
Cognitive dissonance is the mental discomfort experienced by a person who simultaneously holds two or more contradictory beliefs or ideas. It occurs when performing an action that contradicts those beliefs. Many, if not most, interventionalists have cognitive dissonance regarding routine use of intravascular ultrasound (IVUS) or optical coherence tomography (OCT) to guide percutaneous coronary interventions (PCI). As is the case for the use of translesional physiology with fractional flow reserve (FFR) or non-hyperaemic pressure ratios (e.g., iFR), the limited use of IVUS/OCT is not for want of good data. The interventionalist’s reticence to incorporate IVUS/OCT into their daily intraprocedural strategy, despite years of supporting evidence, can be seen not only by industry’s sales statistics showing <20% global use, but also by a clinical practice survey on the subject conducted by the European Association of Percutaneous Coronary Interventions (EAPCI) and the Japanese Association of Cardiovascular Interventions and Therapeutics (CVIT)1. Of the more than 1,000 respondents, only 51% reported using coronary imaging in >15% of cases. The top three reasons limiting use included high cost (66%), increased procedure time (35%), and reimbursement (29%). The major question, “Will IVUS/OCT make an important difference for my patient’s outcomes?” remains to be convincingly answered; even if it does, is IVUS/OCT worth the extra time and effort, especially in the light of minimal reimbursement? (Motivation through reimbursement is seen in countries providing a reasonable reimbursement which have >90% IVUS use for PCI1).
To address the major questions, consolidate the knowledge base and resolve the objections around the use of intravascular imaging, experts from the EAPCI led by Räber et al2 provide a document in two parts worthy of every interventionalist’s full attention.
Part 1 focuses on clinical usage, codifies the technical performance and interpretation of intravascular imaging, provides metrics and methods that should be employed for stent optimisation in a convenient form for the clinician, and sets the stage for the routine incorporation of imaging into daily practice.
The consensus document begins with a comprehensive review, immediately tackling the most pithy questions: “Does intracoronary imaging improve clinical outcomes following PCI?” and “Which patients and lesions should be considered for intracoronary imaging during PCI?”. Later, the experts address, “How (do we) perform intracoronary imaging and which criteria should be used for stent implantation and optimisation with IVUS and OCT?”, emphasising major important procedural updates with some comments on the concerning unknowns in this area (Table 1).
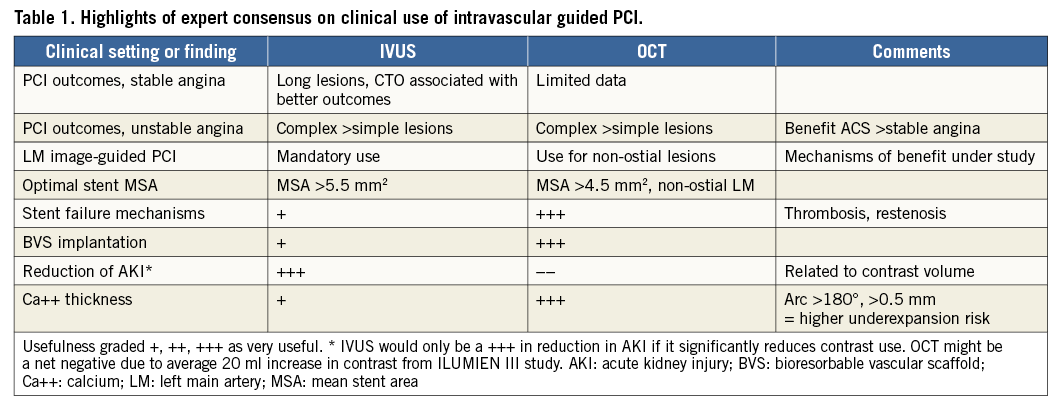
An unappreciated fact, highlighted by the experts and useful to know when one is reviewing major IVUS studies, is that IVUS outcomes differ depending on the stent type used. In the bare metal stent (BMS) era, IVUS had a neutral effect on outcomes, while in the drug-eluting stent (DES) era two out of eight randomised controlled trials comparing IVUS-guided with angiography-guided PCI (the IVUS-XPL3, and CTO-IVUS4 trials) showed significant reductions in major adverse cardiac events (MACE), principally target lesion revascularisation (TLR), with IVUS guidance. OCT’s more recent history precludes meaningful comparisons while the large randomised outcome results are being developed (e.g., ILUMIEN IV). An all-comers randomised study such as the FFR-guidance studies5,6 remains to be completed.
Light or sound?
Many operators are still deciding which imaging tool to use and in which circumstances one is superior to the other1. The experts describe the techniques, advantages and limitations of each modality in concise terms. Although high-definition IVUS is little discussed, the Figures with OCT images demonstrate its specific capabilities in spectacular fashion. OCT documents proposed mechanisms of vascular responses to PCI, stent failure, and PCI complications in ways we would not have anticipated. Examine the OCT/IVUS images (Figure5 in Räber et al) which impressively show scaffold thrombosis of an undersized stent, strut malapposition and an occlusive stent thrombosis due to extensive evaginations, positive vessel wall remodelling, and uncovered stent struts with small multiple protruding thrombi. Unique to OCT is the ability to see features of a fibroatheroma with a ruptured cap and coincidental overlying white thrombus. Stent scaffold discontinuity (i.e., previously apposed scaffold struts that subsequently migrate into the lumen) appears to be a specific finding involved in scaffold thrombosis.
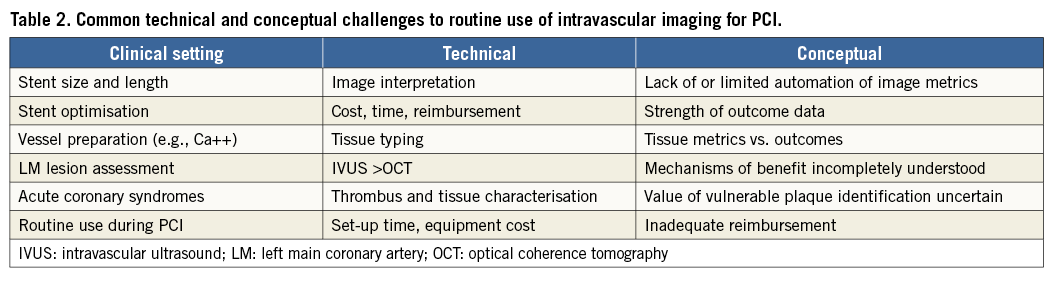
It is worthwhile to peruse the consensus review of the fundamental differences between IVUS and OCT. In the OCT assessment of a stent thrombosis, thrombus mass attenuates the light and obscures the visualisation of the stent struts. For IVUS, the metal struts are strong ultrasound reflectors and are readily seen along with the outer vessel external elastic lamina delimiter, permitting quantitation of positive remodelling, an observation made only by IVUS. More subtle information about stent coverage and associated low-intensity tissue are specific features of OCT but not IVUS. Overall, these images almost by themselves are among the most convincing data that might persuade us to move into visualising our acute and late PCI results on a routine basis. The only thing missing between our imagination and practical application is the strong linkage of the images to the clinical outcomes for each of these unusual findings (Table 2).
Probably the most important contribution of the EAPCI expert consensus document is the concentration of the current state-of-the-art knowledge in one place, providing a comprehensive reference, a document on which we can rely to make imaging ever more relevant to our PCI practice. I look forward to Part II.
In the future, operators will use angiographic co-registration and three-dimensional vessel visualisation, hopefully adding to our ability to improve PCI and increase our knowledge of vascular disease. The clinical implications of novel imaging findings in some settings may be intuitive but most often are not immediately apparent. While we currently lack data to demonstrate the benefits of angiographic co-registration, its value appears both logical and intuitive and, at the very least, will certainly increase the accuracy of intracoronary device placement. For intravascular imaging as a whole, seeing more and seeing it in a quantitative fashion will continue to open doors heretofore unknown, probably leading to improved technical methods and eventually better clinical results.
Conflict of interest statement
M. Kern is a consultant and speaker for Abbott/St. Jude, Philips/Volcano, ACIST Medical Inc., Opsens Inc., and HeartFlow Inc.

