DES with bioabsorbable polymer, bioabsorbable DES, among others).

