
Abstract
It has been 40 years since percutaneous coronary intervention (PCI) was introduced into the clinical setting. Over these years significant advances in device technology and the invention of new therapeutic strategies have broadened its applications in the clinical arena, rendering this treatment the first-line therapy for patients with obstructive coronary artery disease. The evolution of PCI would not have been possible without intravascular imaging which provided unique insights about coronary artery pathology, enabled evaluation of vessel wall response following PCI and allowed meticulous evaluation of the advantages and limitations of emerging devices. This review article appraises the role of intravascular imaging in the evolution of PCI, summarises the findings of invasive imaging studies that examined the efficacy of new therapies and endovascular devices, presents the evidence that supports its use in current clinical practice and discusses its future potential in PCI.
Abbreviations
CFD: computational fluid dynamics
IVUS: intravascular ultrasound
LMS: left main stem
NIRF: near-infrared fluorescence
NIRS: near-infrared spectroscopy
OCT: optical coherence tomography
PCI: percutaneous coronary intervention
POBA: plain old balloon angioplasty
Introduction
It has been 30 years since the introduction of intravascular ultrasound (IVUS), which for the first time enabled detailed assessment of coronary artery morphology and pathology. Even from the first applications of IVUS, it became apparent that intravascular imaging carries a unique potential in evaluating vessel wall response following percutaneous coronary intervention (PCI); therefore, over the last 30 years IVUS and, more recently, optical coherence tomography (OCT) have been extensively used to examine the extent and severity of coronary artery disease, guide PCI in complex lesions, and assess the performance of emerging devices introduced to treat coronary artery disease (CAD). The aim of this review article is to discuss the role of intravascular imaging in the evolution of PCI, present landmark intravascular imaging studies that changed clinical practice (Table 1), summarise the evidence supporting its use in the clinical arena and discuss the potential of upcoming intravascular imaging modalities in guiding PCI.
Role of intravascular imaging in assessing the efficacy of the first percutaneous treatment strategies
In the early days of PCI, IVUS was extensively used to examine vessel wall response following plain old balloon angioplasty (POBA) or atherectomy. Pioneer IVUS-based studies demonstrated that the increase in lumen area noted after POBA is due to an increase in outer vessel wall area, to plaque dissection and to the axial redistribution of the plaque, while in atherectomy lumen enlargement is due solely to plaque debulking and a reduction in plaque area1-4. Serial IVUS imaging in patients treated with these strategies also enabled identification of the causes of restenosis and showed that in both POBA and atherectomy restenosis is due not only to intimal proliferation but also to vessel constrictive remodelling5,6. These findings underscored the need to develop devices that would provide a temporal scaffolding to the vessel wall and safeguard the patency of the vessel.
The introduction of bare metal stents (BMS) reduced the incidence of restenosis but did not eliminate it. In addition, the use of BMS was initially associated with a high incidence of stent thrombosis that was due to the absence of potent antiplatelet agents7. In that era, IVUS studies allowed identification of the mechanical causes of stent thrombosis (i.e., stent apposition, underexpansion, edge dissection)8 and revealed excessive neointima proliferation as the cause of in-stent restenosis9.
In an attempt to overcome these limitations, intracoronary brachytherapy was suggested as it appeared able to reduce intimal proliferation. Again, serial IVUS-based studies provided unique insights about vessel wall response following tissue radiation and showed that brachytherapy can occasionally induce excessive extracellular matrix production and proteoglycan accumulation that appears in IVUS as “black holes”10, while at the edges of the irradiated segment there is often an increase in plaque burden and a reduction in lumen area at follow-up attributed to the low radiation dose in these areas11.
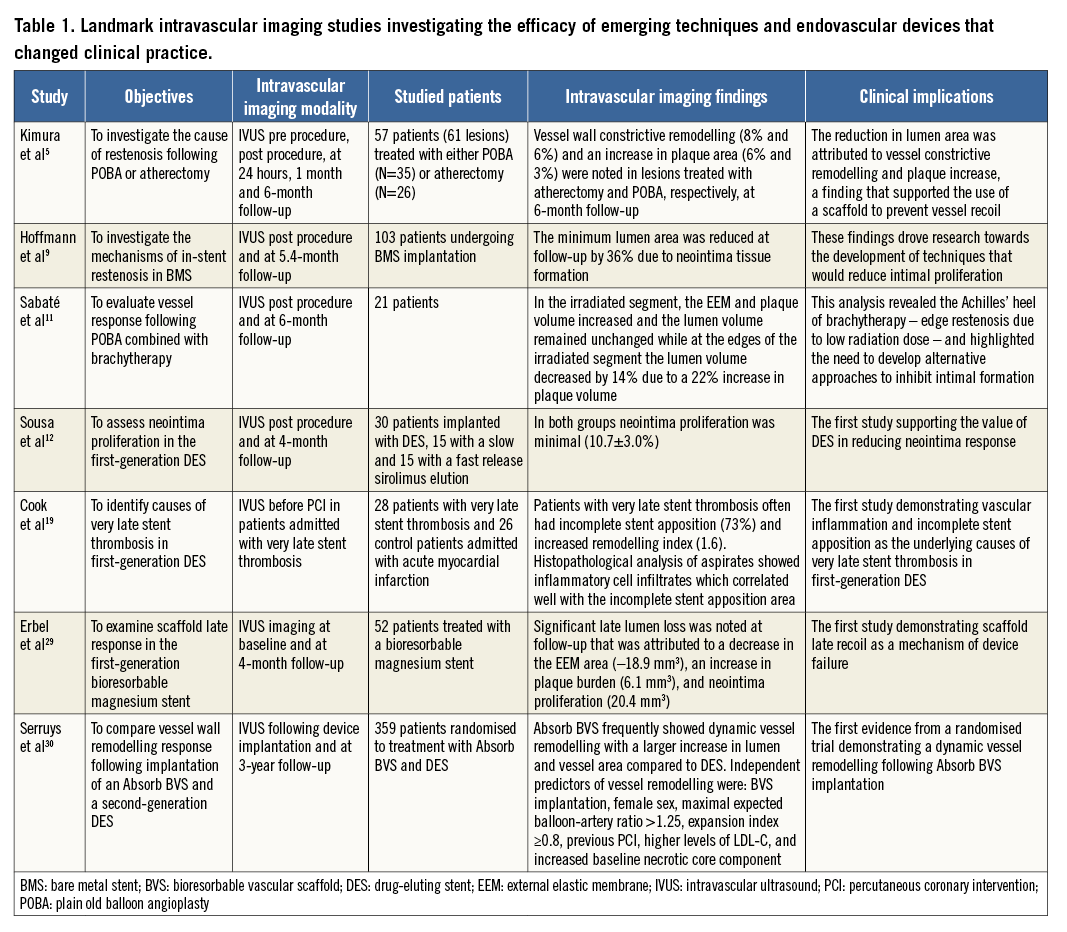
The advent of the first generation of drug-eluting stents (DES) changed the landscape of PCI. These devices minimised the incidence of restenosis, which was the Achilles’ heel of BMS, and enabled percutaneous treatment of complex lesions and high-risk patients. IVUS was again used not only to evaluate the efficacy of different stent designs and antiproliferative drugs and confirm their superiority over BMS12, but also to identify the causes of restenosis (i.e., incomplete lesion coverage13, geographical miss during stent implantation14, stent underexpansion15, and stent fracture14) and stent thrombosis (i.e., stent underexpansion and incomplete lesion coverage16) in DES. The findings of these studies underscored the importance of optimal stent expansion17, while OCT studies that assessed for the first time stent strut coverage in BMS and DES demonstrated a delayed endothelialisation in DES and highlighted the need for prolonged dual antiplatelet treatment in these devices so as to reduce the incidence of stent thrombosis (Figure 1)18. In more recent years, there was a concern about the relatively high incidence of late stent thrombosis in patients treated with a first-generation DES. Pivotal IVUS studies again provided unique insights into the mechanisms involved in this process, revealing the detrimental implications of a late inflammatory reaction induced by different stent polymers (Figure 2)19. These findings resulted in the design of improved revisions – that had more effective antiproliferative drugs and incorporated biocompatible or biodegradable polymers to control the release of the drug – which are currently used in the clinical setting.
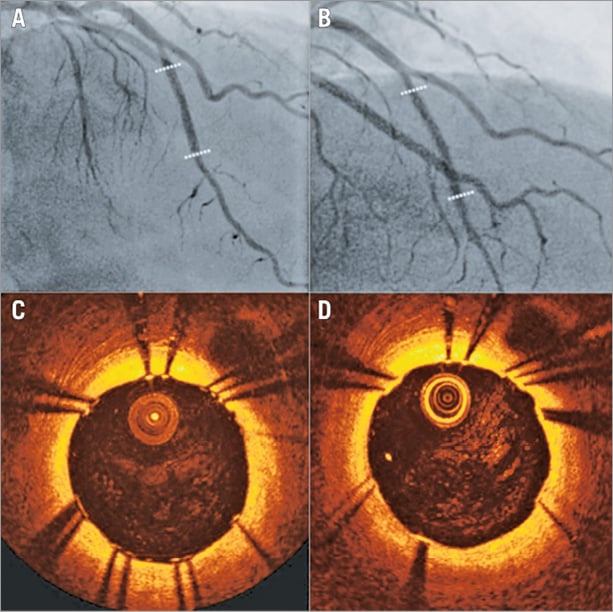
Figure 1. Efficacy of OCT in assessing stent strut coverage. X-ray and OCT images obtained at baseline following implantation (A, C) of a first-generation sirolimus-eluting stent (indicated with dotted lines) and at two-year follow-up (B, D). OCT provides detailed assessment of the deployed stent and enables detection of incomplete stent strut endothelialisation at two-year follow-up.
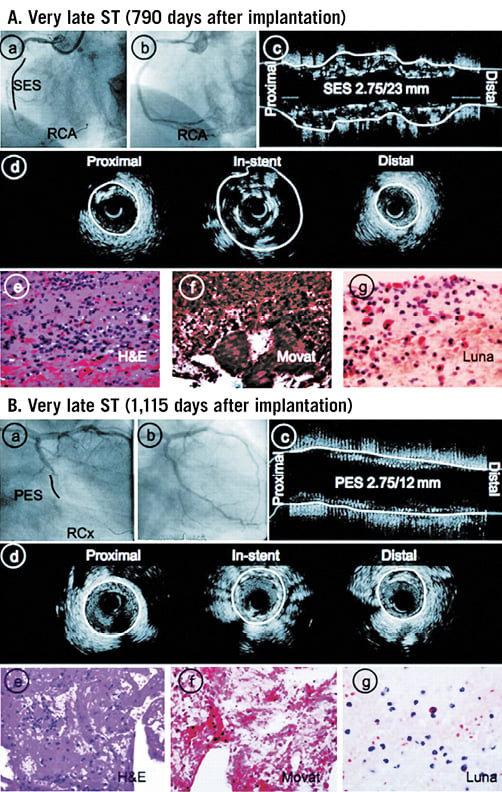
Figure 2. Association between IVUS and histopathological findings of thrombus aspirates in patients admitted with very late stent thrombosis following implantation of two different DES. Panels A (a, b) and B (a, b) correspond to the X-ray angiography of two patients admitted with very late stent failure following implantation of a sirolimus- and a paclitaxel-eluting stent. IVUS in case A (c) demonstrated incomplete stent apposition and positive remodelling, while in case B (c) IVUS demonstrated a good stent apposition and no vessel remodelling. Histopathological analysis in case A (e, f, g) showed thrombus with an intense inflammatory response of white count blood cells and numerous eosinophils, while in case B (e, f, g) it showed thrombus and intense infiltrate of neutrophils but no eosinophils. H&E: haematoxylin-eosin. Image was obtained with permission from Cook et al19.
Role of intravascular imaging in assessing the performance of recent endoluminal devices
SECOND-GENERATION METALLIC STENT PLATFORMS
The second-generation DES, introduced at the beginning of the century, overcame the limitations of the first-generation DES and reduced the incidence of cardiovascular events in patients undergoing PCI. Several IVUS- and OCT-based studies compared first- and second-generation DES and different second-generation DES platforms and permitted quantification of neointima proliferation, assessment of strut endothelialisation20,21, and qualitative evaluation of neointima characteristics22. Similarly to what has been shown in first-generation DES, IVUS revealed stent underexpansion23 as a common cause of stent failure in second-generation DES, while OCT imaging with its superior resolution confirmed that incomplete lesion coverage is associated with future stent-related events24 and revealed new predictors of stent failure such as the presence of thrombus post PCI24 and the formation of evaginations25. Finally, OCT has been extensively used to identify neoatherosclerotic lesions – defined as advanced high-risk plaques in the neointima – and has provided unique insights into their prevalence in different stent types and the impact of local and systemic factors26 on the formation of neoatherosclerotic plaques which constitute a common cause of late stent failure27.
BIORESORBABLE SCAFFOLDS
Bioresorbable scaffolds were introduced to overcome the limitations of metallic stents; these devices have the unique advantage that they provide a physiological therapy of patients with established coronary artery disease as they enable temporary scaffolding that safeguards the patency of the vessel at short term and then disappear, allowing restoration of vessel integrity. The first clinical studies examining their efficacy demonstrated an increased late lumen loss that, as was shown in serial IVUS-based studies, was due mainly to early scaffold recoil, a finding that led the industry to design advanced revisions with increased radial strength and delayed resorption28,29. The updated scaffolds were extensively evaluated in pilot studies that used serial multimodality imaging to study scaffold resorption, vessel wall remodelling30, and neointima distribution31, and to assess neointima, lumen dimensions and plaque burden at short- and long-term follow-up32. The first promising imaging findings supported the broad use of these devices in the clinical arena. However, more recent evidence from registries and large-scale randomised control studies showed an increased incidence of early and late/very late scaffold thrombosis, and raised concerns about the safety profile of bioresorbable scaffolds. Intravascular imaging, and in particular OCT, was again proven useful in understanding the mechanisms of early and late scaffold thrombosis, allowing identification of the indigenous limitations of the existing devices (Figure 3)33.
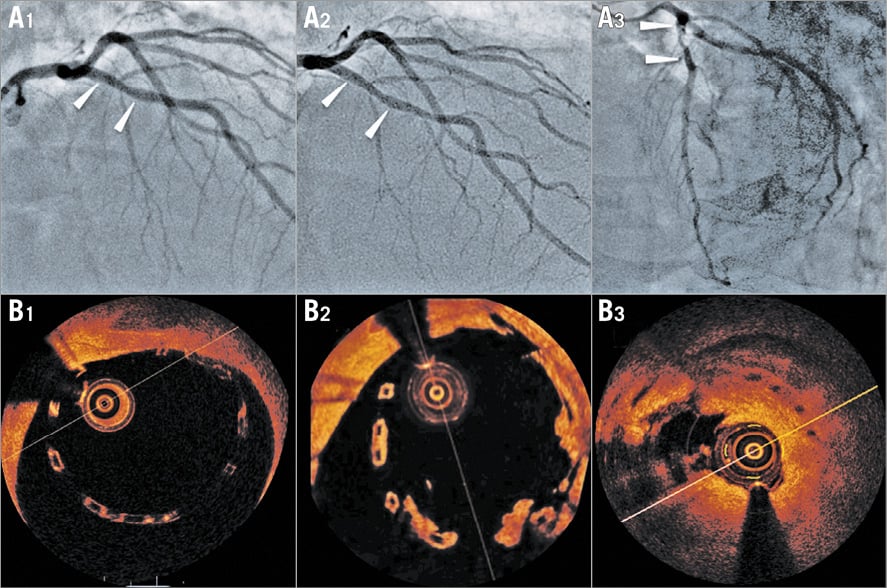
Figure 3. A case example of very late scaffold thrombosis. X-ray angiographic images showing a left anterior descending coronary artery implanted with an Absorb bioresorbable vascular scaffold at baseline (A1), 12-month (A2) and 18-month follow-up (A3). The corresponding OCT frames are shown in panels B1, B2 and B3, respectively. Post-implantation OCT imaging demonstrates scaffold malapposition (B1). Angiography at 12 months shows no significant stenosis, but OCT reveals thrombus formation in the malapposed struts which resulted in scaffold thrombosis at 18 months.
Role of intravascular imaging in the clinical setting
From the above it is apparent that intravascular imaging determined the evolution of PCI and changed practice, which consequently affected the applications of intravascular imaging in the clinical arena. Today, intravascular imaging is used as a diagnostic tool to evaluate the severity of left main stem (LMS) disease where there is prospective evidence showing that an IVUS-derived minimum lumen area >6 mm2 can safely be used to defer revascularisation34, assess hazy lesions in patients with an acute coronary syndrome and examine the geometry of complex anatomies (Figure 4).
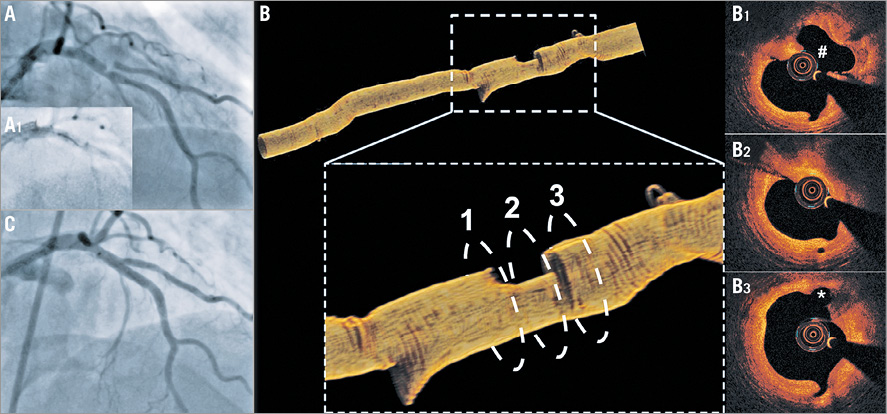
Figure 4. Value of OCT in assessing complex anatomies and planning PCI. A) Coronary angiography showing a hazy lesion in the proximal left anterior descending (LAD) artery immediately before the origin of the 1st diagonal branch which has an ostial stenosis. B) OCT demonstrated a ruptured plaque in the proximal LAD (B1), a tight lesion distal to the rupture site (B2), and a tight stenosis at the ostium of the diagonal (asterisk) which originates immediately after the stenosis (B3). OCT permitted detailed evaluation of coronary anatomy and PCI planning. The maximum lumen diameter of the LAD at the origin of diagonal was 2.9 mm and therefore the SuperCross™ microcatheter (Vascular Solutions, Inc., Minneapolis, MN, USA) with a 120° angulated tip that has the shortest tip to bend length was used to wire the diagonal. C) Final result after double-kiss crush stenting of the proximal LAD-diagonal.
The prognostic value of IVUS in guiding PCI has been challenged in the DES era, as the first randomised controlled trials failed to demonstrate better outcomes in the IVUS-guided groups35-37. However, recent large meta-analyses, registry data and randomised appropriately powered studies provided robust evidence that IVUS guidance is associated with a lower incidence of major adverse cardiovascular events in specific lesion types23,38-40. Therefore, IVUS can be considered today to guide DES implantation in long lesions, chronic total occlusions and in LMS PCI where there are convincing data that IVUS imaging improves outcomes41,42. In addition, operators should have a lower threshold to use intravascular imaging when they consider PCI with bioresorbable scaffolds since these devices have inherent limitations, including their narrower expansion limits, their poor visibility on X-ray angiography, their inferior mechanical properties and their worse haemodynamic profile compared to DES (Figure 5).
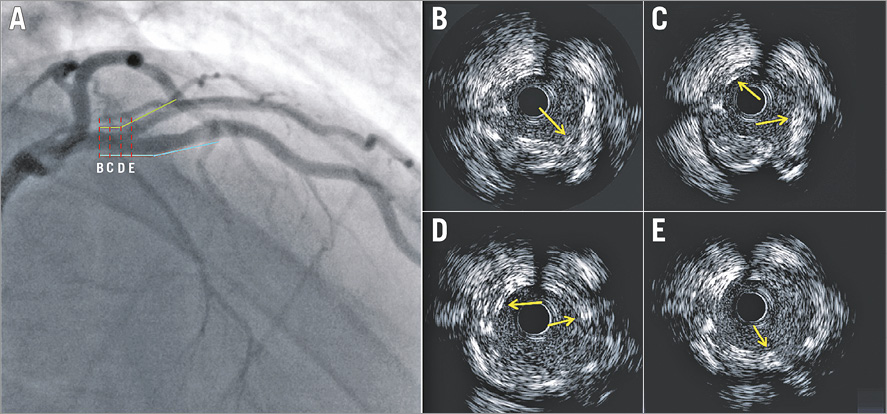
Figure 5. Value of IVUS in assessing the final outcome following complex PCI with the Absorb bioresorbable vascular scaffold in a bifurcation lesion. Coronary angiography portrays a bifurcation lesion in the left anterior descending and 1st diagonal that was treated with two scaffolds (A). The blue line indicates the location of the scaffold deployed in the left anterior descending artery and the green the scaffold implanted in the 1st diagonal. The red dashed lines (A) correspond to the IVUS frames (B-E) which show overlapping and malapposed struts (yellow arrows).
Fewer studies have investigated the effects of OCT as compared to angiography-guided PCI. In a recent report, OCT-guided PCI resulted in a higher post-procedural fractional flow reserve as compared to angiography-guided PCI43; whether this difference is clinically relevant remains open to question. Two randomised trials compared OCT- with IVUS-guided PCI. The ILUMIEN III trial found OCT to be non-inferior to IVUS with regard to in-stent minimum lumen area and resulted in less dissections and major malappositions. It is notable that neither IVUS- nor OCT-guided PCI led to an improved minimum stent area as compared to angiography guidance alone, yet stent expansion was significantly improved when using intravascular imaging44. The OPINION trial compared target lesion failures at one year following OCT- versus IVUS-guided PCI and found an OCT-guided strategy to be non-inferior to IVUS. The key limitation of this study is the lack of an angiographic control group. Therefore, further evidence from randomised controlled trials is mandated to prove the prognostic benefit of OCT during PCI and to identify the clinical setting where this modality will be most helpful.
Future potential of intravascular imaging
Advances in image processing and the miniaturisation of imaging devices have enabled the construction of hybrid imaging catheters which incorporate modalities with complementary strengths, allowing detailed assessment of vessel morphology (Figure 6)45. Over the last seven years, several hybrid intravascular catheters have been developed which allow more accurate evaluation of plaque morphology than stand-alone intravascular imaging, including combined IVUS-OCT, IVUS near-infrared spectroscopy (NIRS), OCT-NIRS, IVUS photoacoustic imaging and IVUS time-resolved fluoresence spectroscopy catheter. Whether the accurate characterisation of plaque pathology will enable better treatment planning and improve clinical outcomes needs to be proven in future studies.
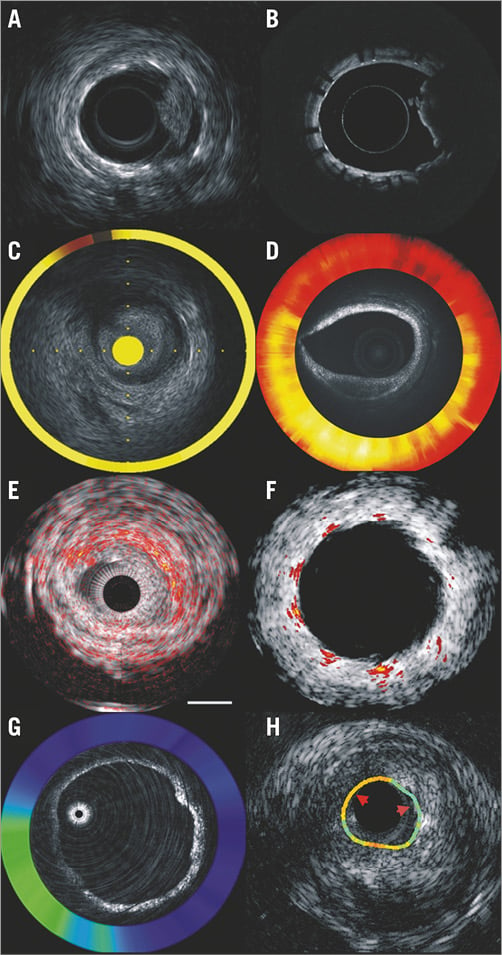
Figure 6. Output of the hybrid intravascular catheters developed to assess plaque morphology and physiology better. Combined IVUS-OCT (A & B), NIRS-IVUS (C), OCT-NIRS imaging (D), IVUS photoacoustic imaging in native and stented segments (E & F), OCT-NIRF (G) and IVUS time-resolved fluoresence spectroscopy imaging (H). Images were obtained with permission from Bourantas et al45.
In addition, two prototypes, the combined OCT near-infrared fluorescence (NIRF) and the IVUS-NIRF catheter, have recently been developed that allow assessment of plaque morphology and detection of fibrin deposition and vascular inflammation46. These devices are expected to enable assessment of local inflammation post stenting, evaluation of its role on clinical outcomes and, depending on the findings of these studies, identification of patients who will benefit from emerging therapies targeting vascular inflammation.
Finally, advances in coronary reconstruction have permitted modelling of coronary anatomy in stented/scaffolded segments and evaluation of the implications of stent/scaffold architecture on the local haemodynamic forces which appear to regulate neointima response and trigger stent/scaffold thrombosis47,48. Recent reports comparing the local haemodynamic forces in different scaffold designs have provided proof of the clinical potential of computational fluid dynamic (CFD) analyses in assessing the implications of different device configurations on local vessel physiology49. In vivo CFD analyses are expected to be used in future to optimise flow and the safety profile of emerging platforms and to improve clinical outcomes (Moving image 1).
Conclusions
Since the advent of the first IVUS prototype, intravascular imaging-based surrogate endpoints have been extensively used to assess the efficacy of emerging therapies and clinical devices, allowing their meticulous validation in small clinical studies. In this way, invasive imaging not only contributed to the faster maturation of PCI but also acted as a compass in PCI evolution, since it enabled identification of the limitations of the existing devices and guided technology towards the development of safe and effective stent platforms that improved clinical outcomes. In future, intravascular imaging is expected to go beyond traditional stereotypes, allowing evaluation not only of plaque morphology but also of its biology, through molecular imaging, and physiology, through CFD processing of intravascular imaging-derived models. These advances are anticipated not only to enrich our understanding about vessel wall response following PCI, but also to enable more reliable detection of vulnerable plaques that will progress and cause cardiovascular events and potentially extend the applications of PCI in the invasive sealing of non-flow-limiting high-risk plaques.
Authors’ perspective
Intravascular imaging has played a key role in the evolution of PCI as it enabled evaluation of the performance of emerging therapies and allowed treatment of high-risk complex lesions. Emerging hybrid imaging modalities are anticipated to allow a more detailed evaluation not only of plaque morphology but also assessment of plaque biology and local haemodynamic forces enabling for the first time precise study of vessel wall response following PCI. These techniques may also allow accurate identification of vulnerable lesions and thus extend the applications of PCI in the invasive passivation of these plaques.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Non-Newtonian blood flow simulation in a model reconstructed from the fusion of OCT and X-ray angiography enables evaluation of the implications of the implanted Absorb BVS on the shear stress distribution in early diastole and identification of areas exposed to a low (indicated with blue colour) and high (shown in red) shear stress environment.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Non-Newtonian blood flow simulation in a model reconstructed from the fusion of OCT and X-ray angiography enables evaluation of the implications of the implanted Absorb BVS on the shear stress distribution in early diastole and identification of areas exposed to a low (indicated with blue colour) and high (shown in red) shear stress environment.

