- coronary disease
- in-stent restenosis
- percutaneous coronary intervention
- paclitaxel-eluting stent
- registries
Abstract
Aims: To examine the incidence of clinical events after implantation of the TAXUS Express paclitaxel-eluting stent (PES) for in-stent restenosis (ISR) in an unselected patient population. Implantation of bare metal stents in native coronary vessels can result in ISR rates exceeding 50%. PES significantly reduces ISR-lesion restenosis and improves event-free survival versus vascular brachytherapy.
Methods and results: The 7,492-patient ARRIVE registry enrolled patients receiving ≥1 TAXUS Express stent at procedure initiation with no inclusion/exclusion criteria, including 489 patients with ISR. Endpoints were independently adjudicated. All major cardiac events were monitored plus a 10-20% sample per site. ISR patient 2-year follow-up was 94% complete (462/489). Compared to simple-use patients undergoing native coronary intervention (N=2698) or other, non-ISR expanded-use cases (N=4305), ISR patients had significantly more baseline comorbidities/complex disease. They had higher 2-year rates for mortality (8.6% vs. 4.2%, P<0.001), myocardial infarction (5.0% vs. 2.2%, P<0.001), definite/probable stent thrombosis (4.0% vs. 1.4%, P<0.001), and target lesion revascularisation (12.3% vs. 5.4%, P<0.001) versus simple-use. Safety outcomes were comparable between the ISR and non-ISR expanded-use subgroups but target vessel revascularisation (TVR) was significantly higher for ISR (16.0% vs. 10.9%, P=0.003). ISR was independently associated with increased risk for TVR (1.4-fold).
Conclusions: ARRIVE ISR, one of the largest studied cohorts of patients receiving DES for ISR, had higher risk for clinical events versus simple-use patients but comparable safety outcomes versus other expanded-use patients except for significantly higher revascularisation. ARRIVE provides a detailed estimate of 2-year outcomes in these real-world patients.
Abbreviations
ARC Academic Research Consortium
BMS bare metal stent
DES drug-eluting stent
ISR In-stent restenosis
PCI percutaneous coronary intervention
PES paclitaxel-eluting stent
Introduction
Implantation of bare metal stents (BMS) in native coronary vessels has been associated with in-stent restenosis (ISR) rates of 10% to >50%, depending on patient and lesion complexity1-3. Observational studies have implicated ISR post BMS implantation with significant morbidities, including presentation as acute coronary syndrome in 36-68% of cases4-9. Repeat BMS implantation has not significantly improved long-term outcomes compared to balloon angioplasty alone10,11. Locally applied vascular brachytherapy (VBT) post angioplasty has increased event-free survival in this setting12-14 but may be associated with restenosis and thrombosis15,16.
Drug-eluting stents (DES) have demonstrated a clear restenosis advantage over BMS in de novo lesions17-20 and have been shown to be effective versus balloon angioplasty in patients with ISR21,22. Paclitaxel-eluting stents (PES) and sirolimus-eluting stents (SES) have also been shown to significantly reduce clinical and angiographic restenosis and improve event-free survival compared to VBT at 9-months and beyond, with similar rates of death, myocardial infarction (MI), and target vessel thrombosis23-26. To explore the effect of PES in the treatment of ISR in an unselected population, we evaluated outcomes through 2-years in 489 ISR patients in the TAXUS ARRIVE Postmarket Surveillance Programme27,28 and compared them to outcomes in patients receiving the same DES in non-complex or complex native coronary lesions.
Methods
Programme design, monitoring, follow-up
The TAXUS® Express2® Coronary Stent System (Boston Scientific Corporation, Natick, MA, USA [BSC]) and the BSC sponsored TAXUS Peri-Approval Registry: A Multicentre Safety Surveillance (ARRIVE) Programme have been described previously27-29. Briefly, the ARRIVE programme included the FDA-mandated ARRIVE 1 (2,487 analysed patients at 48 sites) and the voluntary post-market ARRIVE 2 (5,005 analysed patients at 53 sites) registries, which were similarly designed to consecutively enrol patients determined to be appropriate candidates for a DES. Both studies are registered with ClinicalTrials.gov (NCT00569491 and NCT00569751). Patients were enrolled in ARRIVE 1 from February to March of 2004 and in ARRIVE 2 from October 2004 to October 2005; follow-up visits were scheduled at 30 days, six months, and one and two years. No specific inclusion/exclusion criteria were mandated as patients receiving a TAXUS stent were included, whether or not they also received a non-TAXUS stent during the index procedure. A protocol approved by the local institutional review board in full conformity with FDA guidelines and the Declaration of Helsinki was used to enrol patients who provided informed consent at the beginning of the procedure to minimise the potential bias of excluding complicated or unsuccessful procedures. Follow-up angiography was not mandated, and was performed in accordance with local practice. Dual antiplatelet therapy (aspirin and clopidogrel/ticlopidine) was begun before or immediately after percutaneous coronary intervention (PCI). Aspirin was continued indefinitely and the thienopyridine was recommended for six months per the Directions For Use (DFU).
Major cardiac events (MCE) included all cardiac death, MI, and target vessel revascularisation (TVR). Target lesion revascularisation (TLR) was defined as “TAXUS-stent-related” TVR, given the absence of a central angiographic core laboratory. Data for patients who had subsequent cardiac events were source verified by monitors retained by the sponsor. An additional 10-20% of patients were sampled to confirm the accuracy and completeness of data collection. Cardiac events and their relationship to the TAXUS stent were adjudicated by an independent Clinical Events Committee28. An event was considered related to the TAXUS stent if it occurred at the stented segment or if the relationship to the stent could not be excluded based on existing information. The Academic Research Consortium (ARC) definition of “definite/probable” stent thrombosis (ST) was used30.
Statistical analysis
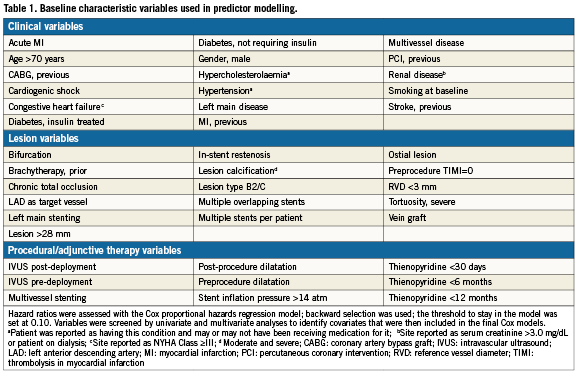
Continuous variables are presented as mean±SD and were compared by Student t-test. The chi-square test or Fisher exact test was used to determine the significance of differences in categorical variables. Time-to-event curves were generated by the Kaplan-Meier product method, and the differences were assessed by the log-rank test. Multivariable Cox models were created with the use of baseline clinical and angiographic characteristics and procedure-related variables (Table 1) in order to identify independent predictors of death, MI, TVR, and ST. Backward selection was used to identify significant predictors; the threshold to stay in the model was set at 0.10. To determine if ISR was a confounder for other risk factors or an independent risk factor in and of itself, the predictor analysis was also carried out with the variables shown in Table1 and ISR forced into the model. All analyses were performed using the SAS System Software, Version 8.0 or higher (SAS Institute, Cary, NC, USA). A 2-sided P value of <0.05 was considered statistically significant.
The authors had access to the data and take responsibility for its integrity. All authors have read and agreed to the manuscript as written.
Results
Baseline and procedural characteristics
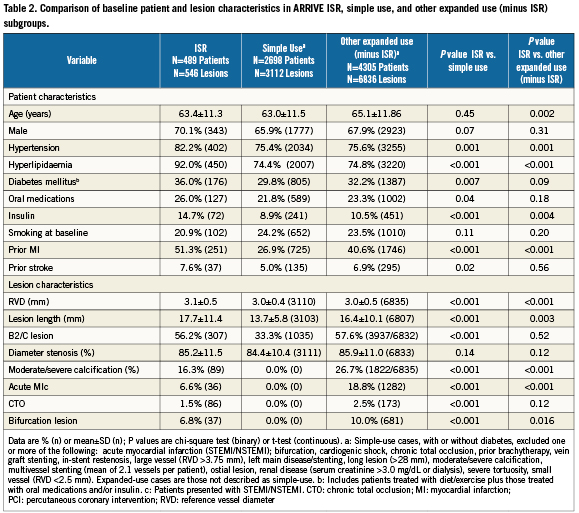
Of 7,492 analysed ARRIVE cases, 489 patients (6.5%) underwent PCI for ISR. They were part of a large subgroup of expanded-use ARRIVE patients (N=4,794; 64%) with patient and/or lesion characteristics outside the simple-use subgroup (N=2,698) who underwent PCI in de novo lesions and would have met the criteria for inclusion in the TAXUS IV pivotal trial18. Patients in the ISR subgroup differed significantly from those in the simple-use subgroup with more baseline comorbidities and complex disease (diabetes, hypertension, hyperlipidaemia, longer and more complex lesions; Table 2). The ISR subgroup also had significantly more insulin-dependent diabetes with more hypertension and hyperlipidaemia and longer lesions compared to the cohort of other expanded-use, non-ISR cases (N=4,305).
Predilatation was performed for 64.7% (353/546) of ISR lesions and average stent implantation pressure was 14.7±3.3 atm. Most ISR patients had a single lesion (89.4%, 437/489) and a single vessel (97.5%, 477/489) treated. The use of pre-deployment intravascular ultrasound (IVUS) was significantly higher among ISR patients (7.5%) compared to simple use (4.1%, P<0.001) or other expanded use (3.7%, P<0.001) patients.
Clinical outcomes
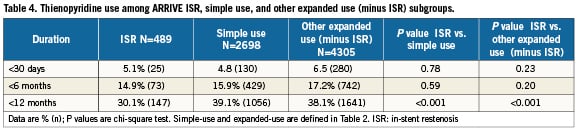
Among ARRIVE ISR patients, clinical follow-up was available in 98% (479/489) at 1-year and 94% (462/489) at 2-years. The ISR subgroup had significantly higher 2-year rates for mortality, MI, and ST compared to simple-use cases but these outcomes in ISR patients were not significantly different from those of the cohort of other expanded-use, non-ISR cases (Table 3 and Figure 1). Interestingly, although ST though two years was significantly higher among the ISR versus simple-use subgroups, significantly more simple-use patients stopped thienopyridine use before 12 months (Table 4). The most common clinical event in the three subgroups was revascularisation; through two years the ISR subgroup had significantly more revascularisation events than either of the other two cohorts (Figure 1). By 1-year, the TVR curves for the ISR cohort and the other expanded-use, non-ISR cohort had diverged noticeably. Rates for TLR, however, were not significantly different between these two subgroups until year two. Among vessels with TAXUS stent-related restenosis, the available angiographic data (n=42) indicated that 23.8% had edge stenosis (16.7% proximal and 7.1% distal), 88.1% had in-stent stenosis (47.6% focal and 45.2% distal), and 2.4% had gap stenosis. As reported by the sites, 1.7% of patients treated with TAXUS for ISR underwent coronary artery bypass grafting for the lesion within two years compared to 0.7% for the other expanded-use, non-ISR cohort and 0.5% for the simple-use cohort.
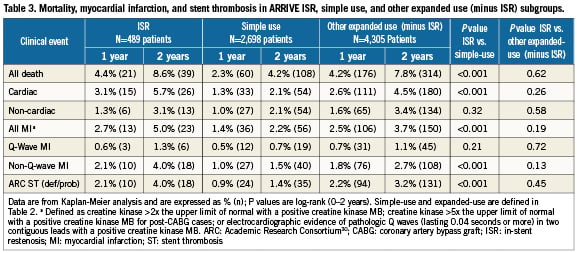
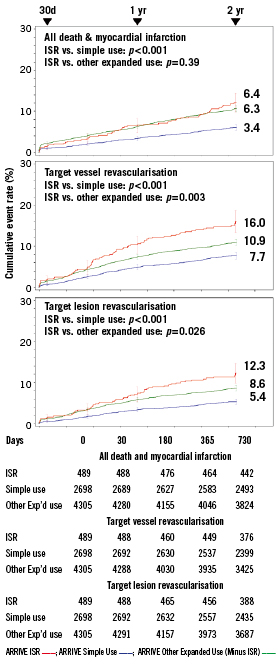
Figure 1. Death / myocardial infarction and revascularisation through two years in the arrive ISR, simple-use, and expanded-use minus ISR subgroups. Simple-use and expanded-use are described in Table 2. Target lesion revascularisation was defined as “TAXUS-stent-related” target vessel revascularisation, given the absence of a central angiographic core laboratory. P-values (log-rank) are for the comparison between in-stent restenosis (ISR) and simple use subgroups and between ISR and other expanded use (minus ISR) subgroups; error bars are ±1.5SE.
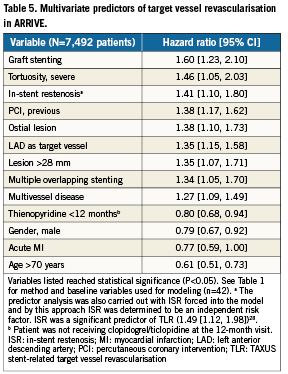
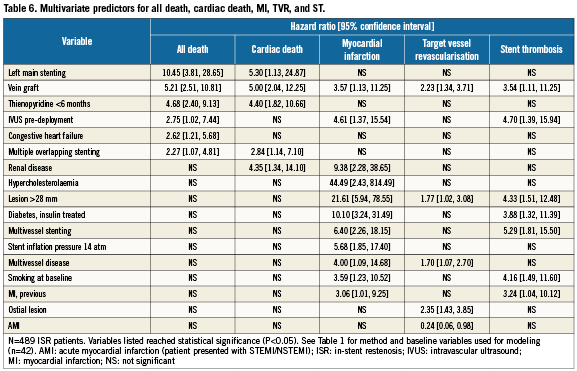
While ISR was not a significant multivariate predictor of 2-year death, MI, or ST in the ARRIVE population28, Table 5 shows that along with other lesion-based variables ISR was independently associated with increased risk for TVR (1.4-fold). Table 6 shows multivariate predictors for all death, cardiac death, MI, TVR, and ST through two years among ARRIVE ISR patients. Predictors for mortality, MI, and ST were patient, lesion, and procedure-based whereas TVR predictors were mostly lesion-based, including vein graft, ostial lesion and longer lesions (>28mm). Stenting of the left main (10.5-fold) and vein graft stenting (5.2-fold) posed the highest risk for death among ISR patients.
Discussion
The ARRIVE registries collected data through two years on 7,492 patients from ‘real-world’ clinical experience, including 489 patients who received the TAXUS Express PES for ISR27,28. This subgroup represents one of the largest cohorts of patients receiving DES for ISR described to date and their inclusion in ARRIVE allowed for direct comparison to the subgroup of simple-use patients (N=2,698) who would have been eligible for the TAXUS IV pivotal trial18 as well as to the cohort of other, non-ISR expanded-use patients (N=4,305). These ISR patients had more baseline cardiovascular comorbidities and more challenging lesion characteristics than the simple-use subgroup, which may have contributed to their significantly higher clinical event rates. They also had similar or greater comorbidities compared to the non-ISR expanded-use cohort but experienced comparable safety outcomes, though revascularisation was significantly higher with ISR. In a multivariate analysis, ISR and other lesion-based variables were independently associated with increased risk for TVR (1.4-fold).
Restenosis of BMS post implantation in a native coronary vessel is a common occurrence with estimated 6-month rates of 20-60%31-34 followed by hospitalisation and/or revascularisation for the 36-68% of patients who present with MI or unstable angina7,8. Observational analyses have found ISR to be a risk factor for future revascularisation procedures, often attributable to increased comorbidities in the restenosis group4,5,9. However, ISR has been reported to be an independent risk factor for BMS TVR6 and DES restenosis35, which is consistent with results reported here, and in one study ISR was associated with increased long-term mortality34. In the overall ARRIVE registry, renal disease and several other comorbid clinical factors were independent predictors of mortality, as also reported by others28,36. In the ARRIVE ISR subgroup, the two strongest predictors of mortality were lesion based (left main stenting [10.5-fold] and vein graft stenting [5.2-fold]). The 2.8-fold increase in mortality with IVUS pre-deployment may reflect the use of IVUS among more complex patients prone to increased event rates. Predictors of revascularisation in the ISR group included lesion related factors similar to other studies37.
The treatment of ISR continues to be a challenge in routine practice38 and there are no established treatment guidelines. Smooth muscle proliferation, extracellular matrix formation, accelerated arterial vessel remodelling, and intimal hyperplasia all contribute to BMS restenosis3. With the logistical complexities of VBT and accompanying late adverse effects, DES have been the preferred therapeutic modality for bare metal ISR38. In a randomised controlled trial (RCT), both SES and PES were significantly better than conventional balloon angioplasty, with SES associated with less late lumen loss and clinical restenosis compared to PES21. Arecent report comparing DES to BMS for treating bare-metal ISR in an unselected population over a median of 3.2 years found follow-up DES use associated with significantly lower mortality versus BMS, with a trend towards reduced MI and TLR39. Results with paclitaxel-coated catheter balloons also have been promising40, as have outcomes with the second generation everolimus-eluting and zotarolimus-eluting stents in the treatment of BMS ISR41.
Mortality, MI, and revascularisation in ARRIVE were higher than reported with SES in the Tuscany Registry of Sirolimus for Unselected In-Stent Restenosis (TRUE)42, but similar to that reported for ISR patients receiving DES in the STENT registry43. The 2-year ST rate in the ARRIVE ISR subgroup was higher than in TRUE or STENT but was not significantly greater than that observed in the cohort of other, non-ISR ARRIVE expanded-use patients and was also similar to rates observed in other ARRIVE high-risk subgroups44. Differences in absolute event rates between studies may reflect differences in methodology, including event ascertainment, monitoring, and adjudication. The significantly higher rate of ST in ISR patients argues for the extension of dual antiplatelet therapy to at least 12 months in patients who can tolerate it, as noted in recent guidelines45.
This study has several limitations. As with all registry studies, there was no control group and the monitoring level was lower than standard for traditional RCTs, although the outcome data are concordant with those from RCTs with 100% monitoring27. Analysis was based on site visual assessments of angiographic data rather than a core angiographic laboratory, possibly allowing for operator variability in the assessment of TVR. There was no routine angiographic follow-up, which precluded analysis of late angiographic events but more clearly reflected “real-world” clinical experience. The absence of serial cardiac enzyme or electrocardiographic measurements may have resulted in an underestimation of the rate of smaller (non-Q) MI. The results of this study apply to patients with ISR who were treated with PES, and do not address other treatment modalities such as balloon angioplasty, cutting balloon atherectomy, brachytherapy, or drugs available for the treatment of ISR. While these limitations must be noted, this large cohort of ISR patients with complex disease provides an important source of information for a group of patients who are typically not included in large randomised clinical trials.
Conclusions
ARRIVE patients undergoing stenting for ISR had significantly more comorbidities and expectedly higher event rates than the simple–use cohort. Compared to other, non-ISR expanded-use patients, ISR subgroup comorbidities were also greater, but safety outcomes were comparable. Revascularisation, however, was significantly higher with ISR patients, likely reflecting the underlying pathology. In the absence of a large RCT in the ISR patient population, our study provides important insight into the characteristics of patients who receive a DES for ISR and their clinical outcomes over an extended period of time.
Acknowledgements
The study was supported by Boston Scientific Corporation, Natick, MA, USA. The authors thank Yun Lu, M.S. and Aijun Song, M.S. (Boston Scientific Corporation) for assistance with statistical analyses.
Conflict of interest statement
Dr. Lee has received speaker’s bureau fees from Boston Scientific Corporation (BSC), BMS, and Schering-Plough. Dr. Lasala has received speaker’s bureau fees and consulting fees from BSC. Dr. Cox has received speaker’s bureau fees from and is on the Medical Advisory Board for BSC. Drs. Bowman, Starzyk and Dawkins are full-time employees and stockholders of BSC. Dr. Yang has no conflict of interest to declare.

