
Abstract
This Consensus Document is the first of two reports summarizing the views of an expert panel organized by the European Association of Percutaneous Cardiovascular Interventions (EAPCI) on the clinical use of intracoronary imaging including intravascular ultrasound (IVUS) and optical coherence tomography (OCT). The first document appraises the role of intracoronary imaging to guide percutaneous coronary interventions (PCIs) in clinical practice. Current evidence regarding the impact of intracoronary imaging guidance on cardiovascular outcomes is summarized, and patients or lesions most likely to derive clinical benefit from an imaging-guided intervention are identified. The relevance of the use of IVUS or OCT prior to PCI for optimizing stent sizing (stent length and diameter) and planning the procedural strategy is discussed. Regarding post-implantation imaging, the consensus group recommends key parameters that characterize an optimal PCI result and provides cut-offs to guide corrective measures and optimize the stenting result. Moreover, routine performance of intracoronary imaging in patients with stent failure (restenosis or stent thrombosis) is recommended. Finally, strengths and limitations of IVUS and OCT for guiding PCI and assessing stent failures and areas that warrant further research are critically discussed.
Preamble
This Consensus Document, which is the first of two reports summarizing the views of an expert panel organized by the European Association of Percutaneous Cardiovascular Interventions (EAPCI), appraises current evidence on clinical indications for intracoronary imaging and provides consensus opinion regarding use, strengths, and limitations of intravascular ultrasound (IVUS) and optical coherence tomography (OCT) based also on the best current practice.
Expert committee: selection criteria, organization, and consensus development
The consensus group was selected by the Scientific Documents and Initiatives Committee of the EAPCI based on the acknowledged expertise in intracoronary imaging and the origin from different geographical areas. During the first meeting in August 2017, the expert committee discussed the documents content, the perspectives and scopes, the methods of data searching and assigned lead authors for each document. Authors conducted literature searches [peer review literature with special attention to the publications in the last 5 years, existing level of evidence, randomized clinical trials, meta-analyses, registries, including a systematic research for the derivation of the meta-analysis (Figure 1)] and drafted the document outline. Consensus on the final document was found during several meetings and conference calls as well as two revisions of the draft document by all expert committee members. The key points of each chapter are summarized in summary boxes. The relationships and industry information for all members, writing committee, and peer reviewers are published in the Supplementary Material.
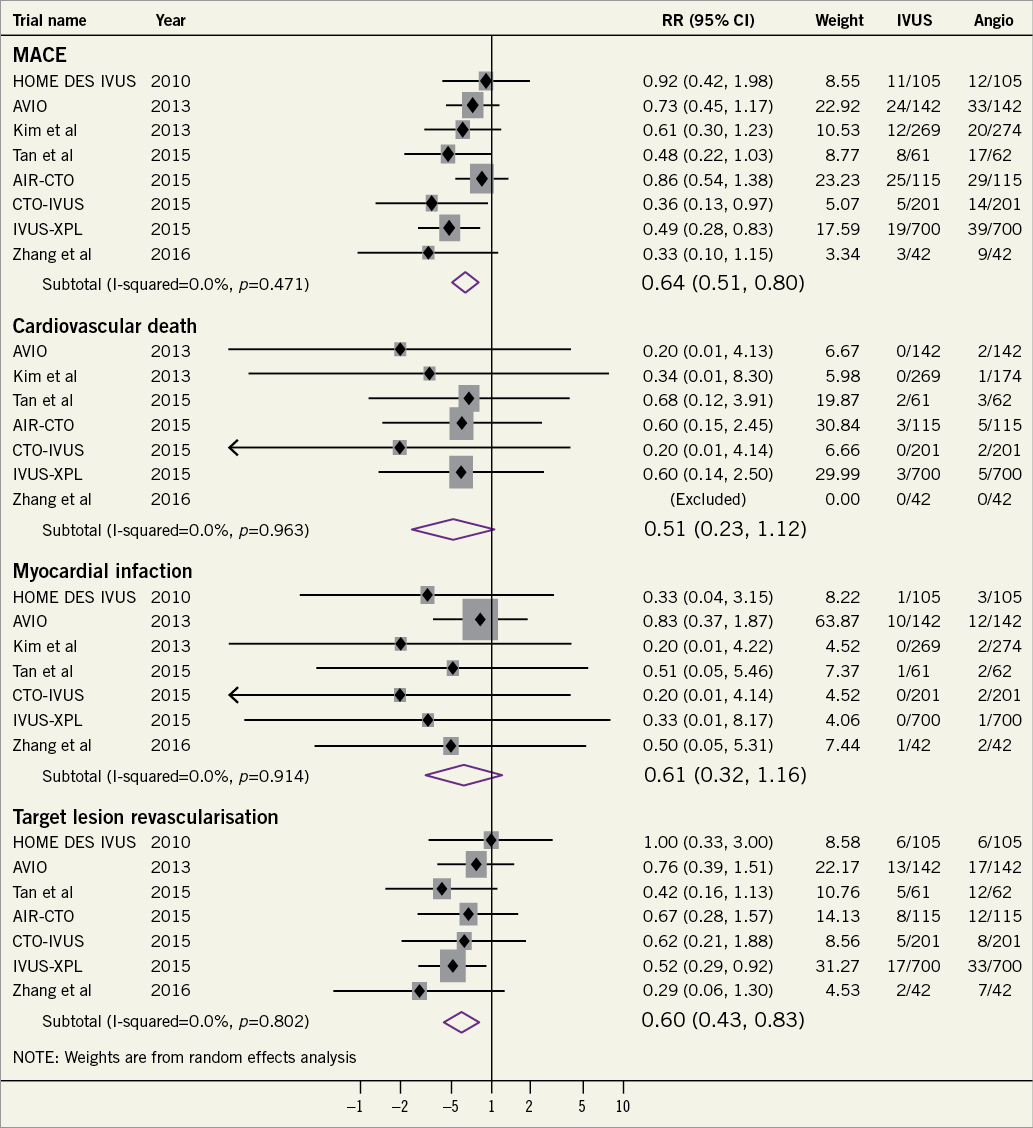
Figure 1. Forrest plot summarizing the effects of intravascular ultrasound-guided percutaneous coronary intervention as compared with angiography-guided percutaneous coronary intervention on cardiovascular outcomes.
Introduction
Coronary angiography is the traditional imaging modality for visual evaluation of coronary anatomy and guidance of percutaneous coronary interventions (PCIs). However, the derived two-dimensional lumenogram cannot depict the arterial vessel wall, and thus evaluate vessel dimensions and plaque characteristics, nor directly assess the result of stent implantation. Intracoronary imaging by means of IVUS and OCT provides valuable incremental information that can be used clinically to optimize stent implantation and minimize stent-related problems1,2. Pre-procedural measurement of lumen and vessel dimensions and lesion characterization can facilitate accurate stent sizing and guidance of the stenting strategy. Post-procedural imaging provides strut-level evaluation of the stent result and guides optimization measures. There is growing evidence from observational studies3, randomized controlled trials (RCTs)4, and meta-analyses5,6 that intravascular imaging guidance by IVUS not only enhances the acute procedural result, but also improves clinical outcomes. In spite of this, the adoption of intracoronary imaging remains limited in routine clinical practice and highly heterogeneous according to geographic region7. Over the past decades, IVUS and OCT have progressively evolved with respect to technical performance (higher resolution imaging), and procedural aspects (faster pullback, automatic vessel/lumen and plaque burden detection and measurements, and co-registration with angiography). This has enabled their use as clinical tools used routinely or in selected cases7.
Does intracoronary imaging improve clinical outcomes following percutaneous coronary intervention?
INTRAVASCULAR ULTRASOUND VS. ANGIOGRAPHY
In the era of bare metal stents (BMS), several RCTs showed significant favourable effect of IVUS guidance over angiographic guidance alone on restenosis and target lesion revascularization (TLR) rates, with a neutral effect on mortality and myocardial infarction (MI) (Supplementary material online, Table S1)8-11.
With respect to PCI with drug-eluting stents (DES), eight RCTs have compared IVUS-guided with angiography-guided PCI to date (Supplementary material online, Table S1)4,12-18. Among these trials, the IVUS-XPL4 (lesion length >28 mm), and CTO-IVUS12 (chronic total occlusions) trials showed significant reductions in major adverse cardiac events (MACE) with IVUS guidance. The benefit was driven by a reduction in repeat revascularization for restenosis in both trials. A meta-analysis of seven RCTs (3192 patients) including only DES-treated patients confirmed the superiority of IVUS guidance vs. angiographic guidance alone in reducing MACE [odds ratio (OR) 0.60; 95% confidence interval 0.46-0.77], cardiovascular mortality [OR 0.46 (0.21-1.00)] and stent thrombosis [OR 0.49 (0.24-0.99)]5. The duration of follow-up in these studies ranged between 12 and 24 months. These findings were confirmed in a subsequent patient-level meta-analysis including 2345 patients from RCTs of new-generation DES19. Significant reduction in MACE, TLR, and TVR was also shown in a meta-analysis of RCTs specifically in patients with complex lesions20. An updated meta-analysis performed by the present group including eight RCTs (3276 patients) confirmed the superiority of IVUS-guided PCI for reduction of MACE and ischaemia-driven TLR following DES implantation (Figure 1).
Several points require consideration when interpreting these findings. First, the fact that most individual RCTs with DES showed a directionally favourable trend but no significant superiority of routine IVUS guidance (despite achieving larger post-intervention stent dimensions) is likely explained by the limited power of the individual studies. The inclusion of non-complex lesions, and at least in part the absence of pre-specified guidance protocol represent additional limitations. Indeed, significant MACE reduction was observed in studies assessing patients with long lesions and chronic total occlusions12, as well as in meta-analyses of all available RCTs5. Notwithstanding these benefits, the effects of the use of intracoronary imaging in an all comers setting remains to be established.
Notably, the pooled benefit emerged despite the fact that predefined stent optimization targets were not reached in many of the enrolled patients (Figure 2). It should also be noted that, although pre-specified expansion targets in imaging-guided PCI are not always achievable, it is reasonable to assume that these targets do guide operators in attempting to achieve the goals and potentially result in increasing minimum stent area (MSA). Whether a higher rate of acute procedural optimization or alternative optimization targets might result in an incremental improvement in clinical outcomes is unclear. Another unknown factor is the potential effect of a systematic implementation of quantitative coronary analysis to assist angiography-guided PCI as compared to visual estimation alone.
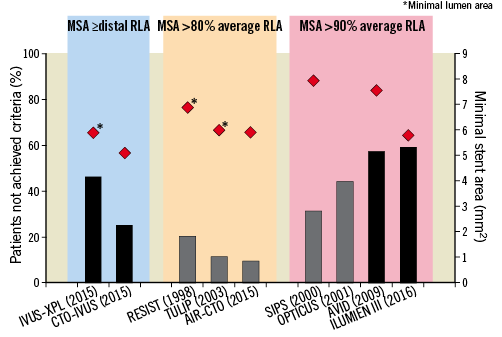
Figure 2. Effects of intravascular ultrasound-guidance and optical coherence tomography-guidance on stent expansion. Proportion of patients who did not achieve predefined criteria of stent expansion in selected randomized trials of intravascular ultrasound-guided and optical coherence tomography-guided interventions are shown by bars and the achieved minimal stent area (or lumen area indicated by asterisks) as indicated by the red diamonds. Trials are grouped according to the predefined stent expansion criteria. Black bars represent drug-eluting stents and grey bars represent bare metal stents. Minimum stent area values are means with the exception of ILUMIEN 3 (median). RLA, reference lumen area.
Observational studies of IVUS-guided PCI with DES reported consistent reductions in ischaemic outcomes21. Owing to the lack of randomization, considerable differences in patient and lesion characteristics were observed at baseline. Moreover, additional unmeasured confounding factors are also likely to be differentially distributed between the comparison groups. The largest observational study including 8583 ‘all-comer’ patients (ADAPT DES) showed most pronounced benefit of IVUS guidance in patients with acute coronary syndromes (ACS) and complex lesions3. In a meta-analysis of 20 studies (including three RCTs)22, the benefit of IVUS guidance with respect to mortality and MACE was particularly pronounced in ACS patients or complex lesions (left main, bifurcation, CTO, or long lesions).
INTRAVASCULAR ULTRASOUND-GUIDED LEFT MAIN PERCUTANEOUS CORONARY INTERVENTION
The clinical value of IVUS-guided PCI has been studied in one small RCT17 including 123 elderly patients (age >70 years) undergoing revascularization with second-generation DES. At 2 years, IVUS guidance resulted in a lower risk of MACE, which was driven by a significant reduction in TLR17. Extensive evidence exists to support IVUS-guided left main PCI in non-randomized studies. In the largest study including 1670 patients with left-main lesions treated with DES, propensity score-matched analyses showed that IVUS guidance was associated with reduced MACE (cardiac death, MI, or TLR) within 3 years (11.3% vs. 16.4%, P = 0.04)23. MACE reduction was largely driven by all-cause mortality and not by MI or TLR, leaving open the question regarding the mechanism of the observed survival benefit. Larger stents with better expansion, more frequent stent post-dilatation and less use of two-stent techniques in IVUS-guided interventions were also observed in this study, which identified IVUS-guided revascularization as independently associated with MACE reduction, predominantly in the subgroup of patients with distal left-main lesions [hazard ratio (HR) 0.54 (0.34-0.90)]23. In the observational MAIN-COMPARE study, a trend for lower mortality was demonstrated, yet again without a difference in MI or TLR24. A meta-analysis of 10 studies showed significantly lower risk of all-cause death, cardiac death and stent thrombosis for IVUS-guided left-main interventions25. The observed reduction in mortality without clear mechanistic explanation suggests that the results in these studies may be influenced by the presence of residual confounding factors.
OPTICAL COHERENCE TOMOGRAPHY VS. ANGIOGRAPHY
Currently, there are relatively limited data for OCT-guided interventions and there is no RCT of clinical outcomes with OCT-guided vs. angiography-guided PCI. One registry study reported a reduced rate of cardiac death and MACE in patients who underwent OCT-guided interventions26. Additional observational studies showed larger final in-stent minimum lumen diameter (MLD)27 and a reduction in the number of stents used with OCT-guided primary PCI28. The importance of pre-stent assessment by OCT was highlighted in the non-randomized ILUMIEN-I study29. Pre-stenting imaging changed the PCI strategy more frequently (57%) compared with imaging performed after stent implantation (27% of cases).
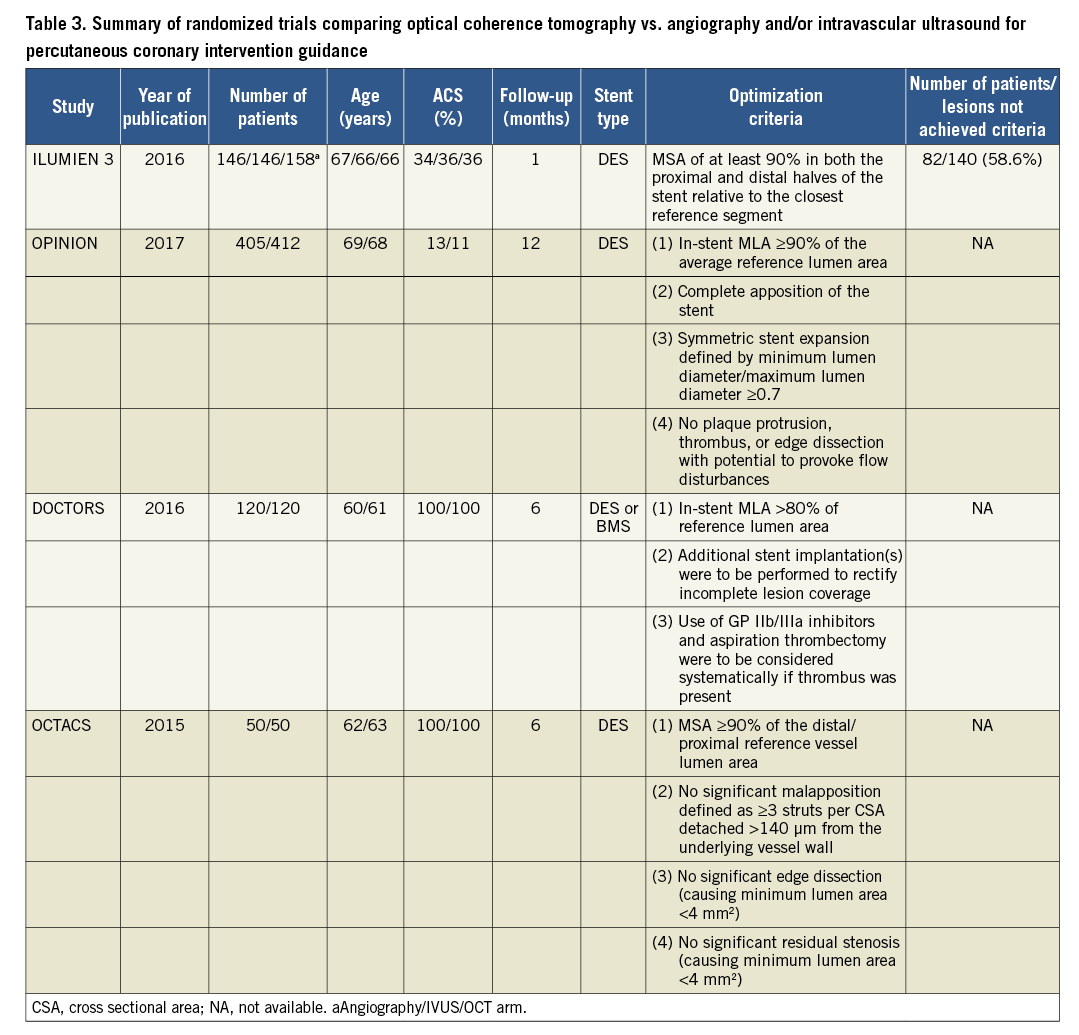
In the randomized DOCTORS trial30 including 240 patients with non-ST-segment elevation ACS, OCT-guided PCI was associated with a small but significant improvement in the primary endpoint, post-procedural fractional flow reserve (FFR) compared with angiography-guided PCI. This benefit was mainly driven by improved stent expansion. In the OCTACS study, 100 ACS patients were randomized to either OCT-guided or angiography-guided implantation of newer-generation DES; OCT-guidance resulted in a lower proportion of uncovered struts at 6 months (4.3% vs. 9.0%, P < 0.01)31. Similarly, the DETECT OCT study showed a superior stent coverage at 3 months (7.5% vs. 9.9%, P = 0.009) when OCT-guidance PCI was applied in 894 stable CAD patients32. The randomized ILUMIEN-III trial33 compared the effects of OCT-guided, IVUS-guided, and angiography-guided PCI on stent expansion. The study was not powered for clinical outcomes, and the primary efficacy endpoint was post-PCI minimum stent area (MSA). Non-inferiority of OCT vs. IVUS and superiority of OCT vs. angiography was tested. OCT was not found to be superior to angiography with respect to MSA but led to significantly improved minimum and mean stent expansion and fewer untreated dissections and persisting major malapposition33. These results have to be interpreted against the background of relatively simple lesion morphology and the efforts to optimize the results in all three groups by experienced operators. The impact of OCT-guided vs. angiography-guided PCI is being investigated further in RCTs currently underway ILUMIEN-IV (NCT0350777/) and OCTOBER trials (NCT03171311).
INTRAVASCULAR ULTRASOUND VS. OPTICAL COHERENCE TOMOGRAPHY
Recently, two dedicated RCTs directly compared OCT-guided vs. IVUS-guided PCI with respect to surrogate33 and clinical endpoints34. ILUMIEN-III addressed the question whether OCT-guided PCI using a specific optimization protocol is comparable to IVUS-guided PCI33. A total of 450 patients were enrolled (median lesion length 15.5 mm; exclusion of left-main and CTO lesions; 36% ACS patients). The primary endpoint, MSA, was non-inferior following OCT-guided vs. IVUS-guided PCI. Minimum and mean stent expansion with OCT-guided PCI was comparable to IVUS-guided PCI and significantly improved vs. angiography-guided PCI. Untreated major dissections [OCT 14% vs. IVUS 26% vs. angiography 19%, P (OCT vs. IVUS)=0.009, P (OCT vs. angio)=0.25] and major malapposition [11% vs. 21% vs. 31%, respectively; P (OCT vs. IVUS) =0.02, P (OCT vs. angiography) <0.0001] were less frequent in the OCT group compared with the IVUS and angiography groups. Post-dilatation was required to achieve a stent expansion of at least 90% in both the proximal and distal halves of the stent relative to the respective reference segment –a unique OCT criterion introduced in this trial33. Notably, the protocol-mandated expansion target was achieved in only 41% of OCT-guided cases and the difference in MSA was minimal compared with the IVUS group, in which no expansion criteria were predefined.
The OPINION RCT included 829 patients with relatively simple lesions (lesion length 18 mm) and tested whether OCT-guided PCI using a lumen-based approach was non-inferior to IVUS-guided PCI with respect to the clinical endpoint of target vessel failure within 12 months post-PCI34. It is thus the first OCT study formally powered for a clinical endpoint. The primary endpoint did not differ between groups (5.2% vs. 4.9%, P for non-inferiority <0.05). In addition, in-stent MLD as assessed by quantitative coronary angiography at 8 months was similar and binary restenosis was identical between groups34.
CRITICAL APPRAISAL OF CURRENT EVIDENCE: INTRACORONARY IMAGING (INTRAVASCULAR ULTRASOUND OR OPTICAL COHERENCE TOMOGRAPHY) VS. ANGIOGRAPHY
The ILUMIEN-III and OPINION trials consistently showed that OCT is non-inferior to IVUS for PCI guidance with respect to the acute procedural result, as well as mid-term clinical outcomes. Although a dedicated RCT is required to address the superiority of OCT-guidance vs. angiography-guidance, the aforementioned studies suggest that the superior clinical outcomes defined by RCTs on IVUS guidance5 in selected patients are likely applicable to OCT-guidance. Consistent with this, a recent network meta-analysis including 17.882 patients who underwent angiography-, IVUS-, or OCT-guided implantation in 17 RCTs and 14 observational studies demonstrated that IVUS- or OCT-guidance was associated with significant reductions in MACE and cardiovascular mortality vs. angiographic guidance, without efficacy differences between IVUS and OCT6.
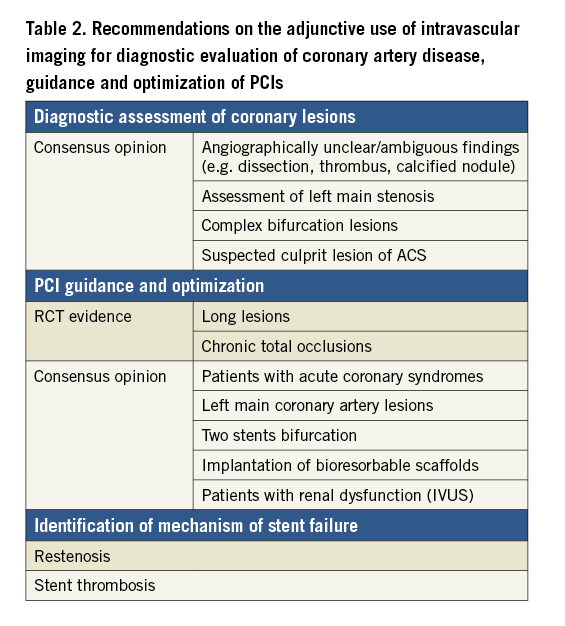
It is the consensus opinion of this expert group that IVUS and OCT are equivalent (and superior to angiography) in guiding and optimizing most PCI procedures. Both modalities can identify features of optimal stent implantation (expansion, apposition, and complications), as well as mechanisms of stent failure that cannot be captured using coronary angiography alone. However, the benefits and limitations of each modality require consideration (Table 1)2.
Owing to lower tissue penetration, especially in lipid-rich tissue, OCT is limited for assessing plaque burden and detecting vessel size [as delineated by the external elastic membrane (EEM)] in the presence of diffuse disease –an approach used for IVUS-guided stent sizing. IVUS is the preferred modality for assessment and treatment of ostial left-main lesions (due to frequent inability of OCT to visualize the ostium as proper blood clearance may be challenging), CTO lesions and patients with renal insufficiency (due to the potential for lower volume or minimal contrast PCI)35,36. In contrast, due to its higher resolution, OCT is more accurate for detecting lumen or stent-related morphologies with potential clinical impact, including thrombus and culprit plaque identification in patients with suspected ACS; residual edge dissection, incorrect wire positions and stent malapposition immediately after stenting. Whether the higher accuracy in the detection of aforementioned findings has the potential to translate into improved cardiovascular outcomes remains unknown. Optical coherence tomography is more user-friendly as the pullback acquisition is faster, and reliable automatic analyses are made available immediately. Furthermore, stent-related findings are easier to interpret with OCT.
The results of available studies should be interpreted in the context of best clinical practice standards. First, newer-generation DES and technical refinements of stenting procedures have resulted in overall improvements of the safety and efficacy of coronary interventions. The SYNTAX II study reported the outcomes in patients prospectively enrolled and treated with a combined protocol incorporating coronary physiology-based revascularization, IVUS-guided stenting, thin strut stent implantation, and contemporary CTO revascularization techniques. The primary analysis showed improved clinical outcomes in comparison with historical control37. Although the contribution of IVUS-guided PCI cannot be exactly defined, it may have contributed to the excellent outcomes in this high risk population (likely in combination with a favourable effect of physiological assessment). Second, although RCTs are of greater value for shaping recommendations in the hierarchy of evidence, practical limitations of performing large, adequately powered RCTs comparing imaging-guided vs. conventional PCI should be taken into account. Along these lines, IVUS was associated with a significant clinical benefit in the largest of the available RCTs12 and in pooled analyses of all individual RCTs5. Third, the benefits of intracoronary imaging depend largely on the interpretation and the operators’ reaction to these findings. Image acquisition alone will not be sufficient to impact on outcomes. Positive impact will require proper technique, correct imaging interpretation and adequate reaction to the findings. Therefore, it is important to implement quantitative measurements, and develop practical algorithms to allow stent guidance and optimization based on standardized criteria. The frequent failure to achieve stent expansion cut-off values (by the study protocols) suggest how relevant pre-stent imaging is to guide appropriate lesion preparation.
Which patients and lesions should be considered for intracoronary imaging during percutaneous coronary intervention?
Guidance of procedural strategy and optimization of the stenting result are major clinical indications for intracoronary imaging. This is in accordance with current guidelines38, and in agreement with the views of interventional cardiologists (see Box 1)7.
| Box 1. Clinical indications and expected effects of intravascular ultrasound- or optical coherence tomography-guided PCI – IVUS-guided PCI improves clinical outcomes in selected patients with long lesions and CTOs. Limited data from RCTs suggest that IVUS and OCT-guided PCI are equally effective in achieving these benefits. – Patients with left-main lesions should be considered for imaging-guided interventions by means of IVUS, or OCT in non-ostial left main lesions, due to particular challenges in angiographic evaluation and procedural complexity, and because of the clinical sequelae of a suboptimal result in this context. – There is stronger evidence on the advantages of intravascular imaging to guide stenting in complex lesion morphology and in patients presenting with ACS, with less benefit in simpler lesions or patients with more stable clinical presentation. – OCT for guidance of PCI is more user-friendly as the interpretation is simpler and automatic analyses are available immediately. – Additional indications favouring OCT include identification of mechanisms of documented stent failure (stent thrombosis and restenosis), and guidance of BRS implantation (Table 2). – Patients at high risk of developing contrast-induced acute kidney injury can benefit from IVUS-guided PCI due to the potential for lower volume of contrast37. |
How to perform intracoronary imaging and which criteria should be used for stent implantation and optimization with intravascular ultrasound and optical coherence tomography?
IMAGE ACQUISITION
Intravascular imaging-guided PCI should start, if possible, prior to stent implantation. Prior to stenting, intravascular imaging can assess plaque composition and distribution (calcification, lipid-rich plaque) and identify the need for more aggressive (rotational atherectomy, cutting, or scoring balloons to induce calcium fractures) or less aggressive (direct stenting to avoid lipid embolization) lesion preparation, and facilitate choice of stent size (diameter and length)3,29,30. Imaging is recommended to be performed using a motorized pullback device, with continued control of the image quality during acquisition. Occasionally, a manual pullback is required with IVUS to verify focal and specific findings detected during the automatic pullback. Low profile, open lumen imaging catheters require purging to exclude air and to ensure optimal image quality. The imaging run should start at least 20 mm distal to the lesion and end at the left main or RCA ostium to include the longest vessel segment possible; using OCT the survey mode (75 mm) is thus preferable for pre-PCI imaging. If the imaging catheter does not cross the lesion prior to stenting, or if the flush is insufficient to clear blood from the lumen (in OCT cases), balloon pre-dilatation may be used to facilitate image acquisition. Establishing a correlation of the intracoronary imaging findings and the angiogram is important for further angiography-guided actions like identification of stent landing zones. Co-registration of IVUS or OCT images with angiography is the ideal technique for this purpose. IVUS and OCT allow the assessment of reference lumen and reference vessel dimensions (as delineated by the EEM) at the proximal and distal, non-diseased, reference sites; IVUS can also assess the vessel dimensions (delineated by the EEM) at the site of minimal lumen diameter. The term EEM is used throughout this document to describe the interface between media and adventitia.
PLAQUE COMPOSITION
CALCIFIC PLAQUE
Coronary angiography has low sensitivity, but a relatively high positive predictive value for detection of calcific plaque39. Intravascular ultrasound and particularly OCT are valuable for detection, localization, and quantification of coronary calcification. OCT can visualize calcified plaque without artefacts40, penetrate calcium to a certain degree, and thus evaluate its thickness more accurately than IVUS41,42. Extensive target lesion calcification may adversely impact the PCI procedure by affecting the ability for effective dilatation of a coronary stenosis and is associated with greater likelihood of stent underexpansion. In lesions with maximum circumferential extension of calcium >180° by IVUS, greater calcific burden was associated with a smaller stent area and greater stent eccentricity43. The presence of OCT-detected fractures following lesion preparation was associated with greater stent expansion in a small-scale observational study44. Similarly, an OCT-based study suggested that lesions with calcium pools with a maximum angle >180°, maximum thickness >0.5 mm, and length >5 mm are at increased risk for stent under-expansion45, but there is no evidence of an impact of lesion calcification on clinical PCI outcomes.
LIPID-RICH PLAQUE
Stenting of attenuated plaque as assessed by greyscale IVUS46-49, or lipid-rich plaques as assessed by IVUS-virtual histology50, OCT51, and near infrared spectroscopy (NIRS) has been consistently associated with a higher risk of post-procedural MI, distal embolization, and no reflow phenomenon. The clinical implications of these observations nonetheless remain unclear. The use of a distal protection filter in NIRS-detected lipid rich lesions did not reduce peri-procedural MI rates in the randomized CANARY study (potentially because of issues caused by the insertion of filters per se)52.
| Box 2. Pre-PCI detection of calcium and lipid plaque by optical coherence tomography and intravascular ultrasound − OCT, in contrast to IVUS, can often assess calcium thickness. − Total calcium arc >180° and increased calcium thickness >0.5 mm are associated with greater risk of stent underexpansion. − Evidence of calcium fractures following lesion preparation is associated with improved stent expansion. − In case of large (>180°) calcium pools and absence of calcium fracture following the initial lesion preparation, a more aggressive lesion preparation should be considered. − Although stenting of lipid-rich plaques is related to an increased risk of peri-procedural MI and no reflow, the procedural consequences of lipid/necrotic pool detection by OCT or IVUS prior to PCI remain unclear. |
SELECTION OF OPTIMAL STENT SIZING
STENT DIAMETER
Stent underexpansion is a powerful predictor of early stent thrombosis and restenosis after DES implantation according to numerous IVUS studies53-56, pointing to the importance of appropriate stent sizing and expansion. Several potential approaches have been proposed for selection of stent diameter (Figure 3). More conservative approaches advocate a stent diameter based on the smallest reference lumen dimensions. Progressively larger stent diameters would be chosen by accounting for the mean (average of proximal and distal) reference lumen dimension; the largest reference lumen dimensions (proximal or distal); or considering the smallest reference EEM area (by IVUS or OCT). Even more aggressive approaches by IVUS (not applicable to OCT) are based on a media-to-media (or mid-wall approach) at the site of the minimal lumen diameter (Figure 3). From a practical standpoint, the use of the distal lumen reference (either EEM or lumen based) represents a straightforward approach to safely apply, with subsequent post-dilatation of the mid- and proximal part of the stent. When applying a lumen based approach, the use of the mean lumen diameter with up rounding the stent diameter for 0-0.25 mm was recommended in the OPINION study. When applying an EEM based approach, the use of the mean EEM diameter (derived from two orthogonal measurements, or only from one measurement in case the visibility of the EEM is limited to approximately 180°) was recommended with down rounding the stent diameter to the nearest 0.25 mm. In terms of feasibility, the distal EEM was visible for >180° in 77% of patients included in the ILUMIEN 3 study. An important exception to this strategy may be long lesions with large diameter changes (e.g. mid-LAD to LM lesion).
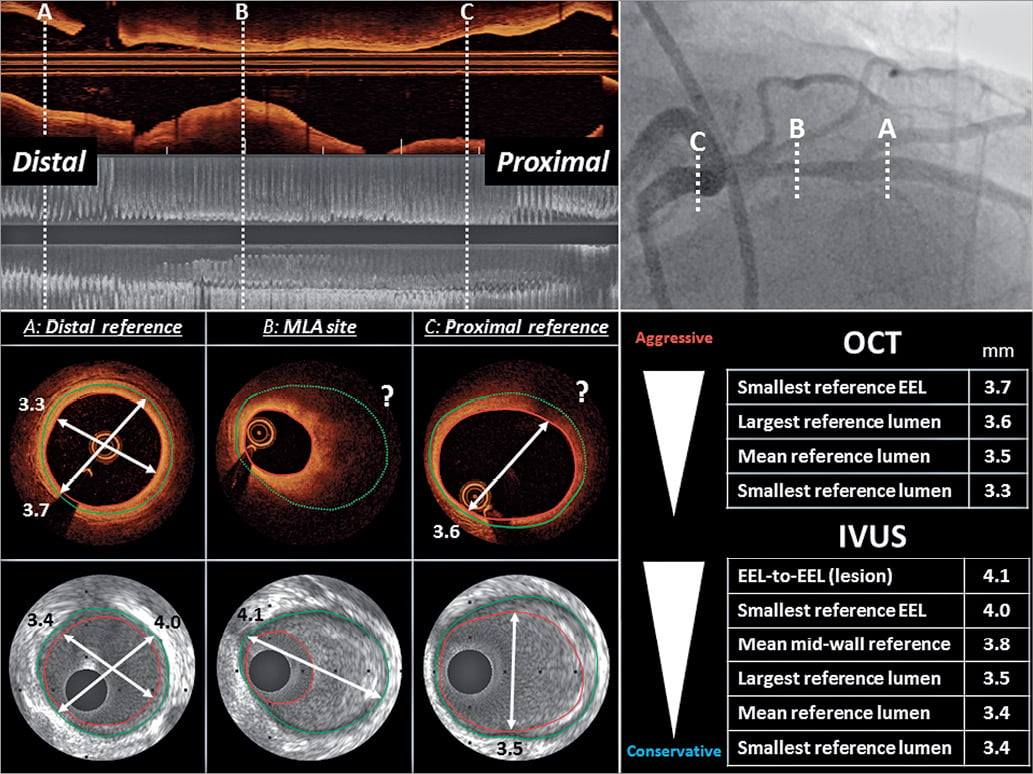
Figure 3. Intravascular ultrasound- and optical coherence tomography-based stent sizing approaches. Illustrative case of a proximal LAD stenosis as displayed by angiography and longitudinal views of intravascular ultrasound and optical coherence tomography. The cross section at the distal reference, the minimal lumen area site and the proximal reference are depicted for intravascular ultrasound and optical coherence tomography. The measurements of various sizing approaches are provided in the lower right panel. The external elastic membrane distance or the mid-wall to mid-wall approach can be used by intravascular ultrasound at the position of the minimum lumen area, whereas optical coherence tomography often fails to visualize the vessel boundaries at this position (?), due to the limited penetration depth in lipid tissues. An external elastic membrane-based approach at the proximal and/or distal reference segment can be used by intravascular ultrasound. The use of optical coherence tomography for such a strategy depends on the visibility of the external elastic membrane within the external elastic membrane segment. A lumen-based approach is similarly feasible for both intravascular ultrasound and optical coherence tomography.
In certain lesion subsets (e.g. long stenoses, vessels located distal to a CTO or location involving a myocardial bridge), assessment of vessel size may be important to rule out negative vessel remodelling, and to ensure that the selected stent size does not imply a risk of vessel rupture.
Criteria for stent sizing need to be viewed in light of certain differences in lumen detection by means of IVUS vs. OCT: minimum lumen area (MLA) derived by OCT is smaller (~10%) compared with IVUS, which may affect lesion severity assessment57,58. Similarly, lumen dimension assessment at the reference site results in smaller measurements by OCT vs. IVUS59 (with the exception of one study57) and this may affect stent diameter selection58,59.
Intravascular ultrasound guidance results in larger stent diameter, greater angiographic MLD and MSA, and implantation of more and longer stents compared with angiographic guidance5,21,60. When comparing IVUS vs. OCT guidance, the largest available study including 809 patients in Japan34 (OPINION) reported a small but significant difference in the average stent size (OCT 2.92 ± 0.39 mm vs. IVUS 2.99 ± 0.39 mm, P = 0.005) when applying a lumen-based stent sizing approach. However, this did not translate into differences in angiographic in-stent MLD immediately after stent implantation or at 8-month angiographic follow-up. In the OPINION imaging sub-study including 103 patients, the lumen-based OCT strategy was associated with a trend for non-significantly smaller minimal and mean stent area61. In the ILUMIEN III study, in which an EEM based distal reference sizing approach was applied, no difference in the stent diameter was observed33.
STENT LENGTH
The importance of proper selection of stent length is highlighted by the consistent identification of incomplete lesion coverage as a one of the predictors of stent failure (stent thrombosis or restenosis)1,62,63 and MACE64. Avoidance of definition of the landing zone within an area of residual plaque burden (e.g. >50%63) and particularly lipid-rich plaque is clinically important, as this has been linked to subsequent stent edge restenosis following new-generation DES implantation65-67. In addition, incomplete stent coverage of lipid pools has been associated with an increased risk of post-procedural MI68.
Co-registration of intracoronary imaging and angiography is an important tool to facilitate stent length selection and precise implantation. This technique is available for clinical practice69 and simplifies imaging-guided stent deployment.
PERCUTANEOUS CORONARY INTERVENTION OPTIMIZATION AFTER STENT IMPLANTATION
Following stent implantation, IVUS/OCT can detect correctable abnormalities related to the stent and underlying vessel wall, such as underexpansion, geographic plaque miss, strut malapposition, and stent edge dissection; these abnormalities have been associated with adverse PCI outcomes1. Findings that represent possible targets for stent optimization are shown in Figure 4 and Take home figure.
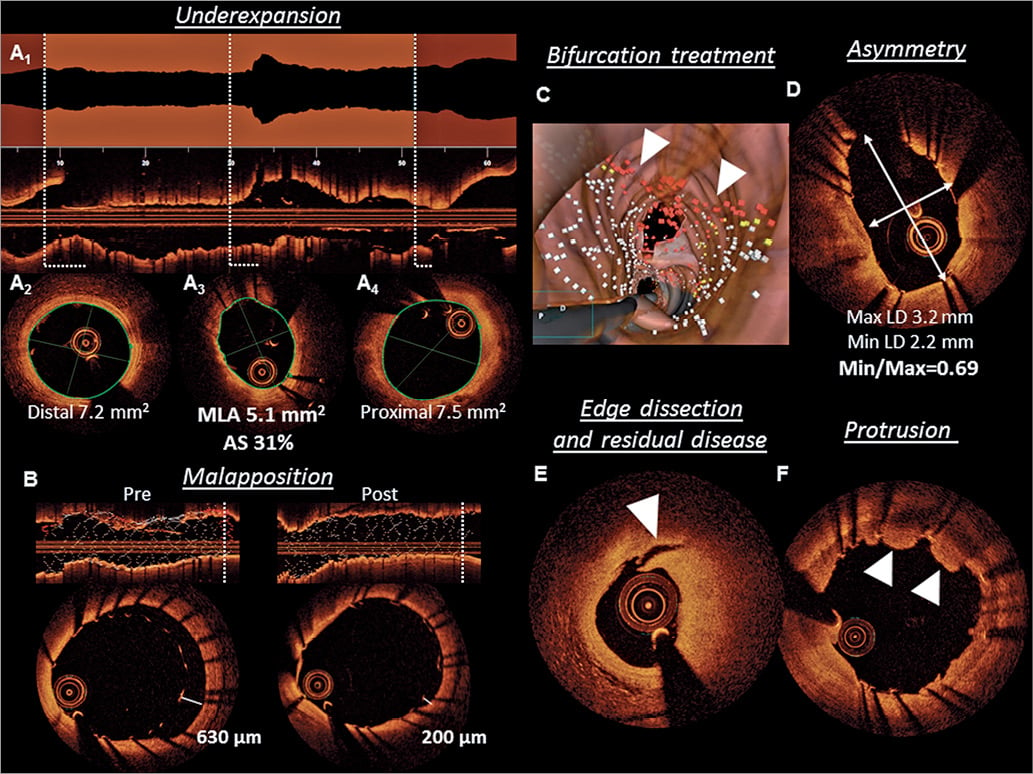
Figure 4. Targets for intracoronary imaging-guided percutaneous coronary intervention. (A) Avoidance of stent underexpansion represents the most relevant target for percutaneous coronary intervention guidance. The use of an automated lumen profile, provided by online optical coherence tomography software, allows detection of the minimal lumen area site. (A1) Graphical representation of the luminal area for every frame and facilitates assessment of stent expansion through operator selection of distal (A2) and proximal (A4) reference boundaries, and automated detection of the minimum lumen area (A3) are shown. In this illustrative case, a residual lumen area stenosis of 31%, required additional post-dilatation. (B) Online optical coherence tomography software allows automatic detection of stent struts, and therefore, identification of acute malapposition according to operator-defined strut distance from the vessel wall (white dots in longitudinal view for apposed and red dots for malapposed struts in C). Indication of malapposed struts may be especially helpful in bifurcation treatment as shown in this case of LAD-D1 bifurcation in which the fenestration of the first diagonal (ostium shown in the upper part) was erroneously performed under the LAD stent without taking note angiographically. The red dots (white arrows) in the three-dimensional reconstruction indicate a grossly malapposed LAD stent despite perfect angiographic result (not shown). (D) Stent asymmetry can be assessed by the quotient of minimal and maximal lumen diameter. (E) Edge dissections (in the context of residual disease burden) and (F) irregular tissue protrusion have both been associated with adverse outcomes.
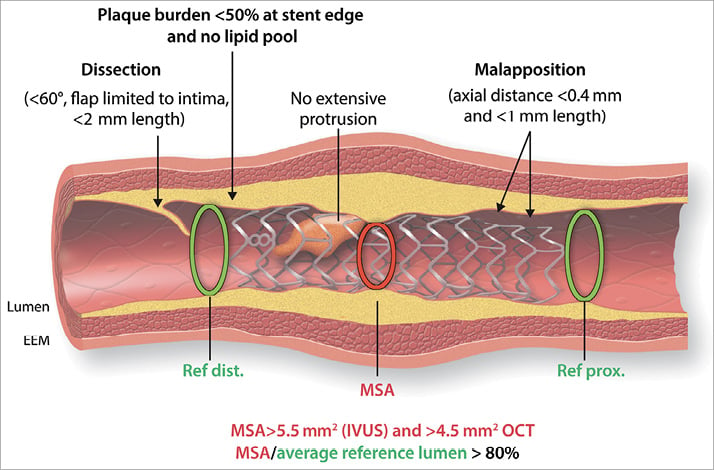
Take home figure. Summary of post-percutaneous coronary intervention optimization targets. The most relevant targets to be achieved following stent implantation in non-LM lesions are shown. These include optimal stent expansion (absolute as well as relative to reference lumen diameter); avoidance of landing zone in plaque burden >50% or lipid rich tissue; avoidance of large malapposition regions, irregular tissue protrusion, and dissections. Thresholds provided reflect the consensus of this group. Some are based on consistent and robust prospective data (e.g. stent expansion, landing zone) and others are less established (e.g. malapposition).
While both techniques can be used in this context, OCT has proven to be superior in the detection of malapposition and stent edge dissections33. Optical coherence tomography as compared to IVUS has a unique value for detecting thrombus, which is often indicative of mechanical or anticoagulation problems.
| Box 3. Stent sizing by intracoronary imaging − The beneficial effect of imaging-guided PCI does not appear to be strictly linked to the algorithm used for stent sizing by IVUS or OCT. − From a practical standpoint, a distal lumen reference based sizing may represent a safe and straightforward approach with subsequent optimization of the mid and proximal stent segments. Specifically, the mean distal lumen diameter with up rounding stent (0-0.25 mm) may be used (e.g. 3.76 → 4.0 mm), or the mean EEM (2 orthogonal measurements) with down rounding to the nearest 0.25 mm stent size (e.g. 3.76 → 3.5 mm). − When using OCT, an EEM reference based sizing strategy appears feasible, although more challenging than a lumen based approach for routine clinical practice. − Appropriate selection of the landing zone is crucial as residual plaque burden (<50%) and particularly lipid rich tissue at the stent edge is associated with subsequent restenosis. − Co-registration of angiography and IVUS or OCT is a useful tool to determine stent length and allows for precise stent placement. |
STENT EXPANSION
Stent underexpansion is established as a major predictor of stent failure70,71. Stent expansion describes the minimum stent cross-sectional area either as an absolute measure (absolute expansion), or compared with the predefined reference area, which can be the proximal, distal, largest, or average reference area (relative expansion). In principle, greater absolute stent expansion has been associated with better long-term stent patency, better clinical outcomes and a lower risk of stent failure55,71-73 and appears to be a better predictor of future stent patency than relative expansion. Intravascular ultrasound studies have been relatively consistent in showing that a stent cross-sectional area of 5.5 mm2 best discriminates subsequent events in non-left main lesions71,73. Consistently, in the DOCTORS trial the optimal cut-off to predict post-procedural FFR >0.90 was >5.44 mm2 by OCT30 and data from the CLI-OPCI registries identified an MLA of 4.5 mm2 as a threshold for discriminating patients with MACEs74. For LM lesions, cut-offs values are higher (e.g. >7 mm2 for distal LM and >8 mm2 for proximal left main by IVUS). Several points require consideration in this respect. Firstly, this cut-off may not be achievable in small vessels or may result in stent undersizing in large vessels. Secondly, there is a step-wise decrease in event rates with larger MSAs. Thirdly, evidence exists that cut-offs of absolute stent expansion that predict future events differ between BMS and DES55. Finally, different criteria apply in the case of left main lesions (larger cut-offs).
With respect to relative stent expansion, there are no uniform criteria regarding recommended targets for PCI optimization in clinical practice. The pre-specified criteria used for stent expansion in currently available IVUS and OCT studies are summarized in Supplementary material online, Tables S1 and 3, respectively. Different targets for stent optimization include either MSA greater than the distal reference lumen area; or >80% or >90% of the average (proximal and distal) reference area. In recent IVUS trials, the presence of a MSA greater than the distal reference lumen area was associated with a very low adverse event rate (1.5% within 1 year)1. Considering that the requirement for achieving >90% expansion was frequently out of reach (Figure 2), this expert group believes that the cut-off >80% for the MSA (relative to average reference lumen area) appears to be a reasonable approach to adopt in clinical practice. In the DOCTORS study, the optimal cut-off value of stent expansion able to predict FFR >0.90 was >79.4%30.
MALAPPOSITION
In contrast to underexpansion (i.e. MSA that is substantially smaller than the average reference lumen areas), malapposition refers to the lack of contact of stent struts with the vessel wall. Stent malapposition and underexpansion can co-exist or occur independently. Malapposition can occur either in the acute, post-procedural period, or it may develop later, possibly as a result of an underlying vascular process of inflammation and positive (outwards) remodelling of the vessel wall. When malapposition is identified at follow-up, it may represent either persistent (i.e. ongoing since the time of implantation), or late acquired malapposition; a differentiation of these two entities is not possible in the absence of imaging immediately post stenting.
While stent underexpansion is a major IVUS predictor of early stent thrombosis or restenosis, no clear link exists between acute malapposition (in the absence of underexpansion) and subsequent stent failure, as acute malapposition may subsequently resolve. Malapposition can be more reliably detected by OCT compared with IVUS, translating into a higher proportion of malapposed stent struts identifiable by OCT33 (up to 50% of stents implanted under OCT evaluation) vs. IVUS (about 15% of stents implanted under IVUS evaluation). Prospective studies have shown little or no relationship between malapposition that is detected during routine imaging and subsequent events. Acute malapposition did not emerge as an independent predictor of stent thrombosis in studies with imaging immediately after stent placement75,76. Still, on the basis of the investigated populations a protective effect of optimal stent apposition in high-risk populations cannot be excluded. In contrast, studies of stents presenting with thrombosis have consistently identified malapposition as a frequent underlying stent abnormality and showed a higher incidence and extent of malapposition in stent segments with vs. without thrombus. Three recent registries performed OCT in patients with definite stent (BMS or DES) thrombosis77-79. In two studies (PRESTIGE77 and PESTO78) malapposition (unclear whether persistent or late acquired) emerged as a frequent finding: 27% and 60%, respectively, in acute stent thrombosis (within 24 h of implantation), 6% and 44%, respectively in subacute stent thrombosis (1-30 days,) and 10% and 44%, respectively in late stent thrombosis (between 30 days and 1 year post-PCI). These observations were consistent with smaller OCT registries80-82. Moreover, malapposition was among the three leading mechanisms in studies investigating patients with very late stent thrombosis77-79 (>1 year following stent implantation). In line with these observations, malapposition has been associated with increased thrombogenicity in in vitro studies83.
Notwithstanding current uncertainties regarding the clinical relevance and potential sequelae of different modes of malapposition, the findings of large stent thrombosis registries, in concert with in vitro investigations, suggest that extensively malapposed struts should be avoided following stent implantation and should be corrected when anatomically feasible. As it relates to clinical guidance (corrective treatment or not), malapposition is not merely a binary (yes/no) phenomenon, as has been addressed in most studies, but can be quantified in a two-dimensional (or even a three-dimensional) fashion. Regarding thresholds for corrective post-dilatation, although there are no robust data, there is informative evidence to provide some guidance. One consideration is the association between the axial distance of incomplete stent apposition (ISA) and the subsequent integration by neointimal tissue; in determining the axial extent of ISA, differences in strut thickness across different stents should also be considered. Serial OCT studies observed that struts with ISA distance <0.35 mm undergo full neointimal integration at follow-up84-86. In agreement with this observation, a detailed analysis of patients with very late stent thrombosis reported minimal ISA distance within the thrombosed segments ranging between 0.3 and 0.6 mm, and longitudinal length between 1.0 and 2.1 mm79. The risk for extensive acute malapposition is increased in bifurcation PCI, a situation in which visualization by three-dimensional OCT may be helpful (Figure 4D). As complex bifurcation stenting requires rewiring of freshly implanted stents, malapposition constitutes a particular problem due to the risk of accidental abluminal rewiring.
| Box 4. Criteria to assess optimal stent result − A relative stent expansion of >80% (MSA divided by average reference lumen area) should be obtained in routine clinical practice. − An MSA of >5.5 mm2 by IVUS and > 4.5 mm2 by OCT should be achieved in non-left main lesions. − The clinical relevance of acute malapposition is uncertain. Nonetheless, extensive malapposition after stent implantation should be avoided and corrected, if anatomically feasible. Early strut coverage may be promoted by full apposition. − Acute malapposition of <0.4 mm with longitudinal extension <1 mm or malapposition should not be corrected as spontaneous neointimal integration is anticipated. This cut-off requires prospective validation. − Late acquired malapposition represents an established cause of late and very late stent thrombosis. − Tissue prolapse in ACS as compared with stable CAD is adversely related to outcomes, likely because of differences in the composition of the protruding tissue. − Large dissections detected by IVUS or OCT are independent predictors of MACE. Presence of residual plaque burden, extensive lateral (>60°), and longitudinal extension (>2 mm), involvement of deeper layers (medial or adventitia) and localization distal to the stent increase the risk for adverse events. − Stent edge haematoma may be detected by IVUS or OCT in case of angiographic appearance of a residual stent edge stenosis. |
TISSUE PROLAPSE
Tissue prolapse (typically defined as tissue extrusion from inside the stent area) may include either lesion protrusion or, in the context of ACS, protrusion of athero-thrombotic material. Optical coherence tomography enables clearer and more frequent visualization of tissue prolapse compared with IVUS33. Tissue prolapse after stent implantation has been identified as an OCT predictor of early stent thrombosis and has been related to adverse short-term prognosis following PCI53,87-89. The volume of prolapsed tissue by OCT has been associated with unstable plaque morphology as well as with post-PCI myocardial injury90. In a large multicenter OCT registry including 780 patients (50% with ACS), irregular protrusion was more common in patients treated for MI and was an independent predictor of 1-year clinical outcomes, primarily driven by TLR91. Evidence exist that tissue prolapse in the context of ACS is more likely to have consequences than in more stable (non-ACS) clinical setting as suggested by the CLI-OPCI and HORIZONS-AMI substudies53.
DISSECTION
Large edge dissections by IVUS have been reported as correlates of early stent thrombosis and these dissections were commonly characterized by their depth (at least disrupting the medial layer), their lateral extension (>60°) as well as their length (>2 mm)53,87. Owing to its higher resolution, OCT can identify less-extensive edge dissections which are missed by IVUS. Therefore, the incidence of OCT-reported edge dissections is at least two-fold higher compared with IVUS-reported dissections; this was confirmed in the OCT vs. IVUS arms of the ILUMIEN-3 trial33. In the CLI-OPCI II Study, dissections >200 μm at the distal (but not proximal) stent edge by OCT emerged as an independent predictor of MACE (HR 2.5)74. In contrast, in an observational study including 780 patients who underwent post-procedural OCT, stent edge dissections (detected in 28.7% of lesions) or in-stent dissections were not associated with adverse 1-year clinical outcomes. In line with IVUS studies, stent edge dissections are considered among OCT-defined predictors of early stent thrombosis. However, subtle abnormalities (i.e. minor edge dissection) are unlikely to be clinically significant and possibly do not require correction92,93. Detection of intra-and extramural haematomas by IVUS or OCT may be relevant, as these findings usually appear as edge stenosis by angiography and can be misdiagnosed as stent vessel mismatch or spasm. The progression of uncovered haematoma may lead to early stent thrombosis.
OPTIMIZATION OF BIORESORBABLE SCAFFOLD IMPLANTATION
In contrast to permanent metallic stents, where optimal implantation techniques have been investigated extensively in imaging studies, intracoronary imaging was not routinely recommended for bioresorbable vascular scaffold implantation. Notably, due to inherent mechanical limitations of bioresorbable materials, and radio-lucency of devices, accurate lesion preparation, device sizing and procedure optimization (i.e. complete expansion without fracture or malapposition) may be even more critical when implanting bioresorbable scaffolds94,95. Retrospective analyses demonstrated that post-procedural lumen eccentricity and asymmetry are related to target lesion failure96. Intracoronary imaging is of importance to detect structural abnormality such as acute disruption and discontinuities at follow-up which are not detectable on angiography due to radio-lucency of devices. Although not shown in prospective trials, malapposition at the time of implantation may adversely impact subsequent tissue coverage and incorporation of the scaffold into the vessel wall, which in turn may create a thrombogenic nidus during the process of scaffold dismantling95.
Assessment of mechanisms of stent failure
This expert panel highly recommends intracoronary imaging in the setting of stent failure. Imaging facilitates identification of the mechanisms of restenosis or stent thrombosis, guides appropriate treatment, minimizes the risk of subsequent stent failure events, and raises awareness of any potential device related concerns.
RESTENOSIS AND STENT THROMBOSIS IN METALLIC DRUG-ELUTING STENTS
Identifiable causes of restenosis other than intimal hyperplasia include chronic underexpansion (in approximately 18-40%54,97) stent fracture (<5%), and neoatherosclerosis (i.e. >1 year of DES implantation). The first two abnormalities can be readily detected by IVUS or OCT, whereas the latter is a finding defined by OCT98. Regarding stent fracture, this can be identified more readily by means of three-dimensional OCT imaging compared with two-dimensional imaging alone. For approximately 60% of restenosis cases, the leading mechanism cannot be assessed beyond the (expected) presence of neointimal hyperplasia. In contrast, stent thrombosis has multiple underlying mechanisms and most of these are recognizable by intracoronary imaging (Figure 5)53,91,99,100. Optical coherence tomography, as opposed to IVUS, can distinguish thrombus from other tissue components, and is therefore, considered the preferred imaging technique for stent thrombosis. However, in some cases the presence of large amounts of thrombus can make the assessment of stent struts and the outer vessel wall challenging by OCT due to light attenuation, and IVUS may be preferred (Figure 5 I). Restoration of TIMI III flow with subsequent administration of GP IIb/IIIa inhibitors and staged OCT represents a strategy previously applied to enhance analysis of the underlying stent thrombosis aetiology78. Three recent cohort studies investigated correlates of stent thrombosis occurring at various time points after stent implantation77-79. One or several leading mechanisms responsible for thrombus formation could be identified in the majority of patients (>90%). Reassuringly, the interpretations of the operating physicians were in agreement with those of experts in 70% of cases in the PESTO registry. The main causes for stent thrombosis in the DES subgroups are reported in Figure 6. In patients with early stent thrombosis, malapposition, underexpansion, and edge dissections were the predominant abnormalities. At variance to previous IVUS studies, malapposition was a relatively frequent finding by OCT. In patients with very late DES thrombosis, malapposition, neoatherosclerosis, uncovered struts and underexpansion were frequently observed. A tailored treatment approach according to the specific OCT findings (e.g. additional stent in case of neoatherosclerosis, post-dilatation in case of underexpansion or malapposition) appears clinically reasonable, although prospective data to support such a treatment strategy are lacking. The 2014 ESC guidelines on myocardial revascularization, which were published prior to publication of the three OCT registries, considered a Class IIa C recommendation for the performance of intracoronary imaging in stent failure by IVUS or OCT38.
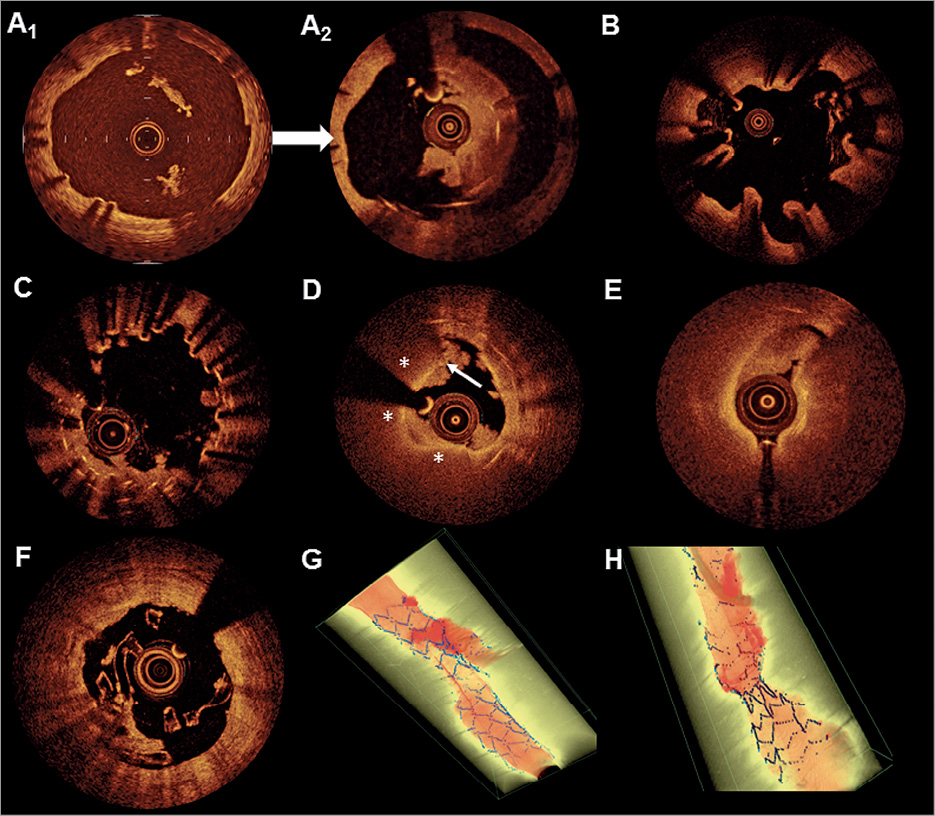
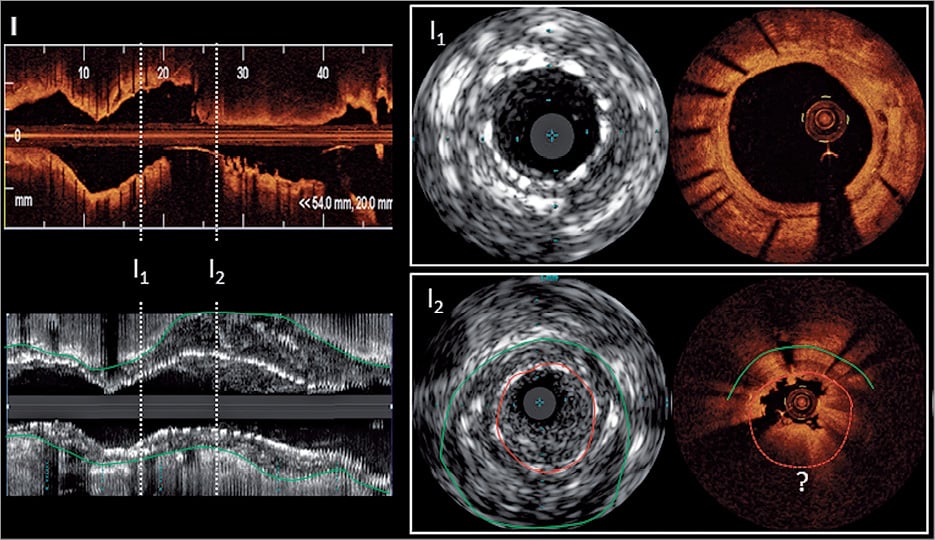
Figure 5. Optical coherence tomography and intravascular ultrasound findings in the context of stent and scaffold thrombosis. (A) A significantly undersized stent suggestive of persistent malapposition 1 year after implantation (A1) was demonstrated. This finding was left uncorrected. Two years later (A2), an occlusive stent thrombosis occurred after cessation of aspirin. (B) Extensive evaginations as an indicator of positive vessel wall remodelling in a Cypher stent implanted 4 years previously. No thrombus is visible as thrombolysis was administered prior optical coherence tomography. (C) Uncovered stent struts in the region of multiple overlapping stents with small multiple protruding thrombi. (D) A typical in-stent fibroatheroma (6-12 o’clock, stars) with a ruptured cap (arrow) and white thrombus is depicted, suggestive of neoatherosclerosis. Disease progression at the stent edge may be a trigger of stent thrombosis (E). Although most mechanisms of scaffold thrombosis are identical with metallic drug-eluting stents, stent discontinuity (i.e. previously apposed scaffold struts that subsequently migrate into the lumen) represents a specific finding in scaffold thrombosis (F). (G) A stent thrombosis occurring at a bifurcation lesion (three-dimensional, thrombus red, and stent blue) is illustrated and (H) a markedly underexpanded stent with thrombus distal to the underexpanded segment. (I) ‘Pros and Cons’ of intravascular ultrasound and optical coherence tomography in the assessment of stent thrombosis is illustrated. The longitudinal view is illustrated on the left hand and cross sections from outside (I1) and inside (I2) the thrombotic region. In the longitudinal view and I2, the thrombus mass is attenuating the light and stent struts are no longer visible (dotted red line and ?) whilst intravascular ultrasound readily depicts the struts. Also, the outer vessel wall (green line), which is indicative of positive remodelling, can only be seen by intravascular ultrasound. Conversely, subtle details like stent strut coverage and peri-strut low intensity regions can only be depicted by optical coherence tomography (I1).
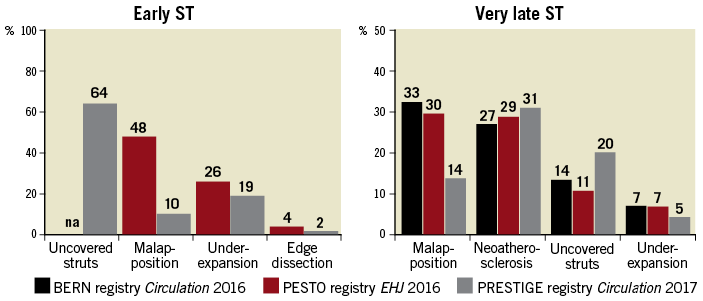
Figure 6. Frequency of presumable causes of early and very late metallic drug-eluting stent thrombosis as assessed in three optical coherence tomography registries.
SCAFFOLD THROMBOSIS
The Absorb BVS scaffold is the only bioresorbable device that has undergone extensive scientific evaluation in adequately powered RCT’s. Individual studies101 and meta-analyses102,103 suggested an increased risk of scaffold thrombosis at all-time points following implantation and particularly beyond one year. Although many experts acknowledge the potential for intracoronary imaging-guided scaffold implantation to mitigate scaffold failures, no RCT to date has addressed the relevance of imaging-guided scaffold implantation and one was stopped prematurely (OPTICO BVS; NCT02683356) following retraction of ABSORB BVS. None of the conducted clinical trials on ABSORB BVS was convincingly able to provide insights into the failure mechanisms. Of note, OCT analyses of scaffold thrombosis by means of OCT within the first year after implantation identified underexpansion and malapposition (due to undersizing or lack of adequate expansion) as predominant findings94,104.
The largest cohort study on very late ABSORB BVS scaffold thrombosis studied by OCT –the Independent OCT Registry on Very Late Bioresorbable Scaffold Thrombosis (INVEST)105–included 36 patients with available OCT at the time point of the thrombotic event; 31% of patients had serial OCT imaging. The leading associated finding was a new bioresorption-specific phenomenon called strut discontinuity (43%). Strut discontinuity or dismantling is present (according to study definition) when struts are dislocated into the lumen despite initial full apposition, and even despite some degree of tissue coverage as shown by intercurrent OCT recordings, or in case of acute malapposition with subsequent discontinuity or in presence of acute scaffold fracture95,105. The newly described mechanism of Absorb BVS failure has not been observed with metallic DES and potentially explains the increased risk of very late scaffold thrombosis. Unravelling of a resorption-specific failure mechanism may permit the development of treatment strategies and provide guidance for improvements in the design of newer-generation devices. Device failures of newer-generation scaffolds should systematically undergo evaluation by intracoronary imaging, preferably OCT.
| Box 5. Stent/scaffold failure analyses by intracoronary imaging − Analysis of stent restenosis and stent thrombosis by intracoronary imaging is essential to understand mechanisms of failure and is highly recommended. − Although prospective data are lacking, tailored treatment strategies based on the exact failure mechanisms appear reasonable (e.g. post-dilatation only in case of malapposition/underexpansion induced stent thrombosis vs. stent implantation in presence of neoatherosclerosis). − OCT is the preferred technique to study in-stent restenosis and stent thrombosis. − Intracoronary imaging should be mandatory in case of any investigational device failure to expedite the identification of potential safety concerns and is recommended for evaluation of any new DES or BRS. − OCT findings from stent thrombosis registries propose the following correctable targets for PCI guidance: malapposition, residual disease burden at stent edge, dissections and stent underexpansion. |
Potential limitations of intravascular imaging
The clinical value of intracoronary imaging for PCI guidance is widely acknowledged1, but potential limitations should be considered. One of the key limitations of intracoronary imaging is the additional time required for imaging. The cost of IVUS and OCT is a notable consideration, and is acknowledged as a potential limitation by practicing interventional cardiologists7. A dedicated analysis addressing the cost-effectiveness of IVUS during PCI with DES showed that IVUS-guided interventions are cost-effective, particularly when used in patients at a greater risk of restenosis106. In view of substantial geographic variability in the clinical use of IVUS and/or OCT in daily practice, ranging from routine use in Japan to selected use in most other countries7 to very limited use in countries with no reimbursement, we recommend and encourage imaging-guided PCI primarily in settings with the most robust evidence of a clinical benefit (Table 3). Adequate training in the acquisition of images and interpretation of findings is an additional essential factor that may be addressed by integrating training in structured interventional fellowships and by ensuring basic imaging skills for all coronary interventionists and advanced experience with IVUS and/or OCT in at least selected operators in each medium and large-volume interventional catheterization laboratory. With current small diameter imaging device iterations, complications directly related to imaging are exceedingly rare, as shown in a systematic review60. In a large registry of patients undergoing OCT-guided or IVUS-guided PCI (>3600 procedures), imaging-related complications were infrequent (0.6%), self-limiting or easily treatable with no major adverse events107. One potential limitation is the deliverability of imaging catheters in some complex lesion subsets, e.g. heavily calcified, tortuous, angulated anatomies, where accurate imaging could be of potential benefit. Refinements in imaging technology such as co-registration [angiography and intracoronary imaging (roadmap)], lower-profile and more deliverable catheters with faster pullbacks, higher resolution technology (IVUS), and fully automated software to support pre- and post-stent assessment are expected to further improve the ease of use and therefore penetration in daily clinical practice.
Acknowledgements
The authors thank Georgios Siontis, MD PhD, for assistance in conducting the meta-analysis included in this document and Yasushi Ueki, MD, for assistance in preparing the figures.
Conflict of interest
Dr. F. Alfonso has nothing to disclose. Dr. Z. Ali reports grants from Cardiovascular System Inc, Abbott Vascular, personal fees from Cordis, Canon USA, Abbott Vascular, Boston Scientific, Acist Medical, Opsens. Dr. R. Bhindi reports grants from National Heart Foundation of Australia, personal fees from Bristol Myers Squibb, Astra Zeneca, Medtronic. Dr. R. Byrne reports grants from HeartFlow, Boston Scientific, personal fees from Boston Scientific, Biotronik, B.Braun. Dr. D. Capodanno reports personal fees from Bayer, Daiichi Sankyo, Pfizer, Astra Zeneca, Abbott, Direct Flow Medical. Dr. R. Carter has nothing to disclose. Dr. A. Colombo has nothing to disclose. Dr. J.M. de La Torre Hernandez reports grants from Boston Scientific, Medtronic, Biotronik, Abbott Vascular. Dr. C. Di Mario reports grants from Volcano, Abbott, Medtronic, Shockwave. Dr. J. Escaned reports personal fees from Astra Zeneca, Philips Volcano, Abbott, Medtronic, Orbus Neich, Boston Scientific. Dr. G. Guagliumi reports grants from Boston Scientific, Abbott, personal fees from Abbott, Boston Scientific. Dr. J. Hill reports grants from Boston Scientific, Abbott Vascular, personal fees from Boston Scientific, Abbott Vascular, Abiomed. Dr. N.R. Holm reports grants from Abbott, Terumo Inc, REVA Medical, Medis medical imaging, Abbott, Boston Scientific, personal fees from REVA Medical, Abbott, Terumo Inc. Dr. H. Jia has nothing to disclose. Dr. T. Johnson reports personal fees from Boston Scientific, Terumo Inc, Abbott Vascular, Daiichi Sankyo, Astra Zeneca. Dr. M. Joner reports grants from European Society of Cardiology, personal fees from Orbus Neich, Biotronik, Astra Zeneca. Dr. K. Koskinas reports personal fees from Amgen, Sanofi Aventis. Dr. N. Meneveau reports grants from Abbott Vascular, Daiichi Sankyo, personal fees from BMS/Pfizer, Abbott Vascular, Boehringer-Ingelheim, Daiichi Sankyo, Bayer Healthcare, Edwards Life Sciences. Dr. G. Mintz reports grants from Boston Scientific, Volcano, Abbott Vascular, personal fees from Boston Scientific, Volcano, Infraredx, Abbott Vascular. Dr. Y. Onuma reports personal fees from Abbott Vascular. Dr. F. Prati reports personal fees from Abbott. Dr. L. Räber reports grants from Abbott, Sanofi Aventis, personal fees from Abbott, Amgen, Astra Zeneca, Biotronik, CSL Behring, Sanofi Aventis. Dr. M. Radu reports personal fees from Abbott. Dr. E. Regar reports grants from Abbott, personal fees from Abbott. Dr. W. Wijns reports grants from Abbott Vascular, MICELL, Microport, personal fees from Microport. Dr. B. Yu has nothing to disclose.
Appendix. Authors’ affiliations
1. Department of Cardiology, Bern University Hospital, Bern, Switzerland; 2. Cardiovascular Research Foundation, New York, NY, USA; 3. Bristol Heart Institute, University Hospitals Bristol NHSFT, Bristol, UK; 4. Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark; 5. Department of Interventional Cardiology, Cardialysis, Thoraxcenter, Erasmus MC, Rotterdam, The Netherlands; 6. The Heart Center, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark; 7. Deutsches Herzzentrum München, Technische Universität München, Munich, Germany; 8. DZHK (German Center for Cardiovascular Research), partner site Munich Heart Alliance, Munich, Germany; 9. Department of Cardiology, 2nd Affiliated Hospital of Harbin Medical University, The Key Laboratory of Myocardial Ischemia, Chinese Ministry of Education, Harbin, China; 10. Department of Cardiology, University Hospital Jean Minjoz, 25000 Besancon, France; 11. EA3920, University of Burgundy Franche-Comté, Besancon, France; 12. Department of Cardiology, IDIVAL, Hospital Universitario Marques de Valdecilla, Santander, Spain; 13. Hospital Clinico San Carlos IDISSC and Universidad Complutense, Madrid, Spain; 14. Department of Cardiology, King’s College Hospital, London, United Kingdom; 15. Department of Cardiology, San Giovanni Hospital, Rome, Italy & CLI Foundation, Rome, Italy; 16. Interventional Cardiology Unit, Cardio-Thoracic-Vascular Department, San Raffaele, Scientific Institute, Milan, Italy; 17. Structural Interventional Cardiology, Careggi University Hospital, Florence, Italy; 18. Department of Cardiovacular Surgery, Zürich University Hospita, Zürich, Switzerland; 19. Division of Cardiology, Cardio-Thoraco-Vascular and Transplant Department, CAST, Rodolico Hospital, AOU “Policlinico-Vittorio Emanuele”, University of Catania, Catania, Italy; 20. The Lambe Institute for Translational Medicine and Curam, National University of Ireland Galway, Saolta University Healthcare Group, Galway, Ireland; 21. Deutsches Herzzentrum München, Technische Universität München, Munich, Germany; 22. Cardiovascular Department, Ospedale Papa Giovanni XXIII, Bergamo 24127, Italy
Supplementary material
Supplementary material is available at European Heart Journal online.

