Abstract
Background: Data are mixed concerning the safety and effectiveness of drug-coated balloons (DCBs) for treating below-the-knee (BTK) lesions.
Aims: The aim of this study was to assess the safety and effectiveness of the IN.PACT 014 paclitaxel-coated balloon catheter versus conventional percutaneous transluminal angioplasty (PTA) for infrapopliteal chronic total occlusions (CTOs) in patients with chronic limb-threatening ischaemia (CLTI).
Methods: The IN.PACT BTK randomised study is a prospective, multicentre, randomised pilot study. Fifty CLTI participants (Rutherford clinical category 4-5) with BTK CTOs were randomised 1:1 to DCB (N=23) or PTA (N=27). The primary effectiveness endpoint was late lumen loss (LLL) at 9 months post procedure. Safety outcomes up to 9 months included all-cause mortality, major target limb amputation, and clinically driven target lesion revascularisation (CD-TLR).
Results: Mean lesion length was 215.41±83.81 mm in the DCB group and 218.19±80.43 mm for PTA (p=0.806). The 9-month angiographic LLL was 0.892±0.774 mm for the DCB group and 1.312±0.720 mm for the PTA group (p=0.070) in a classic analysis, and 0.592±0.944 mm for DCB and 1.260±0.810 mm for PTA (p=0.017) in a subsegmental analysis. The Kaplan-Meier estimated freedom from CD-TLR up to 9 months was 91.1% for DCB and 91.8% for PTA (log-rank p=0.942). At 9 months, 1 patient died in the DCB group and 2 in the PTA group (p=1.000); there were no major target limb amputations in either arm.
Conclusions: The 9-month subsegmental LLL was lower after treatment with the IN.PACT 014 DCB compared with PTA with no differences in safety or revascularisation events in a small complex population of patients with BTK CTOs. ClinicalTrials.gov: NCT02963649
Introduction
Chronic limb-threatening ischaemia (CLTI) is an advanced form of peripheral artery disease with significant below-the-knee (BTK) involvement1. Revascularisation is the guideline-recommended treatment for patients with CLTI2; however, chronic total occlusions (CTOs) are highly prevalent among patients with CLTI and remain a challenging variant for treatment. Although bypass surgery is an important option for the treatment of BTK lesions, endovascular interventions have been on the rise, as many patients suffer from underlying comorbidities that result in high surgical risk. Conventional percutaneous transluminal angioplasty (PTA) has been the primary endovascular intervention used for the infrapopliteal vascular bed, despite the poor long-term patency rates (42-75% at one year) associated with this procedure3.
Paclitaxel drug-coated balloons (DCBs) have been compared to conventional PTA for the treatment of BTK lesions, though studies resulted in mixed outcomes45678910. Inconsistent findings across studies suggest a need for further investigation and an evaluation of opportunities for enhanced rigour in study design. Furthermore, there are no DCB studies that specifically reported outcomes for patients with infrapopliteal CTOs.
The IN.PACT 014 paclitaxel-coated balloon catheter (IN.PACT 014 DCB; Medtronic) is part of the IN.PACT family of paclitaxel DCBs. While similar in design to the IN.PACT Admiral DCB (Medtronic), which is approved for the treatment of femoropopliteal lesions, the IN.PACT 014 DCB is an investigational product compatible with a 0.014” guidewire for BTK vessels. The IN.PACT BTK randomised study was designed to assess the safety and effectiveness of the IN.PACT 014 DCB versus PTA for the treatment of CLTI patients with CTOs in the infrapopliteal arteries.
Methods
STUDY DESIGN
The IN.PACT BTK randomised study is a prospective, multicentre, randomised study to evaluate the safety and effectiveness of the IN.PACT 014 DCB for the treatment of infrapopliteal CTOs in CLTI patients (Rutherford clinical category [RCC] 4-5), as previously described11 (Supplementary Appendix 1). Participants who met all inclusion/exclusion criteria including successful predilation of the target lesion(s) were enrolled from March 2017 to February 2019, across nine study sites (Supplementary Table 1) and randomised 1:1 into DCB (N=23) or PTA (N=27) treatment groups. Participants were followed up to 30 days, 3, 6, and 9 months, and will continue to be followed up to 12, 24 and 36 months, though the sponsor intends to extend the follow-up of the study up to 60 months. Angiographic assessments were performed at the nine-month visit or sooner in case of a target lesion revascularisation (TLR). Participants with ischaemic wounds on the target limb at baseline, or who developed a new ischaemic wound on the target limb during the study, had an additional overlapping follow-up schedule (once per week for the first month, and then once a month until healed) at a dedicated wound care facility or with a wound care specialist. Participant flow through the study is shown in Figure 1.
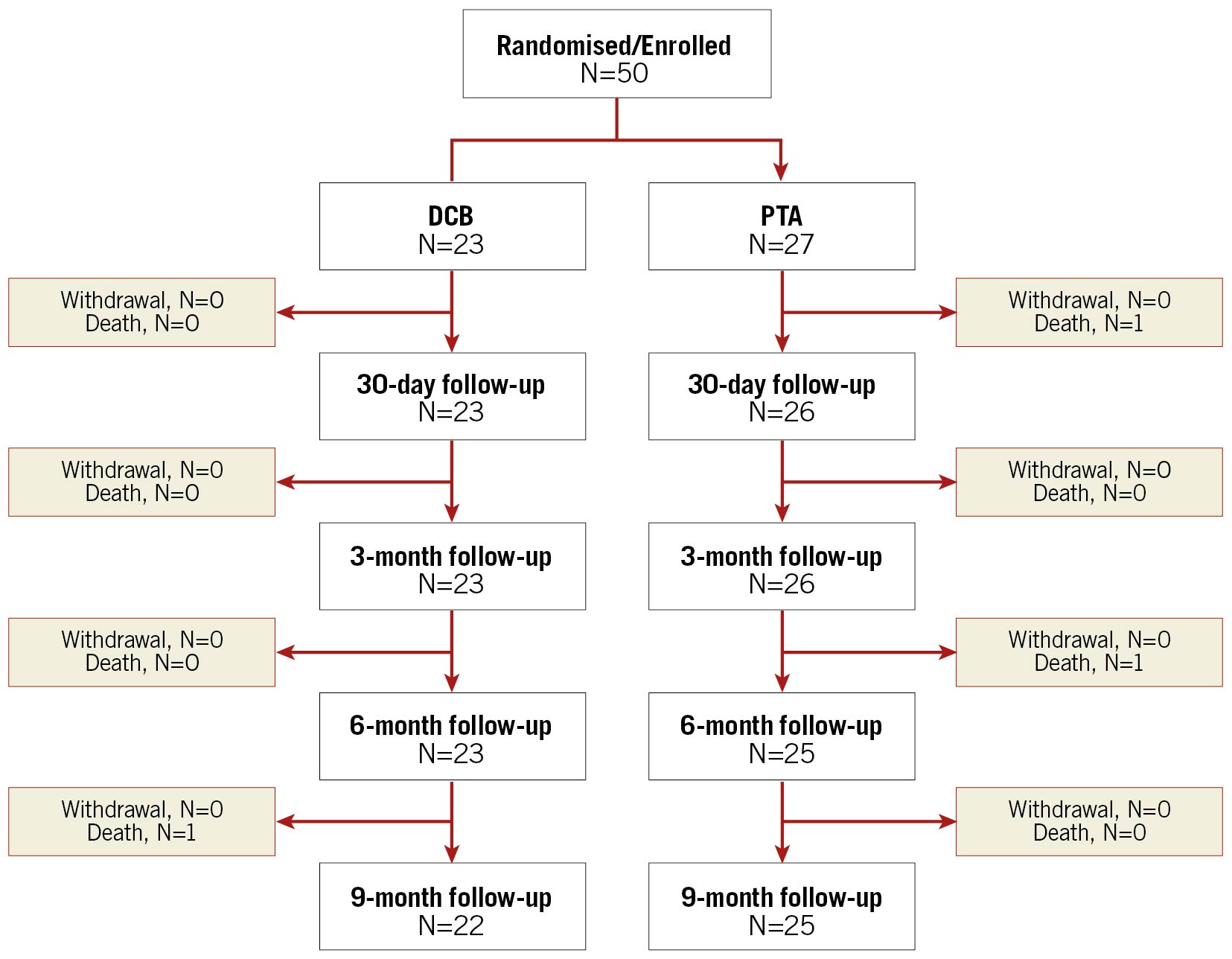
Figure 1. CONSORT flow diagram up to nine months in the IN.PACT BTK randomised study. Participant numbers reported at each time point are eligible participants. DCB: drug-coated balloon; PTA: percutaneous transluminal angioplasty
An independent data safety monitoring board monitored the study and an independent clinical events committee (Syntactx, Herzele, Belgium) adjudicated all major adverse events (MAE) including revascularisation events. An independent duplex ultrasound (DUS) core laboratory (VasCore, Boston, MA, USA) and an angiography core laboratory (Cardiovascular Imaging Core Laboratory, Harvard Medical Faculty Physicians at Beth Israel Deaconess Medical Center, Boston, MA, USA) analysed all images. The clinical events committee and core laboratories were blinded to treatment.
The study is being conducted in accordance with the Declaration of Helsinki, and federal, national and local laws, regulations, standards, and requirements of the countries where the study is being conducted. The study protocol was reviewed and approved by the ethics committees and/or competent authorities, and all participants provided written informed consent prior to enrolment. The study is registered at ClinicalTrials.gov (NCT02963649).
INDEX PROCEDURE
Participants were administered dual antiplatelet therapy per institutional standard of care. Inflow vessels with flow-limiting lesions were treated before the index procedure, with successful treatment defined by a ≤30% residual diameter stenosis. All eligible participants underwent a predilation of the target lesion with a non-drug-coated semi-compliant balloon. According to the core laboratory DUS guidelines for measuring reference vessel diameter, the predilation balloon was sized at a 1:1 ratio to the vessel wall, and a length that covered the entire length of the target lesion. More than one predilation balloon was allowed, and the balloon could be inflated more than once according to protocol. No other vessel preparation devices, such as cutting/scoring balloons, were permitted. Angiography and DUS were used to determine predilation success, which was defined as angiographic residual stenosis ≤30% per visual estimate, and intraprocedural Doppler exam showing a biphasic (with rapid take-off) or triphasic wave signal, with absence of a major (grade D or greater) flow-limiting dissection (observed on two orthogonal views).
For the PTA group, no additional treatment was performed after enrolment and randomisation. Participants randomised to the DCB group received the IN.PACT 014 DCB. Details of the IN.PACT 014 catheter device have been described previously11. If a participant had multiple target lesions, all lesions were treated with the same assigned randomised treatment. Prolonged balloon inflation was allowed to manage a suboptimal result, including >50% residual stenosis, perforation, occlusive complication, or flow-limiting dissection. Bail-out stenting was allowed if prolonged balloon inflation did not provide the expected result. Other adjunctive therapies were not allowed. This included laser, atherectomy, cryoplasty, cutting/scoring balloons, and brachytherapy.
ENDPOINTS AND ASSESSMENTS
The primary effectiveness endpoint was late lumen loss (LLL) at nine months post procedure for DCB versus PTA as measured by the classic and subsegmental angiographic analyses. LLL was defined as the difference between the minimal lumen diameter (MLD) immediately post procedure and MLD at nine-month angiographic follow-up (or at the time of TLR) and assessed using classic and subsegmental angiographic techniques. The classic angiographic method for measuring LLL was suggested to be inadequate in long and diffused lesions (Razavi M. Transverse View Area Loss [TVAL]: An informative angiographic outcome in BTK lesions. The Leipzig Interventional Course [LINC]. 2020, January 28-31, Leipzig, Germany). A subsegmented angiographic method for measurement of LLL was described previously11, and was shown to be suitable for angiographic efficacy evaluation12. Briefly, in this method, each treated length was divided into a tandem array of ten equally spaced subsegments. Subsegmental measures were taken to determine the mean and minimal diameters within each subsegment, which were matched with the baseline, post-procedural, and follow-up angiograms. These values were compiled into a data series and used to generate an overall mean value for the target lesion. Angiographic outcomes obtained by subsegmental analysis at nine months post procedure included subsegmental LLL, subsegmental acute luminal gain (defined as the difference between subsegmental MLD immediately post procedure and subsegmental MLD at baseline), loss index with subsegmental MLD (defined as LLL divided by acute luminal gain), number of subsegments with ≥50% stenosis per lesion, and number of occluded subsegments (100% stenosis) per lesion at nine months post procedure. Other angiographic outcomes assessed included acute luminal gain, loss index with MLD, diameter stenosis, and binary restenosis (≥50%) at nine months post procedure.
Secondary endpoints included: a composite safety endpoint within nine months (composite of freedom from device- and procedure-related mortality within 30 days, freedom from major target limb amputation and freedom from clinically driven TLR [CD-TLR] within nine months post procedure), MAE rate up to nine months (composite of all-cause mortality, major target limb amputation, and CD-TLR), mechanically driven TLR (defined as any TLR due to a flow-limiting dissection or flow-limiting thrombosis at the target lesion that occurs within 37 days post procedure documented by a DUS peak systolic velocity ratio >2.4 and confirmed by angiography), and any TLR, any target vessel revascularisation (TVR), clinically driven target vessel revascularisation (CD-TVR), and thrombosis at the target lesion(s) within nine months. CD-TLR is defined as any TLR of the target lesion with restenosis >70% (confirmed by angiography) associated with at least one of the following: reoccurrence of ischaemic rest pain, worsening of pre-existing wounds or occurrence of new wound(s). Clinical improvement at nine months was assessed with an evaluation of RCC and functional flow (defined as the absence of target lesion occlusion [no flow] on DUS).
STATISTICAL ANALYSIS
This is a pilot study including a new DCB platform, the IN.PACT 0.14, the performance of which in terms of safety and efficacy was unknown. Pilot studies do not have the statistical power of large trials but may provide scientific signals to be validated in larger studies. The number of patients considered valuable for this type of investigation is between 30 and 5013. Analyses were based on the intent-to-treat principle. Baseline demographics and characteristics are summarised on a patient basis, and lesion characteristics on a lesion basis. Continuous variables are described as mean±standard deviation, and comparisons between groups were performed with the t-test or Wilcoxon rank-sum test in case normality was violated. Dichotomous and categorical variables are described as proportions and counts, and comparisons between groups were performed with Fisher’s exact test. The Kaplan-Meier method was used to evaluate time-to-event data for freedom from CD-TLR, freedom from the composite safety endpoint, freedom from MAE, and freedom from all-cause mortality over the nine-month follow-up period. The log-rank test was used for comparison between groups. For event rates that are expressed as a proportion, the number of participants with an event within 270 days was the numerator and the total number of participants with an event or at least 210 days of clinical follow-up was the denominator. Overall missing data were not imputed, and the level of statistical significance was set at 0.05 for a two-sided test. Statistical analyses were performed using SAS version 9.4 (SAS Institute).
Results
PARTICIPANT AND LESION CHARACTERISTICS
Baseline demographic, clinical, and lesion characteristics were similar between the groups (Table 1). The mean age was 73.1±7.4 years in the DCB group and 69.6±9.4 years in the PTA group (p=0.107). Both groups had a high incidence of diabetes mellitus (DCB 73.9% and PTA 96.3%; p=0.039). The mean lesion length was 215.4±83.8 mm in the DCB group and 218.2±80.4 mm in the PTA group (p=0.806). Approximately half of the lesions in each group were calcified (DCB 54.2%, PTA 41.4%; p=0.410), with 8.3% of DCB lesions and 13.8% of PTA lesions having severe calcification.
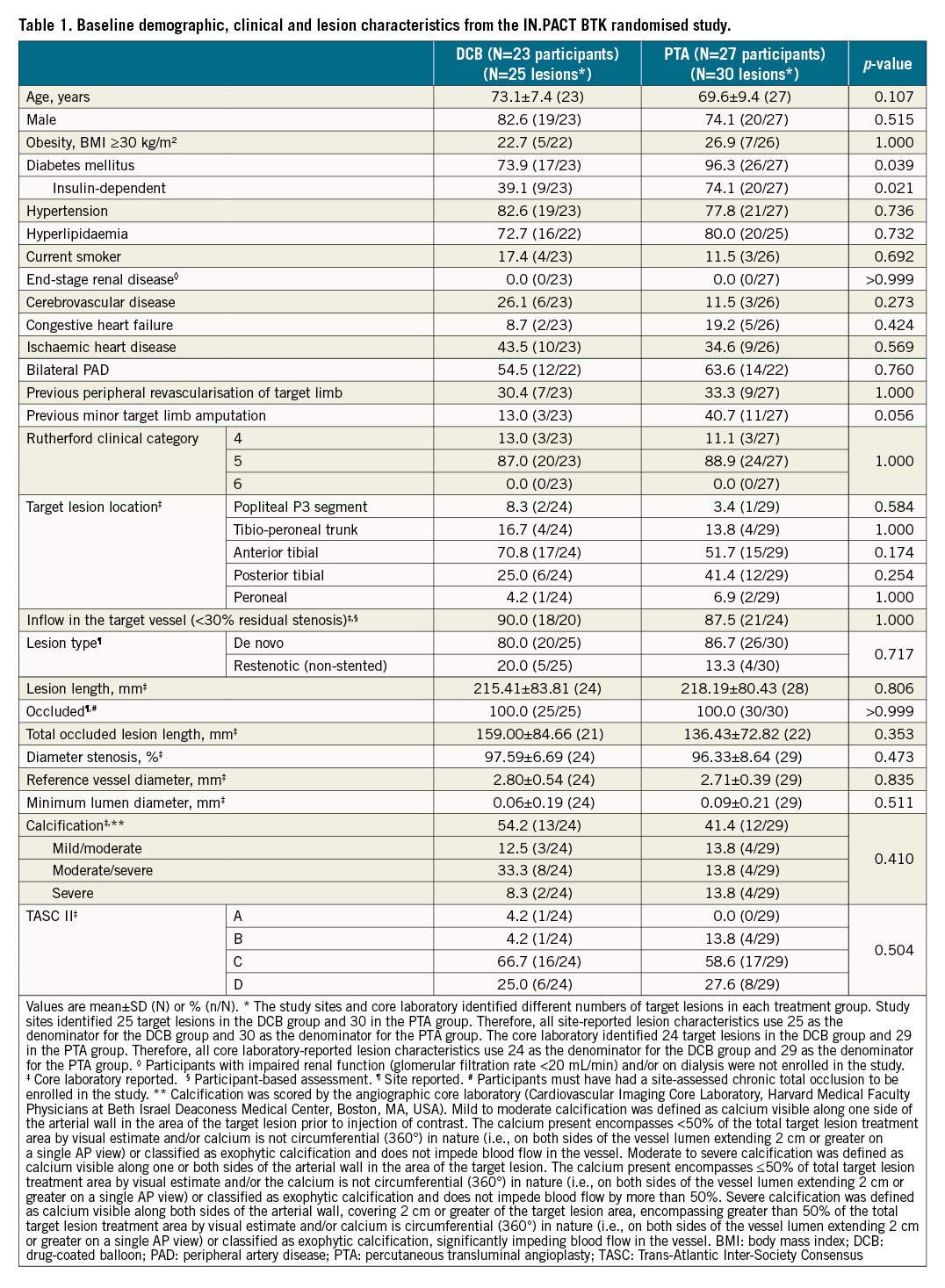
PROCEDURAL CHARACTERISTICS
Most lesions in the PTA group (73.3%) had three or more predilations compared with 48.0% in the DCB group (p=0.087) (Supplementary Table 2). The rate of provisional stenting was 8.0% in the DCB group and 3.3% for PTA (p=0.586). While the occurrence of periprocedural dissections was not significantly different between the groups (p=0.108), there was a trend towards more ≥grade B dissections in the DCB group (54.2%) versus PTA (27.6%) (Supplementary Table 2). Acute angiographic outcomes were similar between the groups. Immediately post procedure, MLD was 1.945±0.412 mm in the DCB group and 1.797±0.462 mm for PTA (p=0.230), and diameter stenosis was 31.843±10.959% for DCB and 34.124±14.368% for PTA (p=0.703). Final residual stenosis was 5.1±7.6% for DCB and 6.8±8.5% for PTA (p=0.432) (Supplementary Table 2).
ANGIOGRAPHIC EFFECTIVENESS OUTCOMES
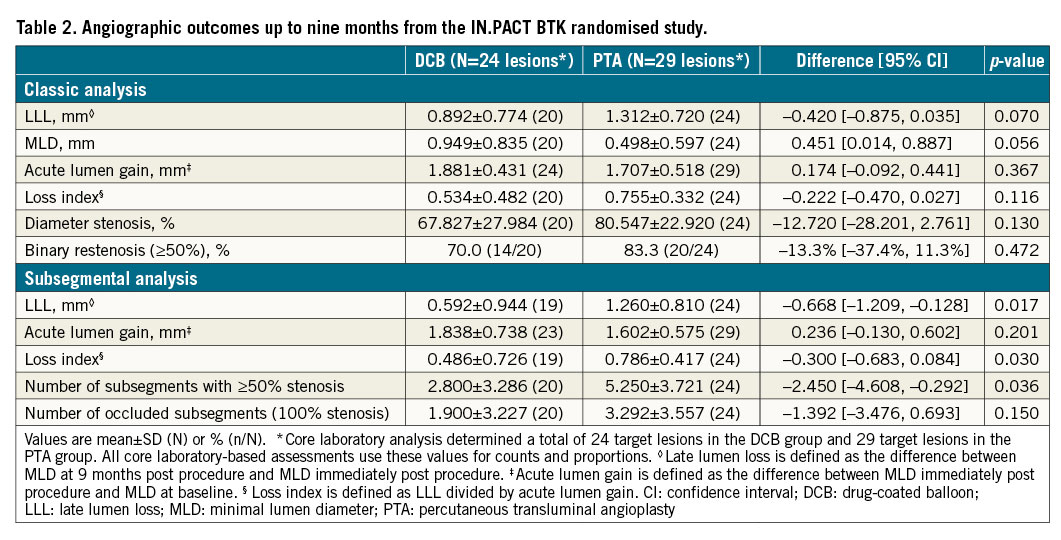
There was a trend of lower LLL at nine months in the DCB group (0.892±-0.774 mm) compared to PTA (1.312±0.720 mm; p=0.070) in the classic analysis, which was statistically significant in the subsegment analysis (0.592±0.944 mm for DCB vs 1.260±0.810 mm; p=0.017) (Table 2). The mean loss index was 0.534±0.482 for DCB and 0.755±0.332 for PTA (p=0.116), and the rates of binary restenosis (≥50%) were 70.0% for DCB and 83.3% for PTA in the classic analysis (p=0.472) (Table 2). The subsegmental loss index was significantly lower for DCB versus PTA (0.486±0.726 versus 0.786±0.417; p=0.030). The number of subsegments with ≥50% residual stenosis was lower in the DCB group (mean 2.800±3.286) compared with the PTA group (5.250±3.721; p=0.036). The number of occluded subsegments (100% stenosis) was 1.900±3.227 in the DCB group versus 3.292±3.557 in the PTA group (p=0.150). The individual subsegmental MLD values measured at baseline (pre-procedure), immediately post procedure, and at nine months post procedure are reported for each group in the Central illustration.
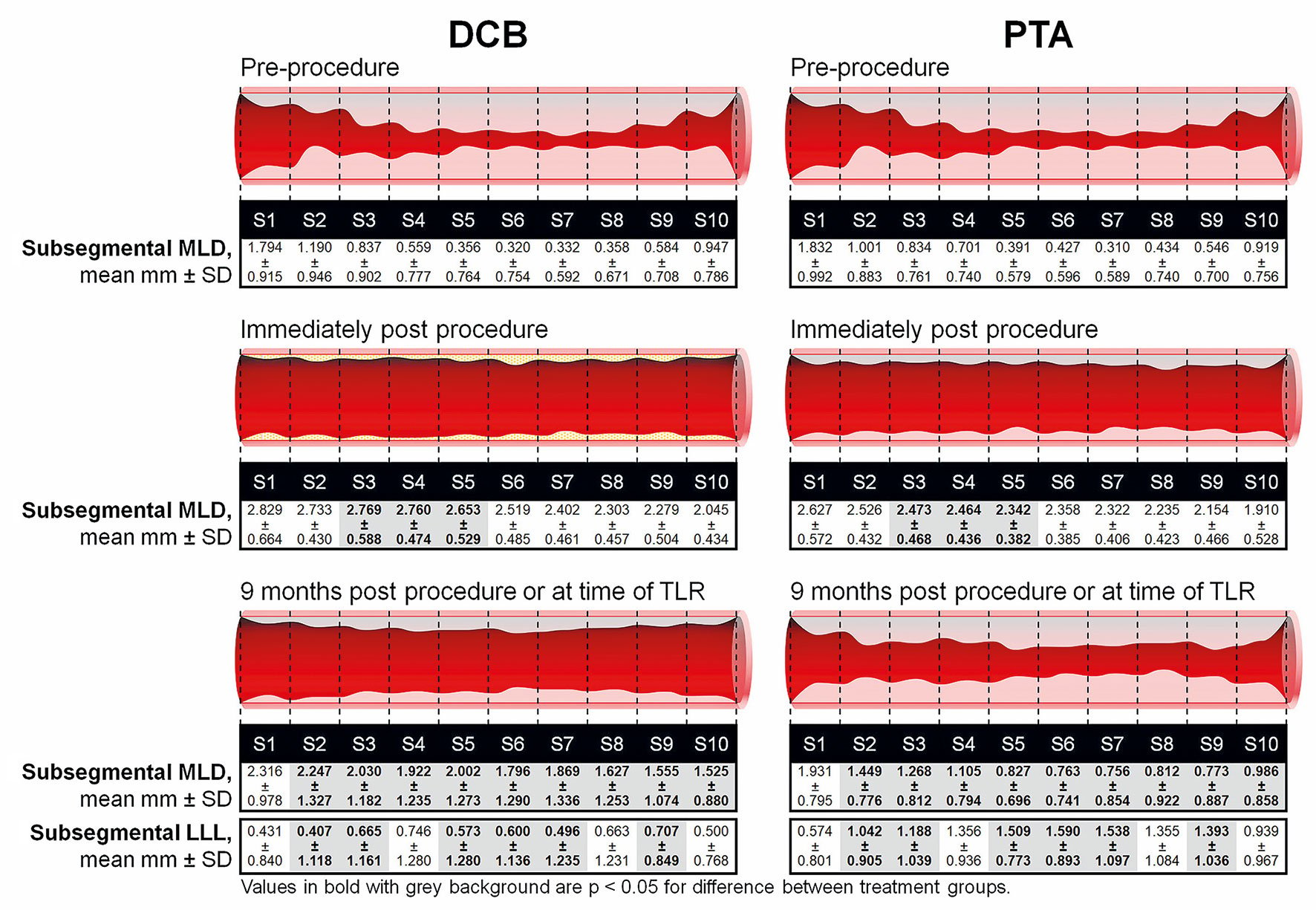
Central illustration. Subsegmental array angiographic analysis from pre-procedure up to nine months in the IN.PACT BTK randomised study. Individual subsegmental MLD values were measured pre-procedure, immediately post procedure, and at nine months post index procedure. Corresponding nine-month subsegmental LLL values are also shown. P-values are for differences in matching subsegmental LLL between groups at nine months post procedure. DCB: drug-coated balloon; LLL: late lumen loss; MLD: minimal lumen diameter; PTA: percutaneous transluminal angioplasty; SD: standard deviation; TLR: target lesion revascularisation
SECONDARY EFFECTIVENESS OUTCOMES
The Kaplan-Meier estimated freedom from CD-TLR up to nine months was 91.1% for DCB and 91.8% for PTA (log-rank p=0.942) (Figure 2). Duplex ultrasound assessments showed that functional flow was persistent in the DCB group up to nine months. In the DCB group, 84.6% of lesions retained functional flow at nine months compared with 60.0% in the PTA group (p=0.341) (Supplementary Table 3).
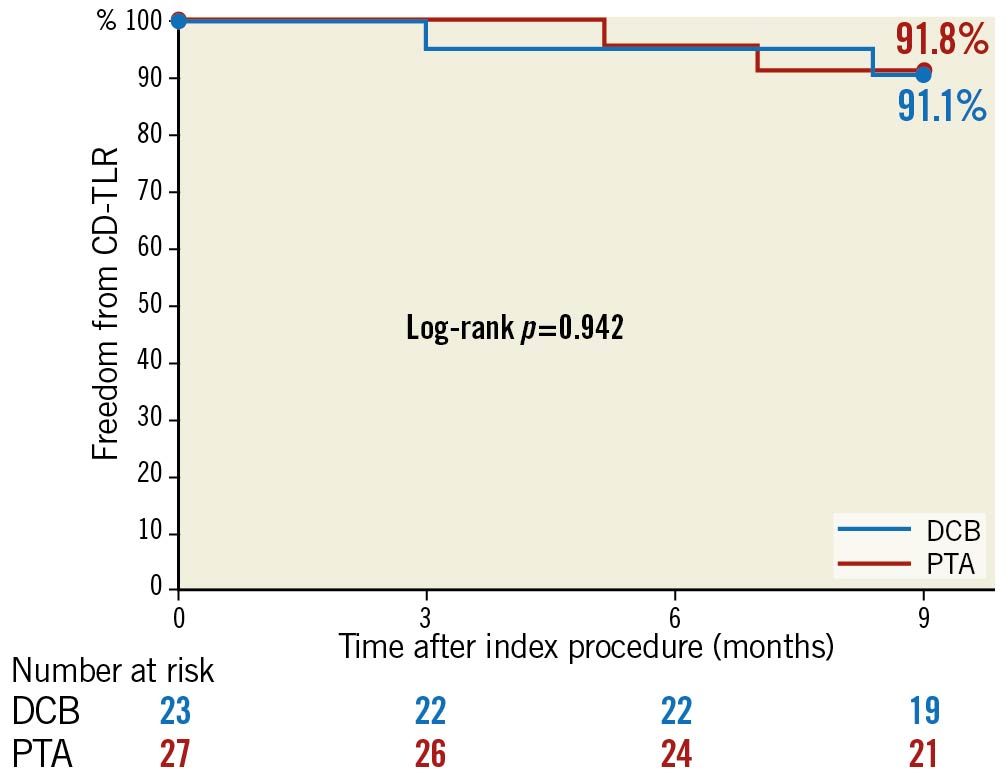
Figure 2. Kaplan-Meier estimates of freedom from CD-TLR up to nine months in the IN.PACT BTK randomised study. CD-TLR: clinically driven target lesion revascularisation; DCB: drug-coated balloon; PTA: percutaneous transluminal angioplasty
FUNCTIONAL OUTCOMES
The differences in RCC between baseline and nine months indicated clinical improvement in both groups (Supplementary Figure 1). At baseline, all participants in each group were in RCC 4-5. At nine months, only 15.0% of the DCB group and 8.7% of the PTA group were still in RCC 4–5. The majority of participants in each group were RCC 0-2 (DCB 75.0% and PTA 82.6%), and more than half in each group were RCC 0 (DCB 55.0% and PTA 60.9%). The RCC distribution was not significantly different between groups at nine months (p=0.895).
SAFETY OUTCOMES UP TO NINE MONTHS
There were no differences between groups in safety events within nine months (Table 3 and Figure 3). The composite safety endpoint was achieved by 91.3% of participants in the DCB group and 87.5% in the PTA group (p=1.000). The rate of MAE was 13.0% in the DCB group and 16.0% for PTA (p=1.000). One death occurred in the DCB group at 232 days post procedure and two in the PTA group, at 17 days post procedure and at 162 days post procedure, all of which were cardiovascular-related deaths (DCB: respiratory failure at 232 days; PTA: myocardial infarction at 17 days, recurrent pulmonary embolism at 162 days). As adjudicated by the clinical events committee, none of these deaths were device- or paclitaxel-related. The Kaplan-Meier estimated nine-month freedom from all-cause death rates are shown in Figure 3. There were no major target limb amputations in either group and the rate of thrombosis at the target lesion(s) was comparable between groups (DCB 4.3%, PTA 4.2%, p=1.000).
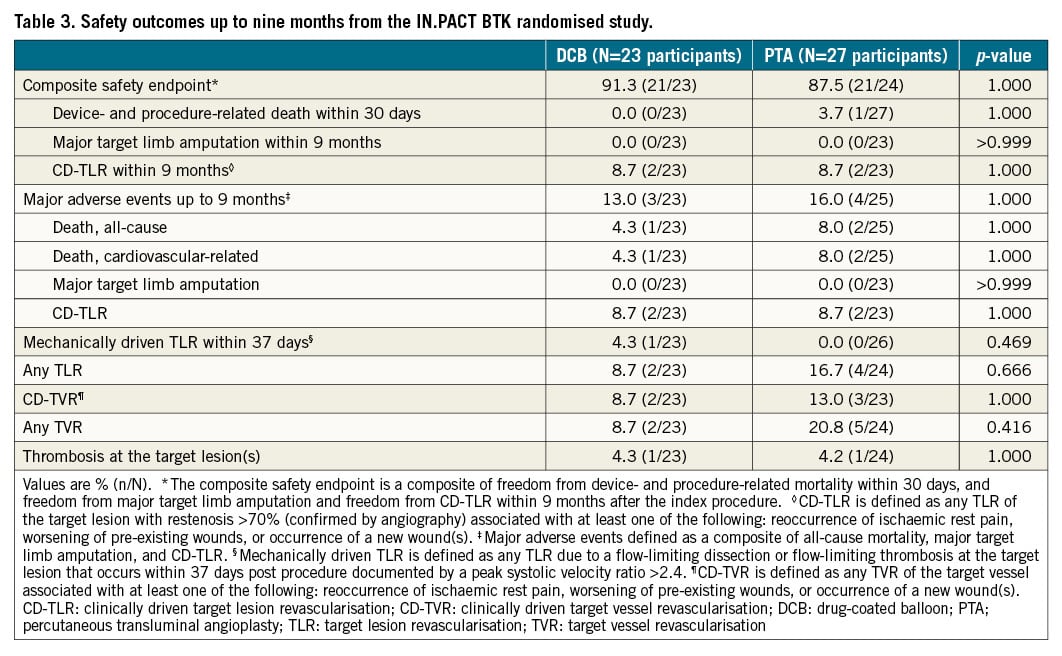
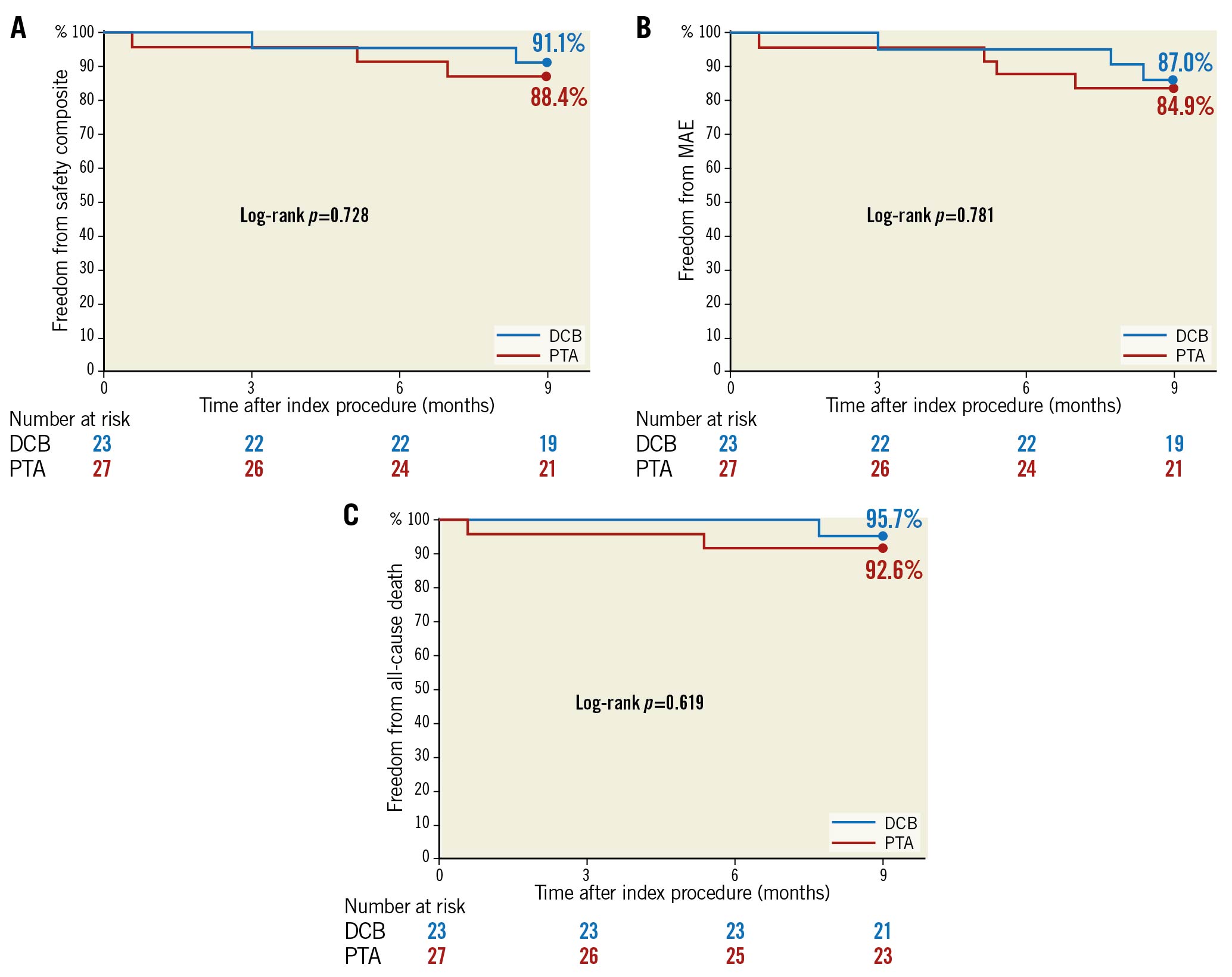
Figure 3. Kaplan-Meier estimates of freedom from composite safety endpoint (A), freedom from MAE (B), and freedom from all-cause death (C) up to nine months in the IN.PACT BTK randomised study. DCB: drug-coated balloon; MAE: major adverse events; PTA: percutaneous transluminal angioplasty
POST HOC SAFETY OUTCOMES UP TO 12 MONTHS
There were three deaths in the PTA arm and one death in the DCB arm, and no major target limb amputations in either arm up to 12 months.
Discussion
The IN.PACT BTK randomised study is a prospective, multicentre, feasibility pilot study evaluating the safety and effectiveness of the IN.PACT 014 DCB versus PTA for the treatment of patients with BTK CTOs. DCB angioplasty was effective in reducing LLL up to nine months compared to PTA, with similar safety outcomes between the groups. There were no major target limb amputations, and mortality rates were low up to 12 months in this complex patient population.
The mean lesion length in this study was over 21 cm in both groups, similar to what is expected in a real-world population with CLTI and diabetes. While direct comparisons are not possible, previous randomised controlled trials of BTK DCBs included simpler lesions with shorter lengths (<15 cm)567814 and often fewer CTOs (<40%)61415. The IN.PACT BTK study design limited randomisation to lesions with successful predilation. This aspect of the study was meant to reduce disparities between study groups and make the comparison more reliable within a small sample size. Patients with suboptimal balloon angioplasty, whether due to significant residual stenosis or flow-limiting dissection, were not enrolled in the study. To ensure an optimal PTA, the predilation balloon was sized at a 1:1 ratio to the reference vessel diameter.
To better evaluate the efficacy of angioplasty in this complex lesion setting, the subsegment method of angiographic assessment for BTK treatment efficacy was previously introduced11. In this subsegmental method, a tandem array was employed to allow better assessment of MLD across the entire lesion as opposed to a single MLD measurement through the classic angiographic analysis. This method was especially helpful in the present study, considering the population of complex long lesions (mean lesion length of over 21 cm), in which the classic angiographic method of assessing LLL is considered inadequate (Razavi M. Transverse View Area Loss [TVAL]: An informative angiographic outcome in below-knee lesions. The Leipzig Interventional Course [LINC]. 2020, January 28-31, Leipzig, Germany). Although there was a reduction of >30% LLL with DCB compared to PTA at nine months in the classic analysis, the difference between groups was not significant, most likely due to the small sample size and the inadequate assessment method. When a subsegmental angiographic evaluation was used to assess LLL, there was a tenfold increase in the number of compared vessel segments that improved the power of analysis. As a result, the difference between DCB and PTA became larger with a relative reduction of >50% in favour of DCB, which was significant. The subsegmental analysis also revealed target lesion details that were otherwise unappreciated with classic analysis: the pattern of restenosis was focal after DCB treatment versus diffuse after PTA, and the number of target lesion subsegments that had restenosis (>50%) in the DCB group was about half that of the PTA group at nine months. This difference may reflect a better distal perfusion irrespective of the absolute maximal LLL and minimal percentage diameter stenosis.
Due to the lesion length and exclusive enrolment of CTOs in this study, it is difficult to compare our results with those of previous trials. In the IN.PACT DEEP study, a trial that evaluated a different DCB (IN.PACT Amphirion; Medtronic), 12-month LLL was approximately 0.6 mm in each study arm among participants who were selected for angiographic evaluation8. In the IN.PACT DEEP angiography cohort, the mean lesion length was restricted to <10 cm and <40% of participants in each group had CTOs at baseline8, which is unusual in patients with CLTI who often have long tibial occlusions16. In addition, 12-month angiography was performed in approximately 50% of the scheduled participants, which is low and weakens the validity of those results8. The primary outcomes of ACOART BTK performed with the Litos DCB (Acotec Scientific Holdings Ltd.) were recently published10. The outcomes between IN.PACT BTK and ACOART BTK are also not directly comparable since there are inherent trial design differences. For example, the primary endpoint was LLL at six months in ACOART BTK as opposed to nine months in IN.PACT BTK. Furthermore, lesion characteristics were more complex in IN.PACT BTK (lesion length, 21.5 cm in the DCB group, 21.8 cm in the PTA group; CTOs, 100% in both groups) compared to ACOART BTK (lesion length: 16.8 cm DCB, 18.7 cm PTA; CTOs: 67.7% DCB, 66.7% PTA). Nonetheless, the nine-month LLL of 0.59 mm (subsegmental analysis) or 0.89 mm (classic analysis) in the DCB arm of IN.PACT BTK was in line with that from ACOART BTK (6-month LLL was 0.51 mm for DCB). LLL in the PTA arms was also comparable in these two BTK trials (1.31 mm at 9 months in IN.PACT BTK and 1.31 at 6 months in ACOART BTK).
The IN.PACT BTK study was not powered to detect a difference in clinical endpoints, such as CD-TLR or other parameters. Recently, a meta-analysis of eight randomised trials, investigating BTK angioplasty with paclitaxel-coated balloons versus PTA, reported significantly worse 12-month amputation-free survival for paclitaxel-coated balloons17. However, there were no differences in the composite safety endpoint at 9 months or all-cause mortality at 12 months, both of which were numerically (but not statistically) better in the DCB group versus PTA. No major target limb amputations were reported in either treatment group up to 12 months. While this excellent finding is encouraging, we acknowledge that this study enrolled only patients with optimal balloon angioplasty and distal run-off to the foot, which excluded patients with high risk of early re-occlusion and insufficient foot perfusion, both risk factors for major amputation.
Strengths of this study include an improved balloon platform and a pre-specified wound management guide, which was absent in the IN.PACT DEEP trial. The investigational IN.PACT 014 DCB shares the same paclitaxel drug formulation of 3.5 µg/mm2 in urea excipient as that of the IN.PACT Admiral DCB used in the IN.PACT SFA and IN.PACT Global trials, and approved for femoropopliteal lesions. The investigational IN.PACT 014 DCB is markedly different than the IN.PACT Amphirion DCB that was used in the IN.PACT DEEP BTK trial8, including changes in balloon material and coating methods. The present study includes a pre-specified foot lesion healing programme with continuous monitoring of the healing process and patency by scheduled visits. This aspect is crucial for prompt diagnosis of lesion worsening and vessel re-occlusion and allows a fast track strategy for reintervention before the foot lesion becomes irreversible.
Study limitations
While the subsegmental method improved the power of analysis, the sample size was small to be statistically powered for events such as reinterventions. Due to the nature of the procedure, it was not possible to blind the operator or study site staff. In addition, participants were not blinded due to inherent differences in procedure between the DCB and PTA groups, for example procedure times, as the DCB group was treated with an additional balloon. While there was a strict schedule for wound care follow-up, wound information was collected by visual estimation only. Furthermore, granular wound care and offloading information for individual patients is not available.
Conclusions
At nine months, participants in the DCB group experienced a large separation (53% lower) in subsegmental LLL compared to those in the PTA group. Similarly, using the classic method, participants in the DCB group showed a trend of lower LLL compared to those in the PTA group. Safety outcomes were similar between the two arms. Future larger studies, utilising improved study design and analytical methods such as those described in this study, are warranted to confirm the safety and effectiveness of DCBs in CLTI patients with infrapopliteal lesions.
Impact on daily practice
Clinical studies, including randomised controlled trials, have shown mixed results on the safety and effectiveness of DCBs for the treatment of BTK lesions in patients with CLTI; study designs have come into question. The pilot IN.PACT BTK randomised study demonstrates that DCB angioplasty is feasible in CLTI patients with infrapopliteal chronic total occlusions, and suggests that the approach is safe with a potential added benefit over PTA by reducing late lumen loss as evaluated by classic angiographic and new subsegmental methods. The improved design of the IN.PACT BTK randomised study embraces inclusion criteria that reduce confounding factors, a new angiographic method that provides detailed subsegment information, a pre-specified wound care programme, and an improved DCB platform.
Acknowledgements
The authors would like to recognise and thank the participants and investigators involved with the clinical study for their participation. The authors also thank Kathleen Cahill, MS, for technical review of the manuscript, Giulia Gatta, MSc, and Wendy Moeyersons, MSc, for clinical study contribution, Pei Li, PhD, for statistical review, and Zachary Harrelson, PhD, and Sangeeta Yendrembam, PhD, for medical writing assistance in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Funding
This study was supported by Medtronic.
Conflict of interest statement
F. Liistro is an advisory board member for Biotronik, Boston Scientific, Medtronic, and Philips. I. Weinberg is a consultant for Magneto Thrombectomy Solutions, a National Principal Investigator for Penumbra, Inc., and Medical Director of VASCORE, the Vascular Imaging Core Laboratory. A. Almonacid Popma receives no personal fees; her spouse, J. Popma, joined Medtronic as an employee after completion of the analysis but prior to publication. M.H. Shishehbor reports consultant income from Abbott Vascular, Boston Scientific, Medtronic, Philips, and Terumo. S. Deckers is a full-time employee of Medtronic. A. Micari is an advisory board member for Boston Scientific and Medtronic.
Supplementary data
To read the full content of this article, please download the PDF.

