
Abstract
This consensus document is the second of two reports summarizing the views of an expert panel organized by the European Association of Percutaneous Cardiovascular Interventions (EAPCI) on the clinical use of intracoronary imaging including intravascular ultrasound (IVUS), optical coherence tomography (OCT), and near infrared spectroscopy (NIRS)-IVUS. Beyond guidance of stent selection and optimization of deployment, invasive imaging facilitates angiographic interpretation and may guide treatment in acute coronary syndrome. Intravascular imaging can provide additional important diagnostic information when confronted with angiographically ambiguous lesions and allows assessment of plaque morphology enabling identification of vulnerability characteristics. This second document focuses on useful imaging features to identify culprit and vulnerable coronary plaque, which offers the interventional cardiologist guidance on when to adopt an intracoronary imaging-guided approach to the treatment of coronary artery disease and provides an appraisal of intravascular imaging-derived metrics to define the haemodynamic significance of coronary lesions.
Preamble
This consensus document, a summary of the views of an expert panel organized by the European Association of Percutaneous Cardiovascular Interventions (EAPCI), appraises current evidence on clinical indications for intracoronary imaging and provides guidance to the interventional community regarding recommended use, strengths, and potential limitations of intravascular ultrasound (IVUS), optical coherence tomography (OCT), and near infrared spectroscopy (NIRS)-IVUS based on existing evidence and the best current practice. The selection of the expert group, the organization of manuscript preparation and consensus development were detailed in Part 1.1
Introduction
The role of intracoronary imaging to enhance the outcome of percutaneous coronary intervention (PCI), particularly through patient selection and criteria for guiding stent optimization was outlined in Part 1.1 Our consensus opinion has been strengthened by recent European guidelines enhancing the recommendation for use of OCT for stent optimization to Class IIa.2 Furthermore, a trial of IVUS- vs. angio-guided PCI has confirmed a reduction in target vessel failure at 12 months through IVUS-guided optimization3; and updated meta-analyses now indicate a mortality advantage.4 Extending the role of intracoronary imaging, requires a shift in focus from the PCI to pre-PCI assessment of the coronary vasculature. Part 2 will focus on the use of intravascular imaging in patients presenting with acute coronary syndrome (ACS), and its role in defining the composition of atherosclerotic plaque, particularly detection of culprit lesions and markers of vulnerability. Additionally, we will emphasize the role of intravascular imaging when angiographic assessment is ambiguous or inconclusive, and its potential for assessing stenosis haemodynamic significance. We believe these extended benefits of intracoronary imaging will provide considerable value to the interventional community and their patients; however, we acknowledge that large scale, robust data is lacking in many of these fields. This strengthens the need for our consensus document to provide clinicians with guidance on the application of intracoronary imaging modalities.
Acute coronary syndromes
The greatest impact of PCI has been observed in the treatment of patients with ACS.5 These patients are at highest risk of major adverse cardiovascular events (MACE) when compared with patients presenting with chronic coronary syndromes.6
IDENTIFICATION OF THE CULPRIT LESION
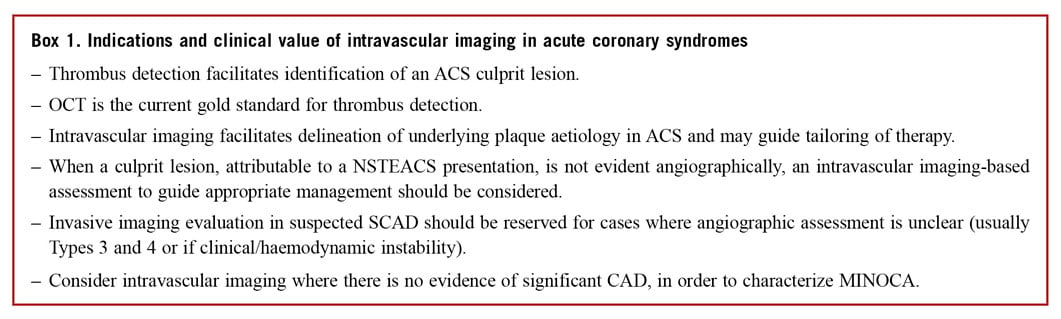
The focus of management for patients presenting with acute chest pain and ST elevation is immediate angiographic assessment.7 In the majority of patients, a culprit lesion is identified and recanalization with stenting is undertaken. However, diagnostic uncertainty can exist and the treating physician should consider non-atherosclerotic aetiologies, if presenting with atypical symptoms, unusual patient demographic/clinical risk profiles or in the absence of significant obstructive coronary artery disease (CAD) on angiography [4-10% of patients presenting with ST elevation ACS (STEACS)8,9]. The exclusion of an atherosclerotic ACS aetiology has important lifelong impacts for the patient, avoiding an erroneous diagnosis, and minimizing exposure to acute anti-thrombotic/anti-platelet and anti-atherosclerotic therapies.
Angiographic interpretation in patients with non-STEACS (NSTEACS) poses greater challenges due to the heterogeneity of presentation with respect to time from symptom onset, electrocardiogram (ECG) changes, and the possible absence of ventricular regional wall motion abnormalities. An identifiable culprit lesion may be absent in >30% of patients and >10% of patients may have multiple culprit lesions on angiography.10 Similar to patients presenting with STEACS, 4-10% of NSTEACS presentations have non-obstructive CAD11 but associated hazard for future events.12
It is important to acknowledge the inherent weakness of coronary angiography to accurately assess vessel and lumen geometry, and its inability to evaluate plaque components and accurately detect the presence of thrombus –information more accurately provided by intravascular imaging.13 Where diagnostic or angiographic uncertainty exists in the setting of ACS, we propose a role for intracoronary imaging to aid diagnosis and guide treatment (Figure 1).
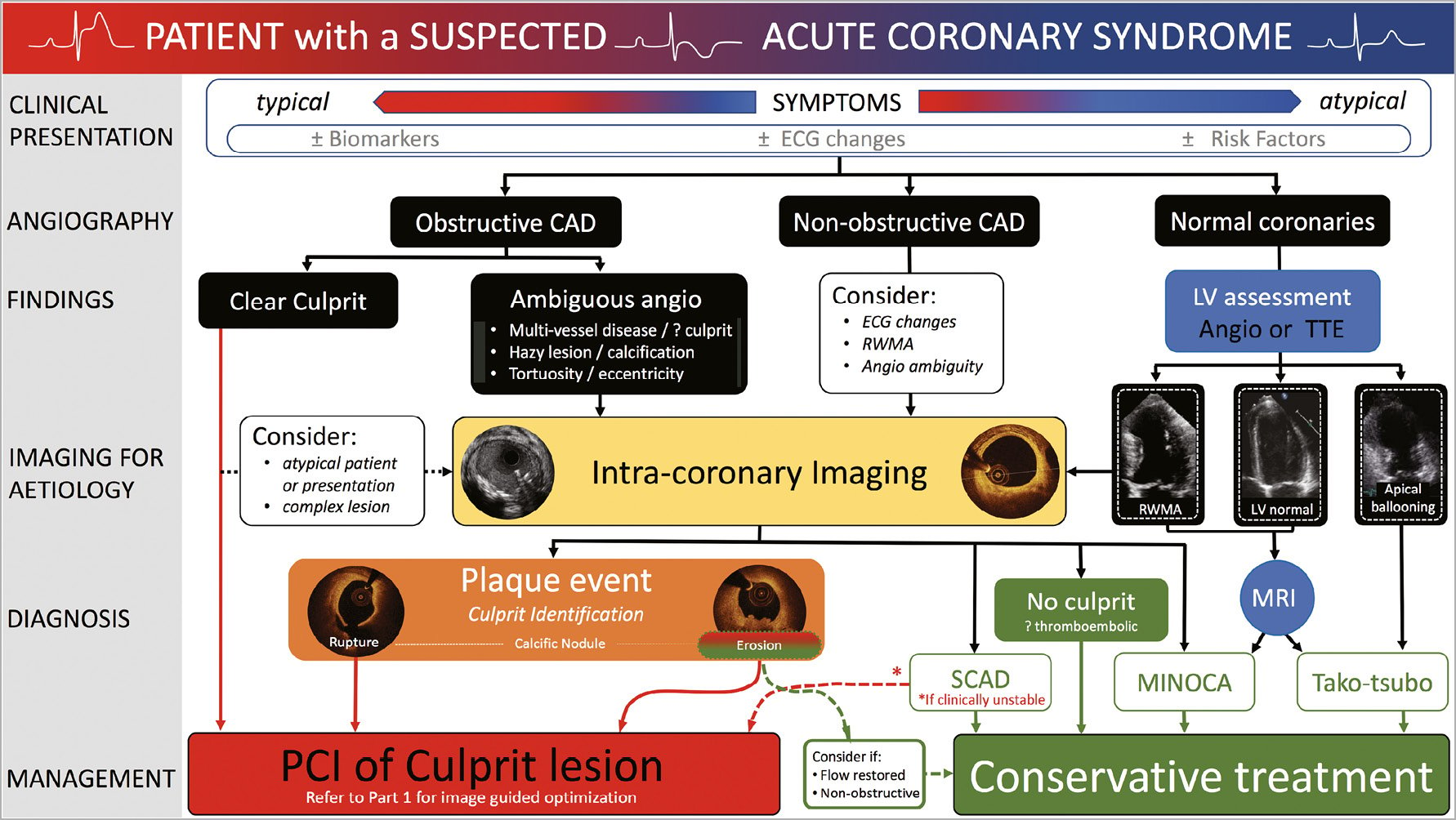
Figure 1. A treatment algorithmto guide the use of intravascular imaging in patients presenting with acute coronary syndromes.
Intracoronary imaging can delineate luminal discontinuity/plaque disruption and associated thrombus, the hallmarks of a culprit lesion. Optical coherence tomography provides accurate detection of intraluminal thrombus14 and is capable of distinguishing red and white thrombus due to the optical attenuating property of red blood cells, abundant within red thrombus (Figure 2 and Supplementary Table 1). Intravascular ultrasound detection of thrombus is more challenging (see Figure 2, Panel 2) but can be improved by stationary imaging at the level of the presumed thrombus and a small injection of contrast to highlight the luminal contour. The advent of high-definition IVUS promises superior resolution and improved diagnostic capabilities, however, at present a data-driven comparison with OCT is lacking.
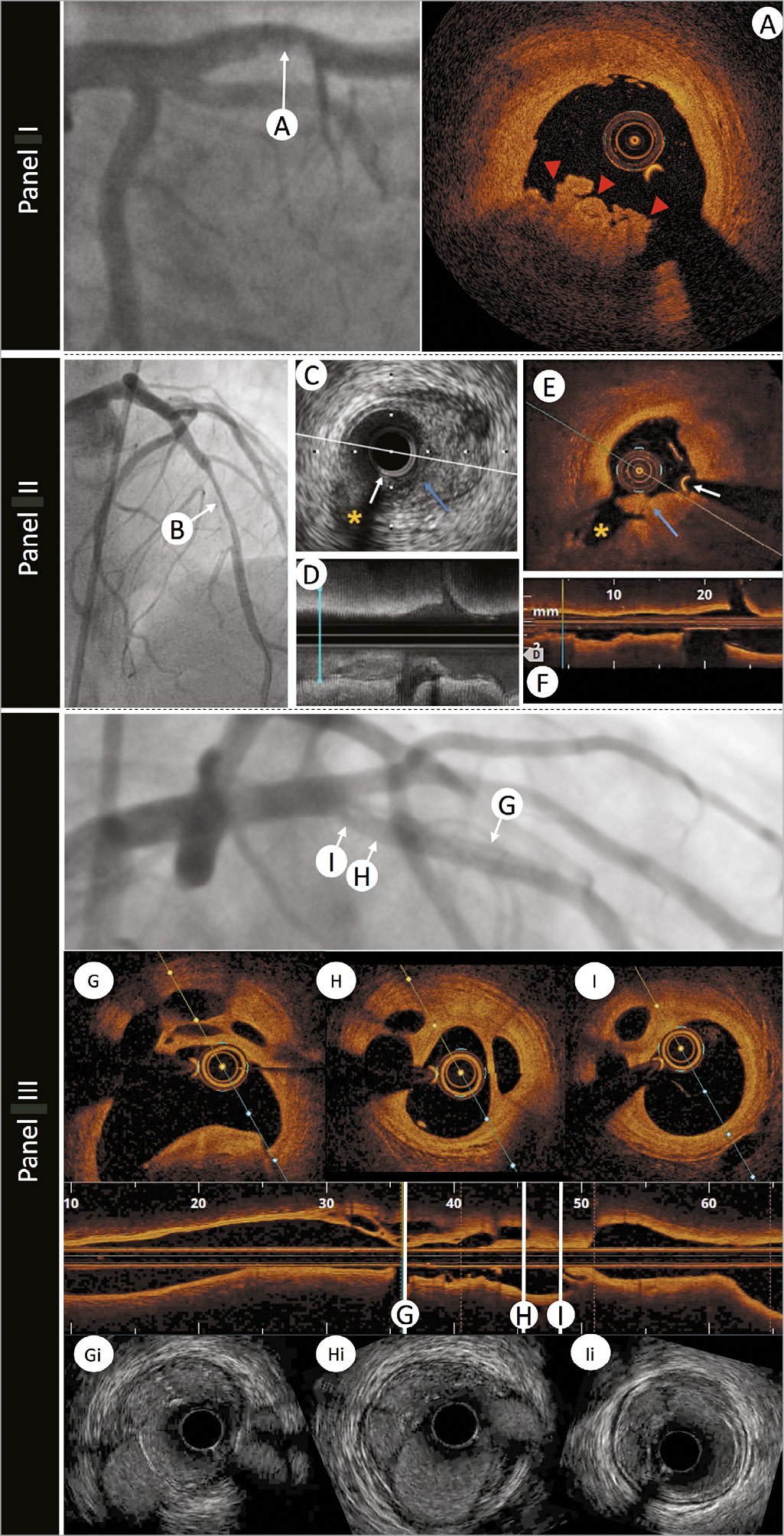
Figure 2. The role of intravascular imaging in delineating thrombus. Panel I: an angiographic image of a left anterior descending artery in a patient with ST-elevation myocardial infarction presentation and anterior ST-segment elevation. A hazy filling defect is evident in the proximal segment of the vessel, highlighted by white arrow A. Optical coherence tomography image (A) demonstrates red thrombus (red arrows) with an irregular surface and adherent to the lumen, attenuating the light, and obscuring deeper structures. Panel II: a 45-year-old woman was admitted with chest pain and anterior ST-elevation. Emergent angiography revealed a filling defect in the mid-left anterior descending artery. After thrombus aspiration angiography showed a tubular stenosis in the mid-left anterior descending artery (B) that was investigated with intracoronary imaging to determine the substrate of the acute coronary syndromes. Corresponding intravascular ultrasound and optical coherence tomography images are shown. (C and D) Cross-sectional and longitudinal intravascular ultrasound images (40 MHz) demonstrating the presence of atherosclerotic plaque (visible in C from 2 to 6). Intraluminal material protruding towards a small side branch was visible (blue arrow). Optical coherence tomography (E and F) confirmed the presence of atherosclerosis (with lipid content given the attenuation observed) and demonstrated the presence of white thrombus (irregular mass protruding into the lumen with optical shadow).White arrow indicates the guidewire artefact. Asterisk indicates the side branch used for matching of corresponding cross-sections. Panel III: left anterior descending artery with mid-vessel filling defects secondary to a conservatively managed anterior ST-elevation myocardial infarction 10 years earlier. Longitudinal optical coherence tomography imaging with three representative optical coherence tomography frames (G, H, and I) demonstrating re-canalized thrombus. Matched HD-IVUS (Boston Scientific) images (Gi, Hi, and Ii) demonstrating the superior delineation of structures with light-based imaging.
LESION MORPHOLOGY IDENTIFICATION IN ACUTE CORONARY SYNDROME AND IMPLICATIONS FOR TREATMENT
Rupture of a thin-cap fibroatheroma (TCFA) with associated thrombus formation has been the historical focus of attention in ACS and treatment has been tailored to stabilize this plaque type.15 However, pathology series and prospective studies conducted with intracoronary imaging demonstrated that one-third of all ACS and one-quarter of STEACS are caused by plaques with an intact fibrous cap16,17 –the majority identified as eroded plaques and a small cohort of calcific nodules. Intravascular imaging, in particular OCT, has enabled identification of these atherothrombotic features in patients presenting with ACS, shedding light on in vivo mechanisms and suggesting tailored therapeutic interventions, as outlined in the subsequent sections. Despite the superior resolution of OCT, enhancing plaque identification, the presence of thrombus in the acute setting can obscure the underlying vessel wall preventing plaque classification in >20% of cases.18 In parallel with identification of culprit plaques, intracoronary imaging has increased the awareness and diagnosis of nonatherosclerotic ACS events, discussed in Non-Atherosclerotic ACS Presentations section.
PLAQUE RUPTURE
Plaque rupture is defined by discontinuity of the fibrous cap overlying a lipid-rich core (Figure 3). Ruptured fibrous cap-ACS is commonly associated with a vessel wall cavity, without IVUS or OCT signal, generated through downstream embolization of the necrotic core. Thrombi are often found overlying the ruptured segment. However, thrombus may be absent at the site of an old plaque rupture or with fresh rupture treated with anti-thrombotic/anti-coagulant therapies. Multiple plaque rupture sitesmay be evident and differences between culprit and non-culprit lesions have been observed, with the presence of thrombus, smaller luminal area, and greater plaque burden associated with culprit lesions.19
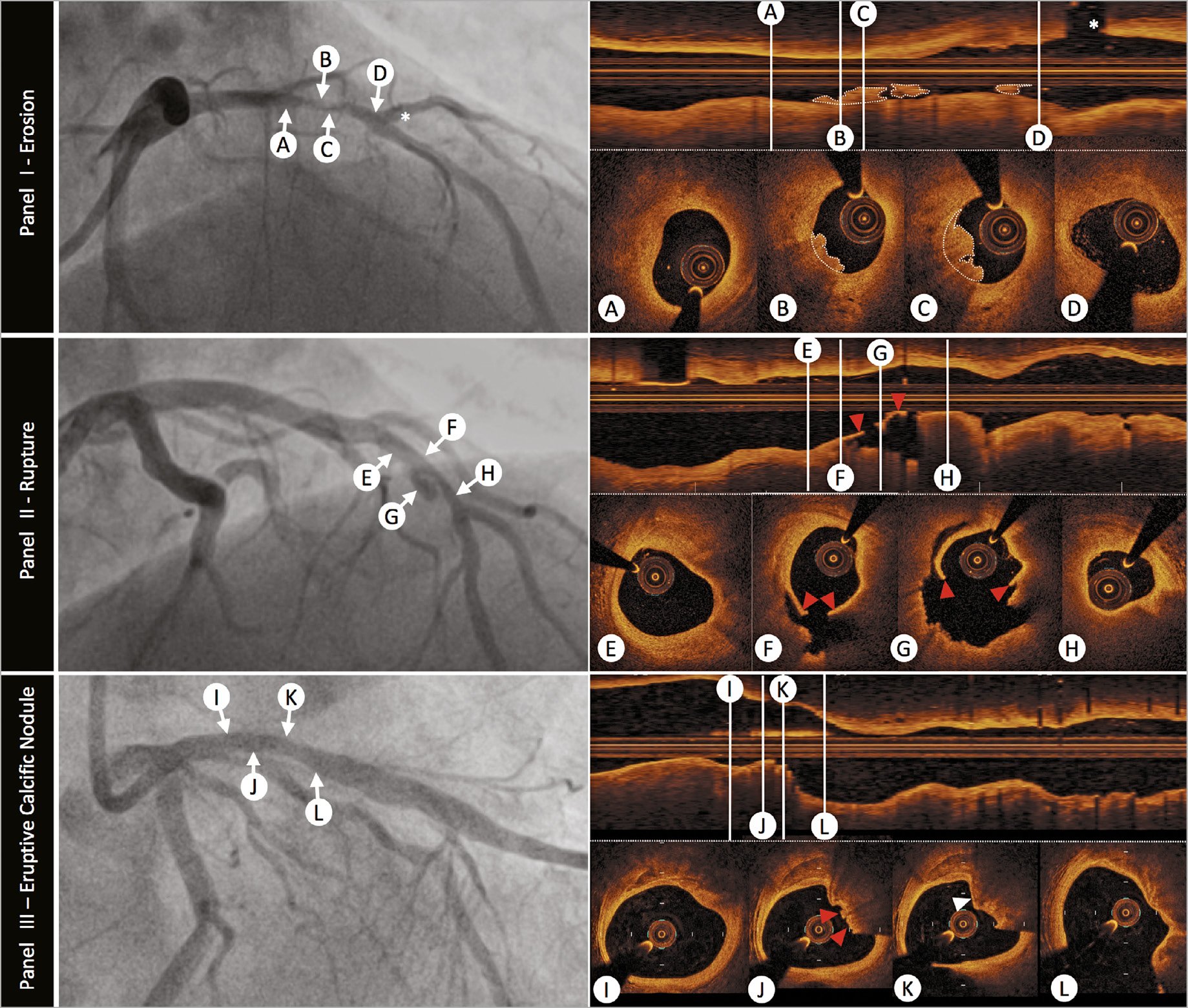
Figure 3. The role of optical coherence tomography to define atherosclerotic acute coronary syndrome plaque aetiology. Panel I: plaque erosion –angiographic image shows a severe stenosis in the mid-left anterior descending coronary artery (indicated by arrows B and C). Serial intravascular optical coherence tomography cross-sectional images indicate that no rupture is detected in the culprit lesion. Optical coherence tomography-erosion is identified as an irregular lumen surface with attached mural thrombus (white dotted outline on cross-sections and longitudinal image) overlying a fibrous plaque (B and C). Cross-sectional images indicate thick-cap fibro-atheroma proximal (A) and distal (D) to thrombus, immediately before a major diagonal branch (white asterisk). Panel II: plaque rupture –angiogram demonstrates a culprit lesion in the left anterior descending coronary artery (indicated by arrows F and G). Plaque rupture is identified on cross-sectional and longitudinal optical coherence tomography images by the disrupted fibrous-cap (red arrowheads) and a cavity formation inside the plaque (F and G). Cross-sectional images indicate optical coherence tomography-defined thin-cap fibroatheroma in the proximal (E) and distal (H) segments of the culprit lesion. Panel III: eruptive calcific nodule –angiography demonstrates a moderate lesion in the proximal left anterior descending artery (indicated by arrows J and K). Optical coherence tomography evaluation of the vessel confirms calcific infiltration of the vessel wall. The distinct margins of a superficial calcific sheet are demonstrated in Panel III-I from 11 o’clock to 2 o’clock. Disruption of the luminal contour with overlying red thrombus (red arrowheads), resulting in image attenuation (12-3 o’clock) is evident in Panel III-J. Immediately downstream the irregular protrusion of the calcific nodule is better delineated (Panel III-K white arrowhead). It is important to note that the nodule generates significant attenuation, obscuring deeper vessel structures, and this can result in misclassification as red thrombus. Endothelial integrity is confirmed more distally (Panel III-L).
PLAQUE EROSION
Plaque erosion is characterized by endothelial denudation, a poorly understood pathological process occurring at a level that is undetectable by current intracoronary imaging modalities. Only OCT has been successfully used, in clinical practice, to identify plaque erosion, although the diagnosis is one of exclusion, where thrombus is associated with non-disrupted plaque. An OCT diagnosis of plaque erosion is considered ‘definite’ in the absence of fibrous cap disruption, in a lesion frequently composed of fibrous tissue, with overlying luminal white thrombus. A ‘probable’ OCT-erosion may lack luminal thrombus but demonstrates an irregular luminal surface, or has overlying thrombus with attenuation of the underlying plaque, without evidence of superficial lipid or calcification in the vessel upstream or downstream of the thrombus site16 (Figure 3). Effective blood clearance is very important to minimize the potential for misdiagnosing OCT-erosion secondary to streaming of blood, particularly when in contact with the lumen wall.
A recent analysis of 51 patients with ACS (STE-/NSTE-ACS) undergoing three-vessel OCT assessment20 demonstrated that patients with erosion, vs. plaque rupture, had a lower percentage of TCFA, smaller lipid burden, and thicker fibrous cap. A large prospective series of 822 STEACS patients investigated the predictors of plaque erosion by OCT.21 Overall, plaque erosion tends to occur with greater frequency in younger patients, especially pre-menopausal women. Additionally, current smoking, absence of traditional coronary risk factors, lack of multi-vessel disease, reduced lesion severity, larger vessel size, and nearby bifurcation were significantly associated with plaque erosion. Nearby bifurcation and current smoking were especially notable in men, while age <50 years was most predictive in women. Existing data suggest that plaque erosion is associated with better outcomes than plaque rupture.22,23
Identification of plaque erosion may facilitate tailoring of patient treatment. A proof-of-concept study, including 60 patients with short (1 month) follow-up, has demonstrated that plaque erosion associated with a residual diameter stenosis <70% may be treated conservatively, with anti-thrombotic and anti-platelet therapy, allowing avoidance of stent deployment.17 A randomized control study is needed to further explore this concept.
ERUPTIVE CALCIFIC NODULE
Discrete calcific nodule with associated plaque disruption is the least frequently observed substrate for ACS but can pose significant challenges for stent deployment and optimization. The process was first identified by IVUS24 with subsequent pathological studies demonstrating that eruptive calcific nodules are responsible for 2-7% of acute coronary events.25 The lesions exhibit breaks in a calcified plate that disrupt the fibrous cap and are overlaid by thrombus.26 Imaging of erupted calcific nodule is possible with IVUS and OCT, with OCT providing superior detection of thrombus, delineation of superficial and deep boundaries of calcium and plaque disruption (Figure 3). However, there are limitations to OCT imaging, for example, the presence of protruding calcium can pose challenges in tissue differentiation, particularly through attenuation of deeper structures resulting in mis-representation as red thrombus and potential misdiagnosis of an acute culprit event. Similarly, distinguishing lipid core from calcium, if the boundaries are ill-defined, or detecting calcium when there is overlying thrombus can be better achieved with IVUS and virtual histology (VH)-IVUS.27
Histopathological comparison has demonstrated that OCT can differentiate various types of coronary calcification and accurately detect calcific nodules. In clinical OCT studies, an eruptive calcific nodule has been defined as a lesion that exhibits evidence of fibrous cap discontinuity and/or thrombus, over a calcified plaque characterized by protruding calcification into the lumen, and the presence of substantive calcium proximal and/or distal to the lesion.16 Intervention in ACS patients presenting with eruptive calcific nodules is associated with higher target lesion revascularization rates, highlighting the complex nature, and challenging treatment of this lesion subset.28 Recently, analysis of a large core laboratory OCT series has proposed additional calcific lesion substrate for ACS, specifically superficial calcific sheets, which were associated with greater post-PCI myocardial injury.29
Delineating the nature of calcific ACS lesions with intracoronary imaging guides adjunctive therapy, including vessel preparation with aggressive pre-dilatation, cutting balloons, rotational or orbital atherectomy, laser therapy, or lithotripsy. Further studies are required to guide selection of plaque modification strategy according to calcium substrate, to ensure effective post-stent optimization, as outlined in Part 1.1
NON-ATHEROSCLEROTIC ACUTE CORONARY SYNDROME PRESENTATIONS
Myocardial infarction with non-obstructed coronary arteries
Confirmation of non-obstructed coronaries in patients presenting with ACS is often considered a reassuring finding. However, MINOCA is not benign. Systematic review has demonstrated a 12-month mortality of 4.7%,30 which far exceeds comparative rates in an equivalent population without ACS. The ESC working group on cardiovascular pharmacotherapy have outlined a differential list including angiographically undetectable plaque disruption, coronary artery spasm, coronary thromboembolism, spontaneous coronary artery dissection (SCAD), takotsubo syndrome, and myocarditis.31
A study of women presenting with myocardial infarction (MI) and non-obstructed coronaries revealed plaque disruption in 38% of those undergoing IVUS evaluation.32 Additionally, the superior resolution of OCT has the potential to detect a thromboembolic or vasospastic aetiology, if thrombus is observed in the absence of atherosclerosis or luminal irregularity (Figure 4). Clearly, the differentiation between a plaque-induced event and the presence of embolic thrombus, despite angiographically non-obstructed coronary arteries, significantly alters the acute and long-term patient management. Consequently, we advocate undertaking intracoronary imaging at the time of index angiography, if there are non-obstructive coronary lesions or if the clinical presentation does not favour other noncoronary MINOCA aetiologies (e.g. myocarditis). Usually imaging will be limited to epicardial territories with coronary lesions or associated ECG/echo features of ischaemia, however, three-vessel imaging may be considered (Figure 1).
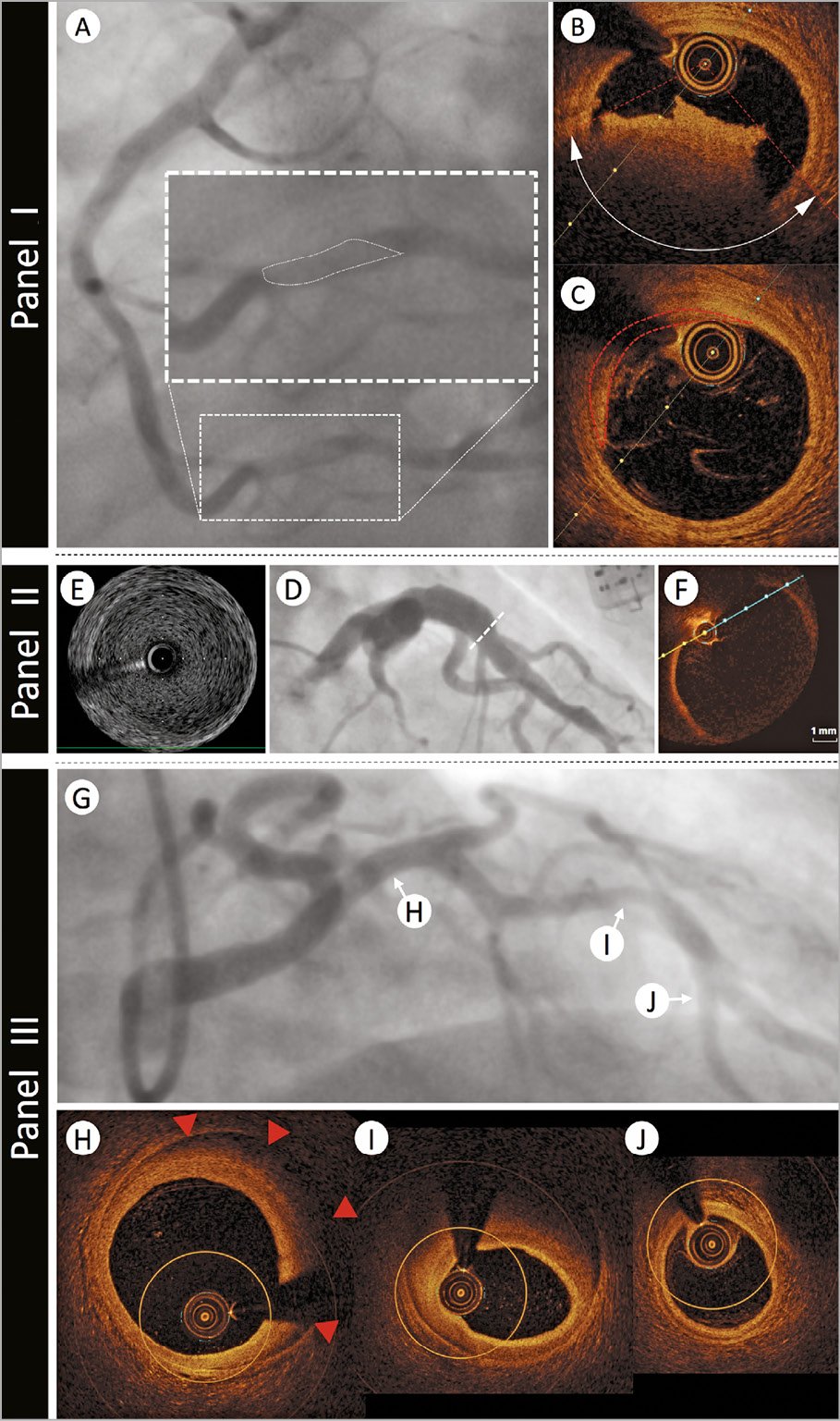
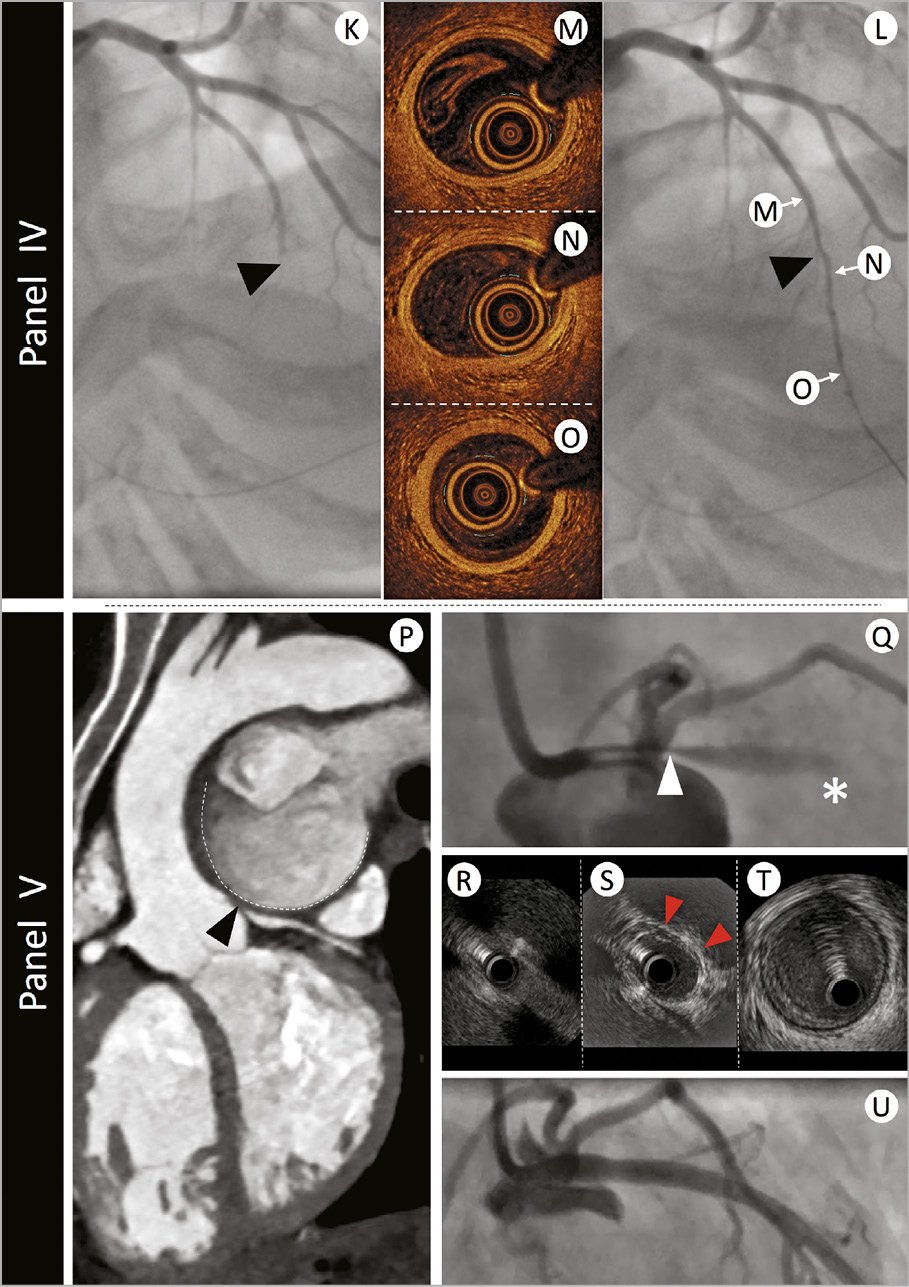
Figure 4. The role of intravascular imaging in non-atherosclerotic acute coronary syndrome presentations. Panel I: a 39-year-old man with no cardiovascular risk factors presented with acute onset chest and abdominal pain and evidence of inferior ST elevation. Immediate angiography demonstrated a filling defect in Segment 3 of the right coronary artery (Panel I-A - filling defect highlighted in enlarged panel by white dotted outline).Optical coherence tomography assessment confirmed fresh red thrombus with associated attenuation [Panel I-B - red dotted lines indicate margins of attenuation (white double arrow head arc)]. Neighbouring regions demonstrated minimal pathological intimal thickening with a tail of thrombus (Panel I-C - red dotted outline). The patient was commenced on a glycoprotein 2b/3a inhibitor and symptoms settled. Further evaluation of his abdominal pain revealed acute ischaemia of his small bowel secondary to thrombotic occlusion. Panel II: a 44-year-old man with history of childhood Kawasaki disease with coronary involvement presented with stable symptoms of angina and underwent investigation by coronary angiography. Panel II-D demonstrates proximal aneurysmal disease of the left anterior descending artery. Intravascular ultrasound evaluation (Panel II-E) highlights a large aneurysm 7 mm in diameter with minimal evidence of atheroma. The size of the aneurysm prevented accurate assessment by optical coherence tomography (Panel II-F). Panel III: A 39-year-old woman with a history of hypertension presented with acute onset chest pain, minor troponin elevation, and dynamic anterior t wave changes on her electrocardiogram. Angiographic assessment (Panel III-G) revealed mild-moderate calibre reduction in the mid-left anterior descending artery. Optical coherence tomography was undertaken to better delineate the nature of her angiographic abnormalities. Panel III-H demonstrates evidence of intima-medial detachment with intra-mural haematoma (red arrowheads) in a segment of the proximal left anterior descending artery that appeared angiographically normal. More extensive intramural haematoma with reduction in lumen calibre was evident at the level of the angiographic stenosis (Panel III-I). Optical coherence tomography analysis of the distal left anterior descending artery segment confirmed normal vessel architecture (Panel III-J). The patient was treated conservatively and made an excellent recovery. Panel IV: a 42-year-old woman without cardiovascular risk factors presented with acute onset chest pain and evidence of anterior ST elevation. Immediate angiographic assessment (Panel IV-K) revealed mid-vessel occlusion of the left anterior descending artery (black arrowhead). Passage of an 0.014” interventional guidewire resulted in recanalization of the vessel (Panel IV-L) and resolution of the ECG changes. Optical coherence tomography evaluation was undertaken to better delineate the aetiology of presentation. No significant vessel abnormalities were detected proximal (Panel IV-M), at the level of occlusion (Panel IV-N), or in the distal vessel segment (Panel IV-O). Panel V: a 47-year-old woman with secundumatrial septal defect and Eisenmenger’s syndrome presented to an emergency department with rapidly worsening dyspnoea and palpitations. Electrocardiogram demonstrated sinus rhythm, normal repolarization, and multiple polymorphic ventricular extrasystoles. The echocardiogram showed normal left ventricle ejection fraction with only mild hypokinesia of the mid and distal anterior left ventricular wall. Immediate double rule-out computed tomography scan (Panel V-P) demonstrated extrinsic compression of the LMS (black arrowhead) from a giant pulmonary artery aneurysm (contour outlined with dashed white line). Urgent angiography (Panel V-Q) confirmed a severe ostial stenosis of the left main stem (LMS - white arrowhead) and TIMI-1 flow in the left anterior descending artery (white asterisk). A percutaneous interventional strategy was adopted and intravascular ultrasound evaluation was undertaken to appropriately size the LMS. Intravascular ultrasound confirmed dynamic compression of the LMS with complete occlusion of the ostium (Panel V-R), evidence of extrinsic compression (red arrowheads) in the shaft (Panel V-S), and normal vessel in the distal LMS segment (Panel V-T). The patient proceeded to intravascular ultrasound-guided intervention with an excellent result (Panel V-U).
Intravascular ultrasound evaluation of vasospastic coronary segments has demonstrated underlying evidence of atherosclerotic lesions with a high incidence of negative arterial remodelling33 and relative absence of calcium.34 An OCT study, including patients presenting with ACS, demonstrated lumen irregularity with thrombus in 25% and evidence of plaque erosion in 26%, supporting the need for anti-platelet therapy in this challenging cohort.35
Takotsubo syndrome was originally described in the absence of CAD but the InterTAK criteria now highlight that CAD does not exclude a diagnosis.36 Intracoronary imaging is, in general, not essential for the diagnosis but may be valuable to exclude a culprit plaque event where there is evidence of angiographic atherosclerotic disease.37
Spontaneous coronary artery dissection
Spontaneous coronary artery dissection is observed in 2-4% of angiograms undertaken for ACS.38,39,40 In pre-menopausal women <50 years old presenting with STEACS, the angiographic prevalence of SCAD is 10.8%.39 The angiographic characteristics can vary considerably, and a classification system has been proposed.41,42 Type 1 represents the classical linear coronary defect with potential contrast hold-up, however, occurs in <50% cases. Type 2 defects are more commonly observed, either with an abrupt calibre reduction and subsequent normalization (Type 2a) or with persistent calibre reduction to the distal vessel (Type 2b). Spontaneous coronary artery dissection can also mimic coronary atherosclerosis (Type 3 – Figure 4) or simply present with abrupt vessel closure (Type 4). The findings from intravascular imaging studies have increased the interventional community’s awareness of SCAD.43 Intravascular imaging has provided insights into the aetiology, with evidence of separation of the intima and media from the adventitia, with or without communication with the vessel lumen44 and can assist in confirming the diagnosis and guiding treatment.45 However, a significant proportion of SCAD can be diagnosed angiographically, thereby limiting instrumentation of a dissected vessel that carries risks of dissection propagation and vessel closure. Therefore, when possible, a conservative treatment approach to treatment should be adopted when flow is restored.
The recent ESC/ACCA position paper on SCAD supports the role of intravascular imaging where a diagnosis by angiography is uncertain.46 If PCI is deemed necessary due to ongoing ischaemia or clinical instability, it is important to acknowledge that there is an increased risk of procedural complications, consequently imaging may provide important guidance, in particular confirmation of the wire position in true lumen, the longitudinal extent of the vessel disruption and vessel dimensions for stent sizing, which can be problematic angiographically, in the presence of extensive intramural haematoma.
Both IVUS and OCT can be used to facilitate diagnosis of SCAD, and both modalities have strengths and weaknesses. Obvious concerns exist regarding the need for contrast injection to achieve OCT imaging, and we would advise that IVUS is preferred where there is evidence of a false lumen (Type 1), and in small calibre and tortuous vessels, where the imaging probe risks being occlusive. Additionally, the imaging penetration depth of IVUS can be advantageous in proximal vessel dissections, where the false lumen stretches the external elastic lamina, increasing the vessel size. However, IVUS resolution can be insufficient for the detection of intima-media complex fenestrations. Despite the vessel disruption associated with SCAD, OCT can be undertaken, where diagnostic uncertainty exists (typically Types 3 and 4) and provides greater diagnostic clarity than IVUS in assessing the distinctive features of intramural haematoma ± intimal flap46,47 (Figure 1).
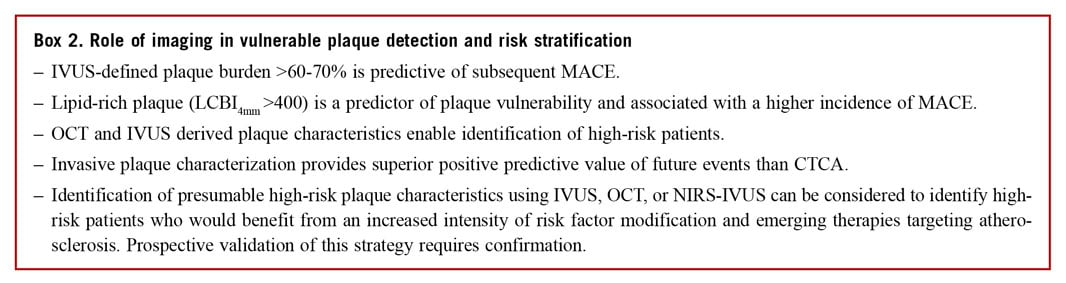
ROLE OF INTRAVASCULAR IMAGING IN RISK STRATIFICATION AND VULNERABLE PLAQUE DETECTION
The term ‘vulnerable plaque’, first coined by Muller et al48 in 1980s was used to describe plaques that are prone to rupture and caused events. Several autopsy studies have shown that the majority of ruptured plaques have a specific phenotype, namely the TCFA. This is characterized by an increased plaque burden,49 positive remodelling, a large lipid core covered by a thin fibrous cap,50 macrophage accumulation,51 and the presence of neovascularization.52
Intravascular imaging enables in vivo evaluation of plaque composition and burden, identifying plaque characteristics associated with increased vulnerability. Numerous histology imaging validation studies have examined the efficacy of different invasive imaging modalities and highlighted the advantages and limitations of these techniques in assessing plaque burden, morphology, and biology53 (Supplementary Table 2). In parallel, several prospective large-scale clinical studies have evaluated the potential value of intravascular imaging in identifying vulnerable plaques and patients who are at risk of suffering cardiovascular events.54,55
INVASIVE DETECTION OF VULNERABLE PLAQUES
PROSPECT and VIVA were the first prospective studies that used three-vessel IVUS imaging to examine it is efficacy in detecting nonculprit lesions that are likely to progress and cause cardiovascular events.54,55 In PROSPECT, a minimum lumen area ≤4 mm2, a plaque burden ≥70%, and the presence of a TCFA phenotype, derived by VH-IVUS, were predictors of subsequent non-culprit MACE. Virtual histology defined TCFA requires >10% confluent necrotic core on three consecutive frames and an arc of necrotic core in contact with the lumen surface for ≥36°, reflecting the inadequate spatial resolution of IVUS to visualize thin fibrous cap.56 Lesions with these highrisk plaque characteristics were eleven times more likely to cause events within a 3.4-year follow-up than simple lesions [hazard ratio (HR): 11.05, 95% confidence interval (CI): 4.39-27.82; P<0.001]; however, the positive predictive value of these three high-risk plaque features for subsequent events was low (18.2%).54
The limited efficacy of VH-IVUS imaging in identifying vulnerable lesions has partially been attributed to limitations of the modality to detect plaque composition.57 To overcome this drawback combined NIRS-IVUS imaging has been proposed. Near infrared spectroscopy imaging relies on the spectroscopic analysis of the backscattered light emitted by a NIR probe which provides information about the cholesterol content in the arterial wall. The output of this analysis is displayed in the chemogram which is a colourcoded map of the probability of the presence of lipid-rich plaques (LRPs). Lipid-rich plaques were originally defined as a lipid core >60° in circumferential extent with >200 mm depth and an overlying fibrous cap thickness <450 µm.58 A measure of the lipid burden is the lipid-core burden index (LCBI): it is calculated by dividing the number of yellow pixels by the total number of pixels available, multiplied by 1000 (LCBI ranges from 0 to 1000). The LCBI4mm is used to quantify the maximal regions of LRP within the interventional target region, divided into 4 mm coronary segments.59 Combining NIRS with IVUS facilitates accurate and less operator dependent detection of LRP with visualization of the lumen and plaque architecture57,60 (Figure 6). To date, there are four prospective studies demonstrating high LCBI as a strong predictor of coronary events on a patient level using either LCBI or LCBI4mm to predict subsequent MACE.61,62,63,64
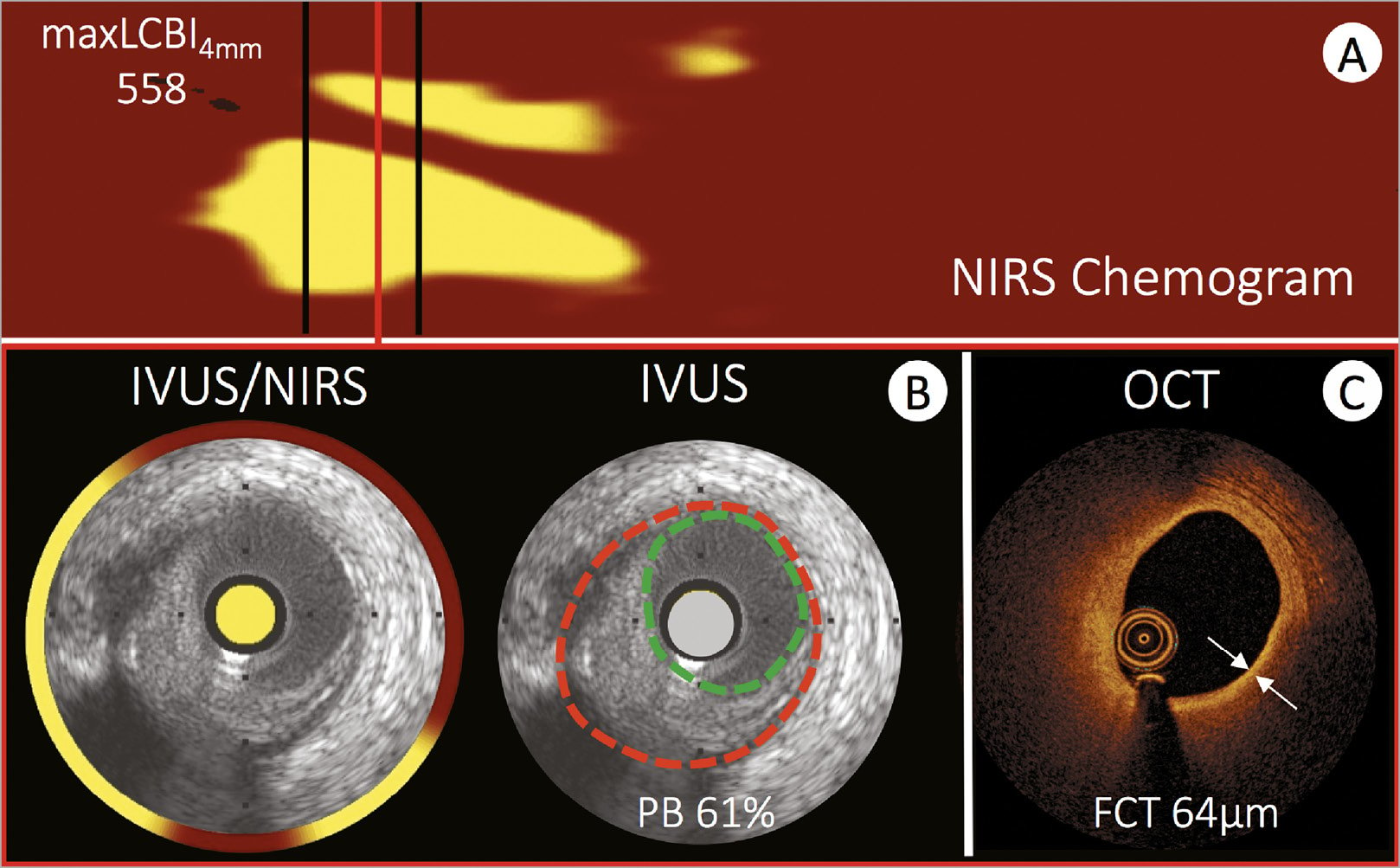
Figure 6. Intravascular imaging evaluation of a high-risk coronary plaque. An example of high-risk coronary plaque evaluated using all three available intravascular imaging modalities. (A) A near infrared spectroscopy chemogram of a bystander vessel segment in a patient presenting with acute myocardial infarction. Yellow areas represent lipid and the region between the black lines highlight the region with a maximal lipid-core burden index of 558. Matched cross-sectional images from intravascular ultrasound to near infrared spectroscopy, greyscale intravascular ultrasound (B) and optical coherence tomography (C) highlight the differences in vessel evaluation with each modality. The circumferential near infrared spectroscopy output highlights lipid signal extending from 4 o’clock to 11 o’clock (interrupted by guidewire artefact at 6 o’clock). The intravascular ultrasound greyscale image facilitates assessment of plaque burden (EEM: red, lumen: green), which amounts to 61%. Optical coherence tomography (C) of the same plaque shows the fibrous cap as a signal rich luminal layer from 3 o’clock to 12 o’clock and a light attenuating signal poor region behind. The fibrous cap thickness amounts to 64 lm. All methods are consistent with a lipid rich fibroatheroma and optical coherence tomography in addition indicates the presence of a thin cap fibroatheroma.
The LRP study was the first prospective large-scale imaging study that examined the efficacy of thismodality in detecting vulnerable plaques on a lesion level. The study enrolled 1241 patients with stable angina (46.3%) or acute coronary syndrome (53.7%) and assessed more than 5000 lesions with NIRS-IVUS.65 Lesion-level analysis demonstrated that the presence of LRP (LCBI4mm >400) was associated with a four-fold higher event rate (HR: 4.11, 95% CI: 2.3-7.34; P<0.001). Also, the adjusted patient-level analysis showed that for each 100 unit increase of maxLCBI4mm the risk of non-culprit MACE increased by 18% and patients with maxLCBI4mm greater than 400 were at 87% higher risk of non-culprit MACE at 24-month follow-up.66 Similarly, a retrospective OCT study demonstrated that OCT detected non-culprit LRP increased the risk of non-culprit MACE, with a more modest two-fold increase in risk (HR: 2.06, 95% CI: 1.05-4.04; P=0.036).67
These studies have demonstrated the diagnostic potential but also highlighted the limitations of intravascular imaging in detecting vulnerable lesions. The complex nature of the studies led to significant levels of patient exclusion due to incomplete data and failed matching of temporal imaging (only 53% of lesions that caused events during follow-up in the PROSPECT trial were studied by IVUS at baseline). Additionally, it is important to recognize that procedural complications were reported in association with multi-vessel imaging (0.3-1.6%67,68,69). In the light of these limitations and an absence of data to support interventional passivation of vulnerable lesions, the routine clinical use of invasive imaging for vulnerable plaque detection cannot be recommended. Studies testing the potential value of vulnerable plaque detection (COMBINE OCT-FFR, NCT02989740; PROSPECT II, NCT02171065) and plaque sealing (PROSPECT II, NCT02171065; PREVENT, NCT02316886) are ongoing.
ROLE OF INTRAVASCULAR IMAGING IN RISK STRATIFICATION
Four prospective studies examined the potential value of intravascular imaging in identifying high-risk patients. In the ATHEROREMO-IVUS study, that included 581 patients who had coronary angiography for clinical purposes and single-vessel VH-IVUS imaging, patients that had lesions with a TCFA phenotype and an increased plaque burden (>70%) had a higher incidence of MACE at 1 year follow-up.70 However, in this study, the event rate was low and thus it was not possible to examine whether VH-IVUS provided additional prognostic information than the well-known clinical risk factors. In addition, the LRP study and the ATHEROREMO-NIRS sub-study showed that plaque composition, and in particular an increased LCBI was associated with worse prognosis.63,66 Finally, in the CLIMA study (presented as a late breaking clinical trial at EuroPCR 2018) that included 1003 patients who were referred for coronary angiography and underwent OCT imaging in a non-diseased proximal left anterior descending coronary artery, the hard composite Endpoint of cardiac death and target vessel MI was 7.5× higher in patients who had lesions with a TCFA phenotype, lipid arc >180°, minimum lumen area <3.5 mm2 and macrophages accumulations, compared with those with plaques without high-risk characteristics (18.9% vs. 3.0%). Non-invasive evaluation of high-risk plaque has obvious advantages in minimizing procedural risks and has demonstrated excellent negative predictive value. However, the positive predictive value of computed tomography (CT) coronary angiography is poor, limiting its role in the prediction of acute coronary events (Figure 5).

Figure 5. A comparison of the positive and negative predictive values of intravascular and non-invasive imaging modalities. Summary of the positive and negative predictive values of coronary imaging-derived variables for prediction of clinical outcomes in the PROSPECT, ATHEROREMO-IVUS, PREDICTION, ATHEROREMO-NIRS, CLIMA, and PROMISE studies. AP, angina pectoris; CACS, CT angiography calcium score; CD, cardiac death; CTA, computed tomography angiography; ESS, endothelial shear stress; LCBI, lipid-core burden index; MACE, major adverse cardiac events; MI, myocardial infarction; MLA, minimal lumen area; PB, plaque burden; PCI, percutaneous coronary interventions; TCFA, thin-cap fibroatheroma; TVMI, target vessel-MI. Adapted from Koskinas et al.71
Identification of high-risk/large burden plaque should trigger intensification of secondary preventative treatment, including lifestyle modification and tailoring of anti-atherosclerotic medication. However, further research is required to confirm the incremental value of intravascular imaging over clinical variables in risk stratification and subsequent treatment guidance.72
Imaging of angiographically ambiguous coronary findings
Invasive coronary angiography remains the most commonly used imaging modality to assess the coronary vasculature. Angiographic ambiguity in the stable population tends to reflect excessive plaque burden (undetectable by angiography), calcification (see Part 11) old (clinically silent) plaque rupture, coronary tortuosity, or aberrant vessel anatomy. Intravascular imaging provides clarity where the angiogram demonstrates haziness, an eccentric or unexpected lesion or prevents delineation of a lesion through aneurysmal/ectatic or overlapping vessels. The availability of angiographic co-registration has facilitated reliable analysis of the intracoronary image acquisition; however, it is important to acknowledge that interpretation requires experience and expertise.
PLAQUE BURDEN
Intravascular ultrasound facilitates a thorough assessment of the entire vessel wall and demarcation of the luminal contour and external elastic lamina allows quantification of plaque burden (Figure 6). It requires a level of IVUS expertize to assess these plaque characteristics especially in the presence of calcific plaques that mask the external elastic lamina borders.
The highly attenuating nature of a lipid pool and necrotic core, as well as the relatively low penetration of OCT, limits its ability to visualize deeper vessel structures and consequently plaque burden cannot reliably be measured. However, work has been undertaken to find surrogates for plaque burden/vessel area with quantification of quadrants of plaque, generating a measure of plaque free wall angle. A plaque free wall angle >220° has been shown to predict an IVUS plaque burden <40% with PPV 78% and NPV 84%.73 Similarly, quantification of LRP by OCT, with measurement of lipid arc and lipid length has been shown to predict MACE, need of revascularization and recurrent ischaemia.67
Part 1 of the consensus document outlined the importance of delineating plaque burden to guide stent placement, aiming to avoid areas with >50% plaque burden.1 Furthermore, both PROSPECT and the ATHEROREMO-IVUS studies have confirmed an association between the presence of plaque burden >70% in non-culprit vessels with higher MACE rates.70,74 Therefore, detection of plaque is important to guide optimal invasive and non-invasive treatment.
CORONARY ANEURYSMS AND ECTASIA
Defining aneurysmal or ectatic coronary segments can be challenging angiographically, especially as most recognized definitions require comparison against a ‘normal’ vessel segment.75,76,77 Consequently, recognition of aneurysmal and ectatic disease by adult cardiologists has been frequently overlooked.78 Historical IVUS evaluation of aneurysmal disease highlights the weakness of angiographic interpretation, as only a third of patients had the IVUS appearance of true or pseudo-aneurysmal disease. In the majority of patients the ‘aneurysmal’ segments represent complex plaque or normal vessel neighbouring significant stenoses.79 Therefore, where uncertainty exists it is prudent to consider intravascular imaging to clarify the underlying vessel morphology. In aneurysms exceeding a diameter of ~5 mm, IVUS is the preferred modality due to its depth of penetration, facilitating evaluation of large vessel dimensions (Figure 3).
Kawasaki disease is the best described cause of coronary aneurysm and the leading cause of acquired heart disease in children in developed countries.80 Surveillance of aneurysmal coronary disease is best achieved non-invasively80; however, the incidence of undisclosed aneurysmal disease, discovered at the time of coronary angiography, has a reported rate of 3-5%.78,81 Kawasaki disease patients may present with ischaemic symptoms precipitated by the development of stenoses at the outlet of aneurysms or giant aneurysm thrombosis.82 Intervention in these patients can be challenging due to calcification of the aneurysm and difficulties in assessing the true luminal dimensions,83 and the use of IVUS should be considered to guide intervention.80
AORTO-OSTIAL AMBIGUITY
The aorto-ostial junction is a unique segment of the coronary arterial tree and provides both diagnostic and interventional challenges. The angle of take-off of the proximal coronary vessels, from the aorta, can result in the angiographic appearance of a significant stenosis and functional assessment may be difficult to perform. For these reasons, morphological assessment [confirmation of atherosclerotic disease/assessment of the minimal lumen area (MLA) (see Role of Intravascular Imaging for Assessment of Lesion Severity section)] should be considered to better determine the ischaemic potential. Furthermore, the aorto-ostial position offers unique tissue characteristics with greater amounts of elastic tissue increasing the risk of stent recoil and subsequent failure.84 Achieving a blood-free field for imaging is challenging in aorto-ostial lesions, consequently IVUS is recommended. Co-axial positioning and disengagement of the guide minimizes over-estimation of the MLA or mis-interpretation of true ostial disease, respectively.85
Angiographic assessment can also be impacted by the anomalous take-off of the coronary vessels or extrinsic compression. There are many patterns of coronary artery anomaly, however, a ‘malignant course’ at risk of ischaemia or sudden cardiac death is associated with an inter-arterial course (i.e. between the aorta and pulmonary artery).86 Most often these anomalies are discovered incidentally in later life, with increasing frequency now that CT angiography is widely used.87 Occasionally younger patients with syncope or angina may require confirmation of the flow limiting potential of the compressive mechanism.88 IVUS may be useful to confirm extrinsic compression and a slit-like lumen. Coronary compression can also result from dilatation of the pulmonary artery due to pulmonary hypertension,89 acute aortic dissection,90 or following heart surgery91 and invasive imaging may be required to provide diagnostic clarity (Figure 4).
TRANSPLANT VASCULOPATHY
Cardiac allograft vasculopathy (CAV) is a leading cause of long-term mortality after heart transplantation. Routine surveillance for CAV is necessary to ensure early diagnosis, as patients are frequently asymptomatic due to allograft denervation. Although non-invasive imaging of the coronaries can be achieved with CT, there are limitations that prevent visualization of the distal vascular bed and image quality can be degraded by high resting heart rates.92 Coronary angiography remains the preferred screening tool for CAV but the diffuse, concentric nature of vasculopathy, combined with positive remodelling, can lead to a failure to diagnose CAV or an underestimation of disease severity. Intravascular imaging allows cross-sectional evaluation of the vessel wall and IVUS has been shown to provide prognostic information, with progression of intimal thickening ≥0.5 mm from baseline to 1 year associated with non-fatal MACE.93 Furthermore, VH-IVUS can provide further characterization of CAV.94 Consistent with findings in native CAD, IVUS-defined attenuated plaque has been shown to be associated with an increased event rate and is predictive of rejection.95 OCT offers higher resolution and initial experience suggests it will provide early detection of CAV and offer insights into the mechanisms underlying the process.96,97 The European Association of Cardiovascular Imaging recommends that IVUS is used in conjunction with angiography, where expertise is available, with a baseline study undertaken to assess for donor heart CAD and at 1 year after transplantation to detect rapidly progressive CAV98 and guide changes to immunosuppressive therapy.
Role of intravascular imaging for assessment of lesion severity
Percutaneous coronary intervention for stable angina is indicated for haemodynamically significant coronary stenoses with insufficient response to optimized medical therapy and/or the improvement of prognosis through treatment of proximal major epicardial coronary disease, multi-vessel disease with impaired left ventricular (LV) function, last remaining vessel, or the presence of significant ischaemia (involving >10% of LV mass).5 The limitations of coronary angiography, for delineating the true nature/extent of coronary atheroma, have already been outlined in Part 1.1 Pressure-derived invasive indices of coronary lesion significance have been accepted as the gold standard method of invasive ischaemia assessment, with a Class I, Level of evidence A, recommendation for the use of fractional flow reserve (FFR),5,99,100,101 or instantaneous wave free ratio (iFR).102,103 Attempts have been made, through comparison with FFR, to identify imaging-based vessel/lesion measurements predictive of ischaemia. Minimal lumen area is the intravascular imaging metric used to define flow-limiting stenoses; however, it has limitations to identify a single cut-off measurement due to variations in patient body mass, heart weight, lesion complexity, and the territory of ischaemic potential. Despite these limitations, in many cases intravascular imaging can detect significant stenoses overlooked by angiography or exclude pseudo-narrowings falsely detected angiographically. The limitations of pressure-derived functional indices for the assessment of left main lesion severity and the proven value of IVUS to guide PCI suggest a liberal diagnostic application.
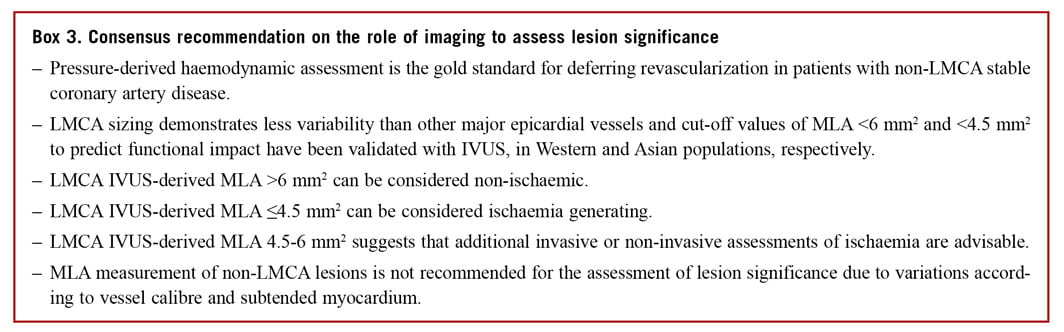
MINIMAL LUMEN AREA AS A PREDICTOR OF ISCHAEMIC POTENTIAL IN LEFT MAIN LESIONS
Interpretation of left main coronary artery (LMCA) disease requires special attention due to its prognostic importance. The aorto-ostial and distal branching pattern of the vessel often makes assessment of lesion significance challenging,104 and the interventional cardiologist must balance the need for confirmation of ischaemia with pressurederived assessment vs. an improved understanding of the anatomical characteristics of the lesion. Ideally, use of both FFR and intravascular imaging would provide a comprehensive assessment of a LMCA lesion but in the real world the equipment costs and time constraints limit this strategy. Additionally, physiological evaluation of the LMCA can be challenging, especially in ostial stenosis or situations with concomitant disease in the left anterior descending artery or LCX, and clinical trial data supporting its use in this specific setting is scant. Consequently, the use of imaging-derived measurements to predict ischaemia can offer benefits for the rationalization of procedural time and costs and was given a Class IIa recommendation in the most recent ESC guidelines onmyocardial revascularization.5
The proximal nature of the LMCA segment limits the variability of its calibre and anatomy, minimizing the impact of body mass and heart size on vessel and lesion measurements, however, a population variation in the LMCAMLA cut-off has been observed.105 An Asian study of IVUS-derived MLA detected a cut-off of 4.5 mm2, correlating with an FFR ≤0.80106; however, a previous US study observed that an IVUS MLA <5.9 mm2 has the best correlation with an FFR <0.75 (sensitivity 93% and specificity 94%).107 The average MLA in patients recruited to these studies differed by 2.8 mm2 (4.8 vs. 7.6 mm2, respectively). In addition to the geographical differences in LMCA size, IVUS has also demonstrated differences in the characteristics of LMCA disease, with a greater burden of atheroma but less calcification in Asian LMCA lesions.108
In a prospective application of the MLA cut-off, the LITRO study guided LMCA PCI by IVUS criteria and 6 mm2 was found to have the highest sensitivity and specificity for detecting ischaemia.109 At 2 years, the outcome of deferred patients was equivalent to that of the revascularized group. Importantly, the outcome of the few patients with LMCA MLA <6 mm2 that did not undergo revascularization was significantly worse. Consequently, it appears reasonable to defer LMCA revascularization if the MLA > 6 mm2, to intervene if the MLA <4.5 mm2 and to consider further evaluation with FFR if the MLA is between 4.5 and 6 mm2, with appropriate consideration of associated comorbidities (Figure 7). The additional advantages to intracoronary imaging-guided LMCA intervention have been addressed in the Part 1 document and includes the ability to reveal pseudo-stenoses at the ostium due to ostial spasm, incomplete vessel filling due to streaming and poor catheter alignment.

Figure 7. A schematic representation of the role of minimal lumen area assessment in the evaluation of left main coronary disease.
Limited data exists supporting the use of OCT for guidance of left main intervention and is not recommended for evaluation of ostial disease or short left main stems. Importantly, the MLA cut-off values established for IVUS assessment of the LMCA cannot be directly translated to OCT.
MINIMAL LUMEN AREA AS A PREDICTOR OF ISCHAEMIC POTENTIAL IN NON-LEFT MAIN LESIONS
Beyond LMCA disease, the variations in patient body mass, vessel calibre, and the subtended myocardium prevent the use of a single MLA cut-off value to define ischaemic potential and guide appropriate intervention. A meta-analysis of >2500 patients confirmed modest diagnostic accuracy for both IVUS and OCT for identification of haemodynamically significant lesions, with marked differences in the median cut-off values for both modalities [1.96 mm2 (1.85-1.98 mm2) for OCT and 2.9 mm2 (2.7-3.1 mm2) for IVUS (area under the curve 0.80 and 0.77, respectively)].110 Similar to the LMCA MLA assessment, a geographical variation has been observed with smaller measurements reported in Asian studies. Direct comparison of OCT, IVUS, and FFR has been reported and demonstrated slight superiority of OCT over IVUS, specifically in smaller vessels (<3 mm) secondary to better delineation of the luminal area.111,112 We recommend the use of pressure-derived assessment of lesion significance, but acknowledge that evaluation of the MLA datamay assist in decision-making where intracoronary imaging has been used.
Conclusion
Since the publication of Part 1 of the expert consensus, the ESC guidelines committee has acknowledged the results of randomized trials comparing OCT with angiography- and IVUS-guided PCI and elevated the recommendation for use of OCT in stent optimization from Class IIb to IIa.2 Furthermore, the first all-comers study of IVUS vs. angiography-guided PCI, appropriately powered to demonstrate clinical superiority, has reported, clearly favouring an imaging-guided approach.3 With an increasing complexity of both patient comorbidity and CAD, considered for percutaneous revascularization, the role of intravascular imaging to guide PCI optimization and improve longterm outcomes will continue to grow. Part 2 has outlined additional indications for the use of intravascular imaging to overcome some of the limitations posed by invasive coronary angiography. As yet, evidence from dedicated clinical trials to support many of these potential uses is lacking but among the community of imaging experts it is believed that intravascular imaging will facilitate a tailored approach to PCI for patients at high risk of recurrent events and stent failure.

