
Intracoronary imaging (ICI) has matured into a well-established clinical and research tool for patients with coronary artery disease (CAD). Intravascular ultrasound (IVUS) and optical coherence tomography (OCT) are now widely accepted for the assessment of lesion morphology in patients presenting with acute coronary syndromes (ACS). The large body of evidence gathered on the clinical value of these techniques cannot be summarised in a conventional clinical practice guideline format. Accordingly, a recent expert consensus document on the importance of ICI to guide coronary interventions1 has now been followed by another expert consensus detailing the value of these techniques to unravel novel diagnostic insights on plaque characteristics and to help in the clinical decision-making process involved in the management of ACS patients2. These documents from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) are comprehensive, timely and complementary1,2.
Over decades, IVUS has accumulated robust scientific evidence supporting its role in assessing coronary anatomy and guiding stent optimisation1,2. IVUS depicts the entire vessel (intima, media and adventitia) and remains unrivalled for visualising the external elastic lamina to determine CAD progression/regression and remodelling phenomena. Heavily calcified plaques, however, induce intense acoustic shadowing on IVUS. The potential diagnostic superiority of novel high-definition IVUS (60 MHz) will require prospective validation. Alternatively, OCT provides a ten-times superior spatial resolution (~15 microns) and is currently unsurpassed for disclosing subtle details on the vessel surface, measuring the thickness of fibrous cap, defining plaque composition (lipid, fibrotic, calcified) and even detecting clusters of macrophage infiltrates1,2. Furthermore, OCT constitutes the gold standard to identify intracoronary thrombus, the hallmark of culprit ACS lesions, allowing differentiation of white (platelet-rich) from red thrombus (rich in erythrocytes and fibrin, obscuring underlying structures)2. Unfortunately, OCT has a limited penetration into the vessel wall and may fail to delineate the external elastic lamina in vessels with significant plaque burden. Finally, in contradistinction to IVUS, OCT requires contrast media to gain a blood-free lumen.
ICI currently represents the best means for comprehensive evaluation of features suggestive of plaque vulnerability (high-risk or thrombus-prone plaque). Likewise, in ACS patients, ICI provides unique information at the culprit site which is difficult to obtain by non-invasive imaging. Finally, ICI enables a systematic appraisal of the dynamic nature of some non-atherosclerotic lesions, clarifies ambiguous angiographic images and delineates the functional significance of coronary lesions, helping in the decision-making process involved in coronary revascularisation2.
Ambiguous angiographic lesions and lesion severity
The superior value of tomographic techniques to clarify coronary anatomy in patients with CAD (ultimately a disease of the vessel wall) as compared with the two-dimensional angiographic lumenogram, is well established. The limitations of angiography are particularly evident in the left main, ostial lesions, bifurcations, aneurysms and transplant vasculopathy1,2. In addition, the classic image of angiographic “haziness” may be caused by intracoronary thrombus but also by eccentric plaques, dissections, intramural haematoma or calcium. These distinct anatomic substrates are readily distinguished by ICI to make informed decisions and to tailor treatment. The consensus document favours the use of IVUS over OCT in ostial lesions, aneurysms and left main disease2. First, blood clearance is challenging in these lesions. Second, scientific evidence on the value of IVUS to defer revascularisation or optimise results of interventions in left main lesions is stronger (class IIa, B) as compared with OCT3.
Alternatively, OCT provides a more accurate measurement of lumen area than IVUS. This is relevant as minimal lumen area and lesion length are key determinants of the hydrodynamic behaviour of coronary lesions. Although the anatomy of epicardial stenosis can only be considered a surrogate of its physiological consequences, several studies have demonstrated a reasonable correlation between minimal lumen area and fractional flow reserve and, even more importantly, with long-term clinical outcomes1,2,3. This correlation appears to be more robust in the left main (an IVUS-derived minimal lumen area <4.5 mm2 mandates intervention whereas areas >6 mm2 suggest deferral). In other coronary segments, a cut-off of minimal lumen area of ~2 mm2 and ~3 mm2, for OCT and IVUS, respectively, has been suggested to inform decisions, although correlations with physiology remain less precise1,2,3.
Refining the pathologic substrate and clinical management of ACS patients
The consensus document incorporates refined algorithms and didactic illustrations to highlight the role of ICI in ACS patients2. These are of particular practical value in patients with diagnostic uncertainties and in the absence of obstructive CAD. Myocardial infarction with non-obstructive CAD (MINOCA) has recently generated major interest and in this scenario ICI may be instrumental to unveil the hidden aetiology3. In some of these patients, an angiographically silent yet classic ACS pathological substrate, such as plaque rupture, erosion, calcified nodule, plaque fissure or intraplaque haemorrhage, may be found. However, the latter two entities, occurring especially in patients with unstable angina, are not discussed2,3,4,5. Alternatively, non-atherosclerotic causes may be implicated in other patients. Spontaneous coronary artery dissection may be comprehensively evaluated by ICI, depicting the location and extension of the true and false lumens. IVUS is better suited to visualise large expanded false lumens fully6. However, the superb resolution of OCT provides striking images of the dissection (medial-adventitial border) and is able to identify the entry tear6. Notably, an intramural haematoma (mimicking CAD), rather than a classic dissection flap, causing a double lumen on angiography, is the most frequent presentation of this entity. Accordingly, only tomographic techniques may provide a definitive diagnosis. In this scenario, the superior resolution of OCT is preferred by most investigators, although special care should be taken to prevent extension of the dissection by contrast injection. Takotsubo cardiomyopathy represents another interesting condition where ruling out angiographically silent culprit atherosclerotic plaques may be important7. MINOCA may also result from coronary embolisation. In these patients, a completely normal vessel wall is visualised once thrombus is removed. Finally, ACS may also be caused by coronary spasm. Most patients show a normal vessel wall or just moderate intimal proliferation when the spasm is reverted but, occasionally, plaque erosion with thrombus may be detected at these active coronary segments2,3,4,5.
VULNERABLE PLAQUE
Thin-cap fibroatheromas are readily detected by OCT due to its ability to measure cap thickness and lipid arc accurately2 (Figure 1). On OCT, clusters of lipid-filled macrophages generate characteristic bright spots, which may be located within the fibrous cap or deep in the plaque. Scattered microcalcifications and free cholesterol crystals may be detected within lipid cores. Virtual histology-IVUS may identify the confluent necrotic core of thin-cap fibroatheromas and has demonstrated its clinical value to identify high-risk patients in prospective studies8. Likewise, near-infrared spectroscopy provides a chemogram with information on lipid content in the coronary wall that has been correlated with subsequent adverse events in prospective studies2. Currently, however, ICI is unable to identify vulnerable plaques with high enough positive predictive value to justify “preventive” coronary interventions2.
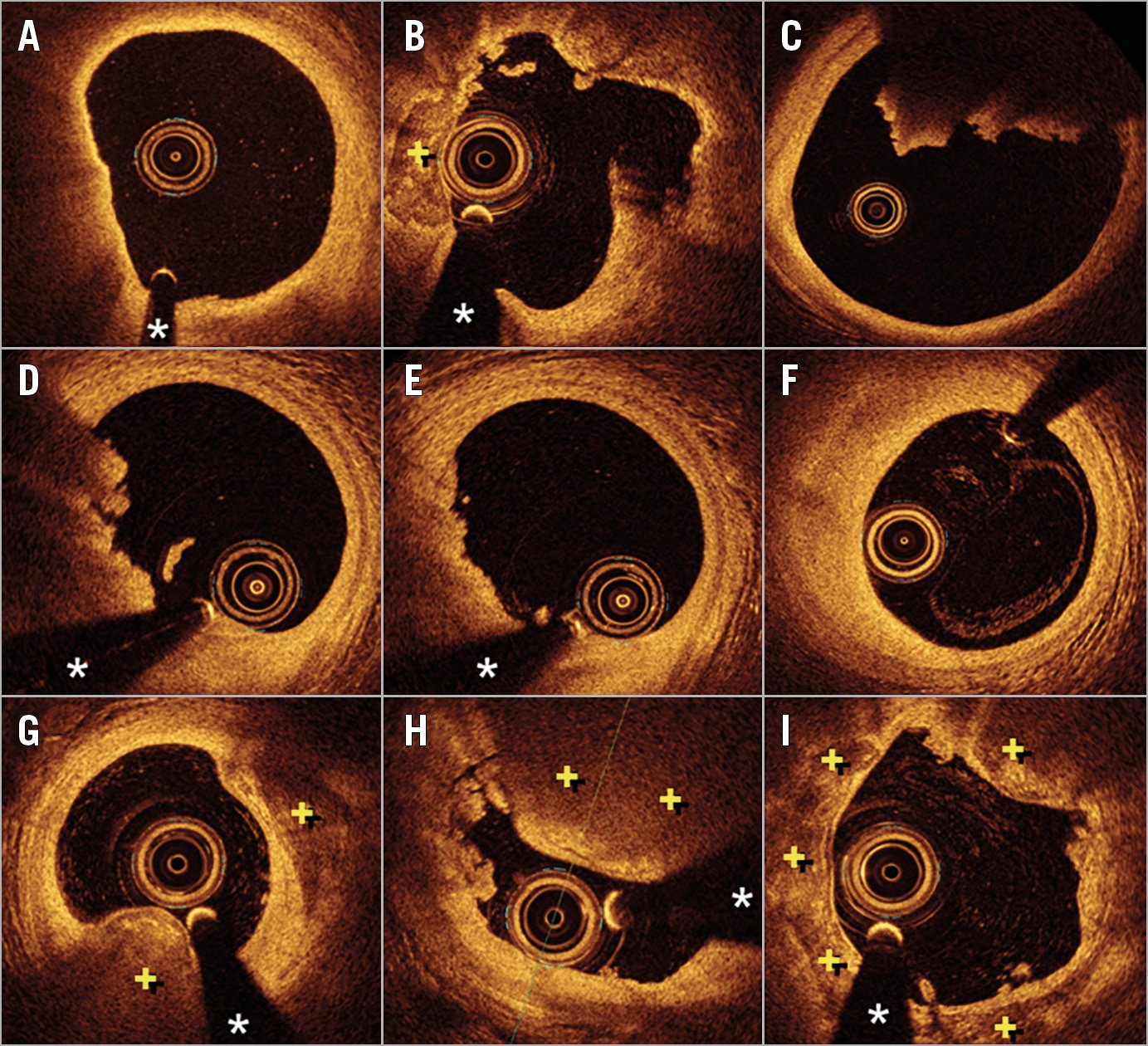
Figure 1. Optical coherence tomography (OCT) images. A) Thin-cap fibroatheroma. Notice the bright thin cap and the underlying large lipid pool (from 7 to 11 o’clock). B) Plaque rupture with a cavity at 2 o’clock. C) Large protruding red thrombus completely obscuring the posterior vessel wall (from 11 to 2 o’clock). D) Plaque erosion with significant mixed thrombus (from 6 to 11 o’clock). E) Plaque erosion with small laden thrombus (from 6 to 10 o’clock). F) Fibrotic plaque at the site of a previous plaque erosion after complete healing and thrombus disappearance. G) Nodular calcification (from 5 to 7 o’clock). Notice the protrusion of calcium with an undisrupted surface free from luminal thrombus. H) Disrupted calcified nodule obscuring the vessel wall (from 11 to 2 o’clock) with associated intracoronary thrombi (from 9 to 11 o’clock). I) Superficial calcific sheets with areas of disruption and tiny intracoronary thrombus. + denotes calcified plaque. * denotes wire artefact.
PLAQUE RUPTURE
Plaque rupture is the most frequent cause of ACS. By OCT a discontinuity of the fibrous cap overlying a lipid core is detected2,9 (Figure 1). The size of the resulting cavity is variable but the vessel frequently exhibits positive vessel remodelling9. Interestingly, the longitudinal pattern of the cavity may have clinical consequences. Indeed, large cavities facing the proximal aspect of the vessel (against the flow) tend to be associated with ST-segment elevation ACS, whereas cavities looking towards the distal vessel usually cause non-ST-segment elevation ACS9. Acutely, a red thrombus is systematically associated with plaque rupture and may hamper complete lesion assessment by OCT. Otherwise, the details of rupture are much better appreciated by OCT than with IVUS.
Layered phenotypes represent a marker of prior plaque destabilisation and are detected in healed plaque ruptures or erosions10. Silent episodes of non-oclusive thrombosis may evolve to thrombus organisation and eventually replacement by collagen. However, layers of different optical density have also been described in patients with intraplaque haemorrhage, a uniquely elusive diagnosis. Both substrates are implicated in plaque progression with histological validation2,10.
PLAQUE EROSION
Endothelial denudation remains beyond the resolution of ICI techniques2,11. Nevertheless, the diagnosis of plaque erosion is now possible - with different levels of certainty (definite, probable, possible) - with OCT11 (Figure 1). However, this remains a diagnosis of exclusion; some investigators prefer to use the term “intact fibrous cap” rather than plaque erosion12. Interestingly, ACS caused by plaque erosion differ from those resulting from plaque rupture. The clinical presentation (young, women, smokers), inflammatory milieu (high myeloperoxidase levels) and type of thrombus (smaller, predominantly platelet-rich) differ from those seen in patients with classic plaque rupture4,5,9,11. Small white thrombus casts little shadow, facilitating adequate characterisation of the underlying plaque to exclude rupture. These plaques are usually fibrotic (rich in collagen/proteoglycans) rather than lipid-rich and show constrictive remodelling2,4,5,9. In some patients, however, subtle irregularities on the lumen surface may be detected with OCT. In cases with large thrombus burden, the diagnosis of erosion can only be considered as possible or probable, because a rupture might be obscured behind the thrombus. A repeated OCT study, days after intense antithrombotic therapy, may confirm the absence of plaque rupture once thrombus has disappeared (Figure 1). Recent data suggest that patients with ACS secondary to plaque erosion have a relatively benign prognosis12. Many of these patients have only mild lumen compromise and show favourable clinical outcomes with aggressive antithrombotic treatment only (without stent implantation)12. If confirmed by larger studies, this approach might herald a paradigm shift in the management of ACS patients, opening new avenues for a tailored and personalised management.
CALCIFIED NODULE
This is the least frequent pathologic substrate still with elusive clinical implications11. IVUS may identify calcified nodules but does not have the required definition to identify surface disruption or associated thrombus that only can be detected by OCT2 (Figure 1). We must differentiate the calcified nodule from “nodular calcifications”, innocent bystanders not responsible for an acute thrombotic event. On OCT, calcium is depicted by low backscattering areas with sharply delineated borders. Until recently, an associated protruding irregular image casting a dorsal shadow was considered definitively diagnostic of thrombus associated with a culprit calcified nodule (i.e., disrupted, complicated or eruptive calcified nodule)11,13,14. Interventions on lesions caused by calcified nodules may result in suboptimal expansion and have a high rate of target lesion revascularisation during follow-up. However, benign “nodular calcifications” may occasionally present as an irregular surface and attenuate deeper structures, potentially leading to a misdiagnosis of red thrombus by OCT14. This diagnostic dilemma (culprit lesion versus incidental, innocent bystander) is acknowledged in the consensus document2. Finally, very recently, a distinct type of calcified plaque has also been proposed as a novel pathologic substrate of ACS15. Superficial calcific sheets have been detected by OCT in some patients with ACS15 (Figure 1). However, the pathologic correlate and the precise pathophysiology of these lesions still remain unclear. Further studies are required to confirm that this distinct substrate should be considered in the differential diagnosis of the aetiology of ACS.
Final remarks
The authors of this expert consensus should be commended for providing a systematic and comprehensive review that summarises current knowledge on the value of ICI in ACS patients very well2. We believe that this information will help interventional cardiologists to obtain a more precise pathophysiological diagnosis of the culprit lesion and to improve management of ACS patients. The statements provided in this consensus document are based on both evidence and experience. Further studies are warranted to provide additional scientific evidence to advance our understanding of ACS.
Conflict of interest statement
The authors have no conflicts of interest to declare.

