Despite the widespread use of drug-eluting stents (DES) in coronary interventions, the incidence of in-stent restenosis (ISR) is still an issue, albeit less so than in the era of bare metal stent use. The most severe type of ISR is the total reocclusion of a stent. In the classic paper by Mehran this was observed in 8% of cases with BMS1. Today with DES the rate of total reocclusions is low, but it is still an issue in complex lesions such as chronic total coronary occlusions (CTOs), being in the range of 1 to 10%2. There is a hope that, with second-generation DES, the incidence of reocclusion may be further reduced but, on the other hand, the complexity of CTOs treated with the improved techniques available today is increasing3. In fact, recent reports from the USA and Japan on the use of everolimus-eluting stents showed reocclusion rates within one year that were still in the range of 5-10%4,5. We should therefore have strategies in place to treat these complex in-stent reocclusions successfully.
The cause of a total chronic reocclusion within a stent (ISR-CTO) is most likely a late thrombotic stent occlusion, which is compensated for by the reappearance of the original collateral supply6, and which therefore does not always lead to an acute coronary syndrome. Alternatively, in late reocclusions, slow diffuse restenosis may lead to an eventual reocclusion, possibly triggered by stent fractures where multiple DES are implanted.
There are two technical challenges when treating ISR-CTOs which are distinctly different from treating de novo CTOs: first to pass the lesion within the stent struts, especially when we must expect underexpanded stents with incomplete stent apposition as one of the major reasons for late stent reocclusion7, leaving some space beneath the stent struts and the vessel border; and second, to apply a therapy to prevent lesion recurrence in the setting of often already extensive previous stenting.
In this journal Wilson and co-workers present a retrospective analysis of a new technical approach to overcome one of the problems inherent in ISR-CTOs, which is the deviation of a recanalisation wire beneath the stent struts8. They apply the CrossBoss™ catheter device (Boston Scientific, Natick, MA, USA) to cross an ISR-CTO. The authors describe in detail the use of this device and some of the technical subtleties required to handle it in the setting of an ISR-CTO. The nature of this device, with its blunt rounded front end with a tip diameter of 1 mm, would avoid the risk of passing beneath the stent struts. It is advanced with the central wire retracted by pure mechanical rotation generated by the operator’s wrist, and then the wire is advanced again for realignment of the device or when it comes close to the distal end of the occlusion. Limitations of the successful application result from the bulky nature of the device itself, which makes it hard to use in tortuous anatomies, and when back-up from the guiding catheter is not sufficient.
The technical success rate with the CrossBoss catheter is 81% and, with additional techniques applied in case of failure, the total success rate reaches 87%. However, about 40% of the ISR-CTOs treated by the authors during the observation period were not performed with the CrossBoss catheter, and we do not know the outcome in these cases as compared to this new technical approach, nor the basis of this selection process. So, the interesting question is how we should rank this new device in the increasingly refined armamentarium of CTO techniques for ISR-CTOs. The available alternatives are the combination of wires and microcatheters, and also high-resolution X-ray machines, preferably biplane, to monitor the progress of dedicated wires through the occluded stents. Although the stent struts present one problem when underexpansion precipitates wire crossing underneath, the radiographic visibility delineates the vessel course better than in native CTOs. The number of dedicated wires for CTOs has increased considerably over recent years, and they also have an application in ISR-CTOs9.
One specific advantage of the CrossBoss catheter, in the experience of Wilson and co-workers, is the swift progress through the occluded stent and the high likelihood of achieving a distal lumen access. The CrossBoss catheter achieved a complete passage to the distal true vessel lumen in 22 of 31 CTOs (71%). In the other cases, additional techniques were required including two cases with a retrograde approach. In the successful CrossBoss cases, the procedure time was lower than in unsuccessful cases, which is an important argument for using a specific device. However, it remains to be determined whether there are some lesion specifics which may favour this device over another approach, given the additional costs of any additional device.
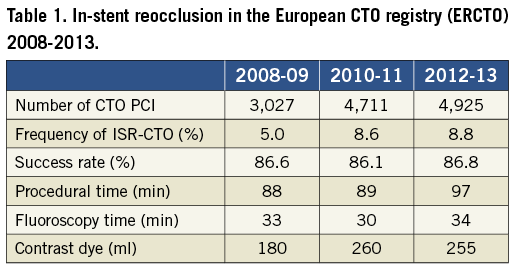
The available literature on ISR-CTOs is limited, as rightly stated by the authors. Therefore, comparisons with other techniques are difficult or impossible in the absence of direct comparisons. The largest CTO database at present is the European CTO registry (www.ercto.org). In the initial publication from the early entries of this registry, the number of ISR-CTOs was reported to be only 2%10, but now the number of entries is more than 12,000 CTO procedures performed by around 45 operators over six years, and the data set has been refined to address issues like ISR-CTOs. An analysis of the database reveals that ISR-CTOs make up 7.2% (924 of 12,763 procedures). The success rate was 86.3% in ISR-CTOs as compared to 83.7% in native CTOs. The procedure time was 93 minutes on average, and the fluoroscopy time 32.6 min (Table 1). Given the limitation that these data are self-reported observations by the operators participating in the registry, the data still provide a comparator to the data presented by Wilson and co-workers. The success rates are surprisingly comparable, and in addition, the procedure times are similar, with a slightly longer fluoroscopy time but a lower use of contrast dye in the registry.
One particular issue with ISR-CTOs is the information on the original procedure during which the stent was implanted. A decisive question is whether there is clear evidence that the distal true vessel lumen had been gained during that initial procedure, or whether the stents ended up in a false lumen with poor run-off which was then the reason for the stent reocclusion. Such ISR-CTOs are less likely to be re-treated successfully by an antegrade approach, as this would require a re-entry into the distal vessel lumen from a stent that is occluded in the false lumen. In these situations a retrograde approach may be required and may be the only possible way to overcome this anatomical problem.
Finally, there remains the issue of how to prevent a lesion recurrence once an ISR-CTO has been successfully crossed. This will require the implantation of DES or the option of drug-eluting balloons, but it is advisable in all these cases to use intravascular ultrasound to understand the reason for the reocclusion in the first place, and to optimise the procedural result based on this observation as the first step to avoid a repeat lesion recurrence.
There is no doubt that, in the setting of CTOs in general and ISR-CTOs in particular, operators are eager to find new devices that facilitate these complex procedures; however, based on the current data, there is still no definite answer to the question as to where the CrossBoss device should find its place in ISR-CTOs. The comparison between the data from Wilson and the European CTO registry shows that the current approach to ISR-CTOs is already very successful, and we require more and specific information on the lesion characteristics that are especially favourable for the CrossBoss device.
Conflict of interest statement
G. Werner is a member of the speakers’ bureaux of: Abbott Vascular, ASAHI Intecc, Biosensors, Boston Scientific and Terumo.

