Abstract
Aims: This study sought to compare angiographic endpoints at one-year follow-up after a drug-eluting stent implantation guided by either intravascular ultrasound (IVUS) or angiography in patients with chronic total occlusion (CTO) lesions.
Methods and results: Patients with at least one CTO lesion recanalised successfully were randomly assigned to the IVUS-guided or the angiography-guided group. The use of IVUS for penetration of the true lumen and optimisation of stent expansion was only done in the IVUS-guided group. The primary endpoint was in-stent late lumen loss (LLL) at one-year follow-up. A total of 230 patients with CTO lesions after successful recanalisation were enrolled and followed with office visits or telephone contact up to 24 months. In-stent LLL in the IVUS-guided group was significantly lower compared to the angiography-guided group at one-year follow-up (0.28±0.48 mm vs. 0.46±0.68 mm, p=0.025), with a significant difference in restenosis of the “in-true-lumen” stent between the two groups (3.9% vs.13.7%, p=0.021). The minimal lumen diameter and minimal stent cross-section area significantly and negatively correlated with LLL (all p<0.001). The rates of adverse clinical events were comparable between the IVUS- and angiography-guided groups at two-year follow-up (21.7% vs. 25.2%, p=0.641).
Conclusions: The IVUS-guided stenting of the CTO lesion was associated with less LLL and a lower incidence of “in-true-lumen” stent restenosis. Additional study is required to identify the clinical benefit of the IVUS-guided procedure for CTO lesions. [ChiCTR-TRC-10000996]
Introduction
Percutaneous recanalisation of chronic total occlusion (CTO), defined as an occlusion duration of >3 months, in the coronary artery tree is the most challenging lesion subset in the interventional community1-5. In several prior studies, the patients who underwent successful CTO-percutaneous coronary intervention (PCI) derived more benefit compared with the patients who had a failure of CTO-PCI1-5. The successful recanalisation of a CTO lesion depends on the lesion’s morphological features which include CTO length, the presence of a side branch proximal to the fibrous cap, a non-tapered stump and calcification6-8. Recently, several novel approaches, including parallel wire1, controlled antegrade and retrograde tracking procedures9, have increased the rate of successful recanalisation.
Moreover, an intravascular ultrasound (IVUS)-guided wiring technique might facilitate the technical success by using a side branch that arises from the occlusion site10-12. A meta-analysis has demonstrated that IVUS guidance is associated with significant reductions in death, major adverse cardiac events (MACE) and stent thrombosis (ST) compared to angiography guidance in an entire patient cohort who received PCI using drug-eluting stents (DES)13. However, most good quality randomised clinical trials on IVUS-guided PCI have failed to demonstrate a benefit of IVUS guidance on clinical outcomes14. Unfortunately, there is no randomised study that shows the superiority of IVUS guidance over angiography guidance in terms of angiographic or clinical outcomes for patients with a CTO lesion. Accordingly, the present two-centre, randomised, prospective study was designed and aimed to investigate the difference in late lumen loss (LLL) between IVUS-guided and angiography-guided stenting CTO lesions.
Methods
PATIENTS
Between October 2010 and November 2011, patients who had at least one CTO lesion (defined as TIMI grade 0 and occlusion duration >3 months) that had been successfully recanalised (defined as a wire-crossed CTO lesion and at the distal true lumen according to angiograms) were included and randomised by the central computer (in a ratio of 1:1) into two groups, the IVUS-guided and angiography-guided groups, at two participating centres. The inclusion criteria were as follows: age 18-80 years, diagnosis of documented silent ischaemia, stable angina, unstable angina or previous myocardial infarction (MI). The exclusion criteria were as follows: age >80 years, pregnant women, liver dysfunction, creatinine >2.5 mg/dl, major bleeding or stroke within six months, platelet count <8×109/L, white blood cells <40×109/L, life expectancy <12 months, allergy to the study medications, failure of recanalisation in a CTO lesion, or presence of STEMI <24 hours from the onset of chest pain to the time of admission to the hospital, and intolerance to dual antiplatelet therapy. The study protocol was approved by the ethics committee of each centre. All patients gave written informed consent.
PROCEDURES
During the index procedure, the patients were treated according to contemporary guidelines and local practice. The CTO-PCI was performed according to routine practice with the retrograde technique used when indicated.
In the angiography-guided group, the lengths of CTO lesions (Online Figure 1) were measured from sufficiently long injections (unilateral or bilateral) that showed the proximal segment and collaterals, which supplied sufficient blood distal to the CTO. After the wire was directed to the segment distal to the CTO lesion, the operator selected the appropriate balloon diameter to dilate the lesion’s segment according to the reference vessel diameter (RVD) using visual estimation. A stepwise dilation was allowed. When the operator determined that the vessel lumen was sufficient to advance the stent, the diameter and the length of stent were selected according to the RVD by visual estimation. The length of stent had to cover the entire lesion including the CTO segment (from normal to normal). The stent/vessel ratio was 1:1, and the stent had to be post-dilated by at least 18 atm using a non-compliant balloon. IVUS images, to which the operators were blinded and which were used solely for analysis by core lab technicians, were recorded post procedure in the angiography-guided PCI group.
In the IVUS-guided group, IVUS examinations were performed before and after DES implantation with a phased-array catheter (Volcano Corp., San Diego, CA, USA). IVUS guidance criteria before the implantation of DES included: 1) if a wire was in the false lumen (defined as the lack of a normal vessel structure with three layers of vessel wall), efforts should be made to manipulate another stiff wire to penetrate into the true lumen (Online Figure 2); 2) the stent was required to cover the entire diseased segment (including distal, CTO and proximal segment) with the landing zone at the sites with minimal plaque burden; 3) the ratio of stent/vessel diameter was 0.8:1; and 4) post-dilatation with a pressure of at least 18 atm using a non-compliant balloon should be performed for all lesions. Post-procedural IVUS was checked in the IVUS-guided group and we attempted to achieve the following IVUS-defined success criteria: good apposition, stent minimal stent area (MSA) >80% of reference vessel area, symmetry index >70%, and no >Type B dissection15.
ANGIOGRAPHY AND IVUS ANALYSIS
The on-site assessment of post-procedural IVUS images was performed by experienced technicians. The analyses of angiograms and IVUS images were performed by core lab technicians (Nanjing Heart Centre, Nanjing, China) who were blinded to the study design. The TIMI flow was classified by grade, 0 to 316. Angiographic in-stent restenosis (ISR) was defined as diameter stenosis >50% measured by quantitative coronary angiography (QCA). LLL was defined as the difference between post-stenting minimal lumen diameter (MLD) minus MLD at the time of follow-up. The stented vessel was divided into a proximal segment 5 mm proximal to the stent, the CTO stent and a distal segment 5 mm distal to the stent, respectively. An angiographically defined false lumen was diagnosed as a lumen with relatively lower blood flow, side branch disappearance, separating from and parallel to a true lumen supplied by collaterals. Angiographic success was defined as the achievement of TIMI grade 3 and residual stenosis of <30%. Procedural success was defined as the achievement of angiographic success and the absence of in-hospital MACE.
FOLLOW-UP
The clinical follow-up was performed with office visits or telephone contact scheduled at one, six, 12 and 24 months. Follow-up coronary angiography and IVUS were scheduled at the 12th month (±30 days) after the index procedure, unless clinical reasons indicated an earlier schedule in both groups. All the data were collected and entered into a dedicated computer database by trained personnel of the Clinical Data Management Centre (Nanjing Heart Centre, Nanjing, China). An events committee blinded to treatment allocation adjudicated all adverse clinical events.
The primary endpoint was in-stent LLL at one-year follow-up. The secondary endpoints included all-cause death, cardiac death, MI, ISR, target lesion revascularisation (TLR) and target vessel revascularisation (TVR). The rate of definite/probable ST served as a safety endpoint. Periprocedural MI (PMI) was diagnosed when the plasma level of troponin I/T increased to >3 times the upper reference limit (URL) in no fewer than two blood samples. Subsequent MI was defined as CK-MB >threefold the URL. All deaths were determined to be cardiac in origin unless non-cardiac reasons were indicated. TLR and TVR were defined as any repeated revascularisation (PCI/CABG) for target lesions and target vessels, respectively, in the presence of symptoms or objective signs of ischaemia. ST was defined according to the Academic Research Consortium definition16.
STATISTICAL ANALYSIS
We hypothesised that the rate of in-stent LLL would be significantly different in the two groups13,17, favouring the IVUS-guided group over the angiography-guided group. Accordingly, a total sample size of 200 patients was needed to detect a power=0.8 (Type II error=0.20, α=0.05, two-sided). To accommodate a 15% (N=30) loss because of the considerable uncertainty regarding the expected endpoint rates, enrolment was extended to 230 patients. The treatment group differences were evaluated using the t-test or Wilcoxon rank-sum scores for continuous variables as appropriate. The χ2 test or Fisher’s exact test was used to analyse categorical variables. Survival rates free from events were generated using Kaplan-Meier analysis, and they were compared using the log-rank test. A two-sided p<0.05 was considered to indicate statistical significance. All analyses were performed using the statistical programme SPSS 16.0 (SPSS Institute Inc., Chicago, IL, USA).
Results
Of 316 patients with at least one CTO lesion, 47 patients were excluded from the present study because they were candidates for CABG (n=33) or they had CTO in the distal obtuse marginal artery (n=4) or AMI <24 hrs (n=10). Among the remaining 269 patients with 357 CTO lesions, 230 patients (85.5%) with 310 CTO lesions (86.8%) successfully recanalised were randomly assigned to the IVUS-guided and angiography-guided groups (Figure 1).
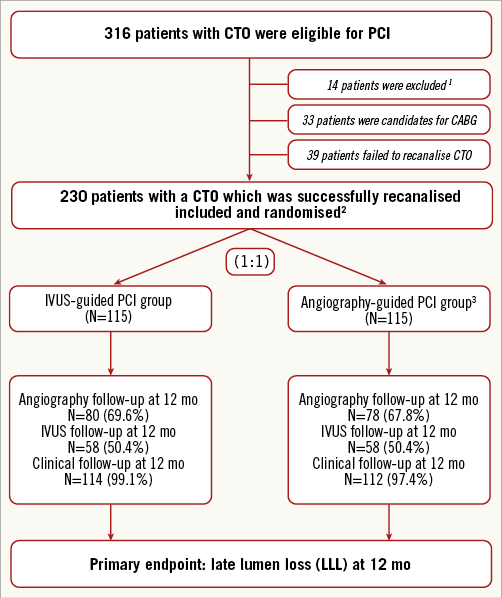
Figure 1. Study flow chart. 1Patients were not eligible for the study according to the inclusion/exclusion criteria. 2The IVUS-guided entry point was used in 21 patients (n=12 in the IVUS group, n=9 in the angiography group). 3Post-procedural IVUS images were recorded in the angiography-guided PCI group, but no additional treatments were performed after IVUS. CTO: chronic total occlusion; IVUS: intravascular ultrasound; mo: months; PCI: percutaneous coronary intervention
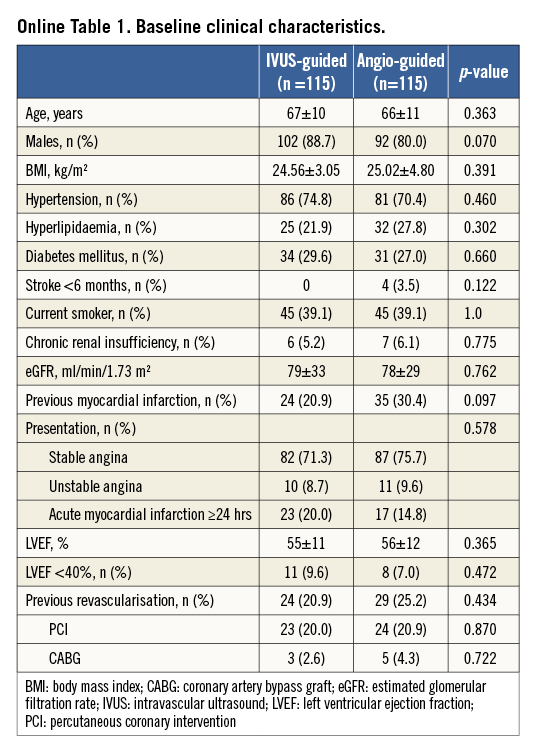
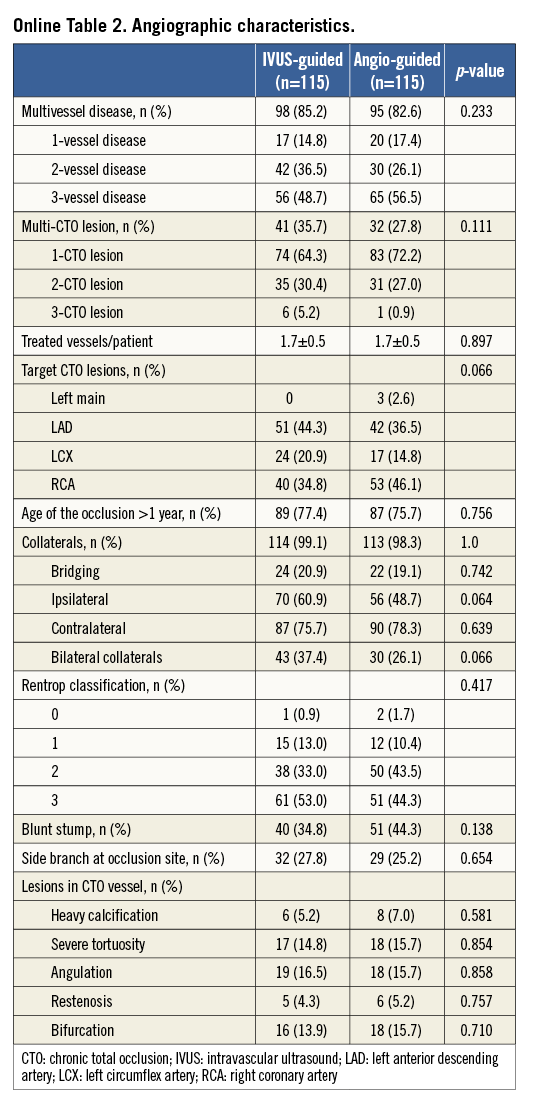
The baseline and angiographic characteristics were well matched between the two groups (Online Table 1, Online Table 2). Multiple CTOs were observed in 31.7% of the patients. A non-tapered stump was noted in 34.8% of the patients in the IVUS-guided group and in 44.3% in the angiography-guided group (p=0.138). Approximately 25% of the patients had a side branch at the occlusion sites.
IVUS was used to identify the entry point in 7.8% of lesions in the IVUS-guided group (Table 1). The average length of CTO segment was approximately 30 mm, and 31.7% of the CTO lesions had a proximal RVD of ≤2.5 mm. Actually, no differences were recognised in the STAR technique used in the two groups (12.2% vs. 8.7%, p=0.314). However, the retrograde approach was used more frequently in the angiography-guided group (10.4% vs. 19.1%, p=0.032). In the IVUS group, the wires were successfully guided by IVUS to the true lumen in two (12.5%) of 16 lesions. Fifteen stents (13.0%) were deployed in the false lumen in the angiography-guided group, compared to 14 stents (12.2%) in the IVUS-guided group (p=0.843). Finally, the length of the false lumen was significantly longer in the angiography-guided group compared to the IVUS-guided group (p=0.002). Notably, the stent diameter in the IVUS-guided group was significantly different from the angiography-guided group (p=0.001). After stenting, the IVUS-defined success rate was 91% in the IVUS-guided group, which was significantly higher than the 68% in the angiography-guided group (p=0.024).
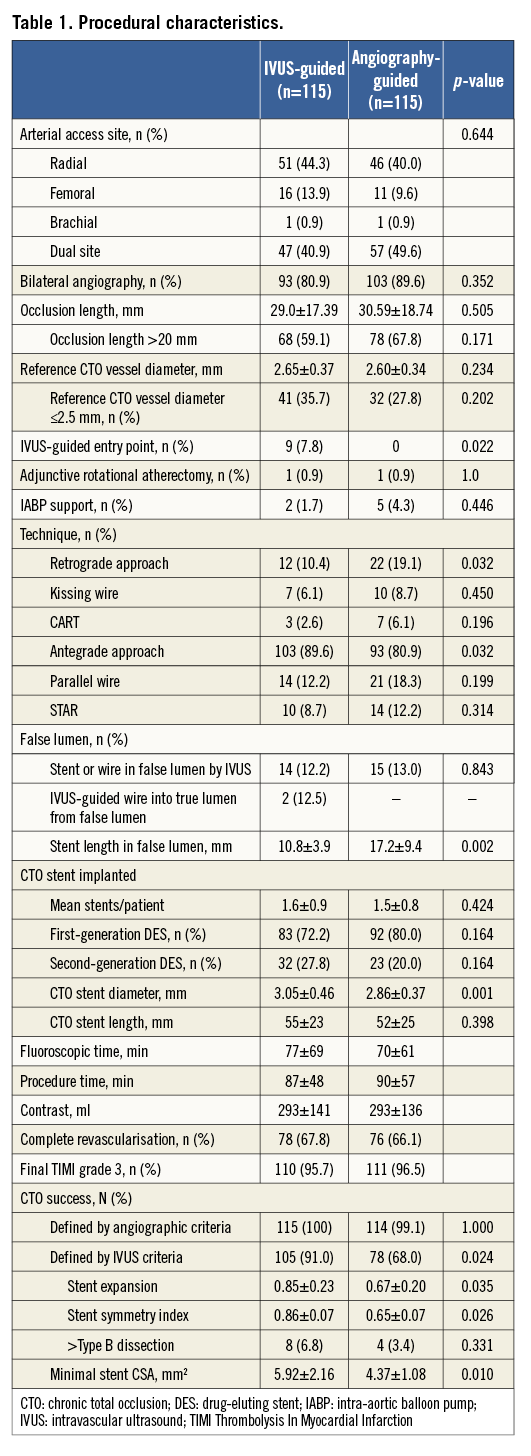
Angiographic follow-up was available in 178 (77.4%) patients (Table 2). For the CTO stent segment, there were lower LLL (0.28±0.48 mm, 95% CI: 0.003-0.355) and less diameter stenosis (21.1±19.9%) in the IVUS-guided group, compared to 0.46±0.68 mm (95% CI: 0.001-0.356) and 29.2±24.6% in the angiography-guided group (p=0.025 and p=0.002, respectively). Relative to the distal segment (Figure 2), the vessel diameter after stenting increased by 23% in the IVUS-guided group, which was similar to the 19.6% increase in the angiography-guided group (p>0.05) but was significantly different from that in the proximal RVD (p=0.001 and p<0.001). Post-stenting MLD by angiography and MSA by IVUS were negatively correlated with LLL (p<0.001) (Figure 3).
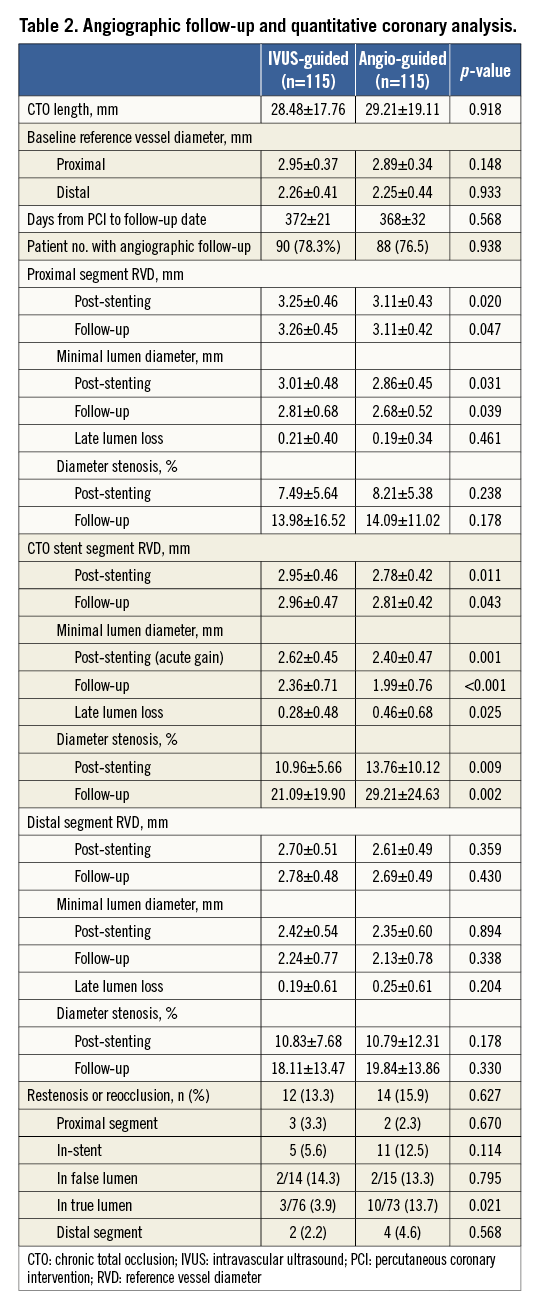
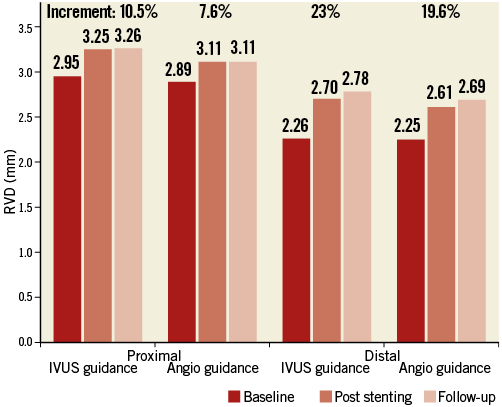
Figure 2. Enlargement of a vessel segment 5 mm proximal and distal to the stent. Immediately after stenting, vessel segments 5 mm proximal to or 5 mm distal to the stent enlarged over time. Notably, the segment 5 mm distal to the stent increased most significantly in both the IVUS-guided group and the angiography-guided group, compared to proximal segments. IVUS: intravascular ultrasound
Figure 3. Correlation of MLD and MSA with late lumen loss. MLD (A) and MSA (B) were negatively correlated with late lumen loss (r=–0.413 and r=–0.438; all p<0.001). A cut-off value of 2.75 mm for MLD and 5.9 mm2 for MSA correlated with a late loss of 0.40 mm and a predicted increased ISR rate. MLD: minimal lumen diameter; MSA: minimal stent area
The rate of restenosis in the IVUS-guided group was not significantly different from the angiography-guided group (p>0.05). Notably, the ISR rate for stents in the true lumen, but not in the false lumen, in the IVUS-guided group was 3.9%, which was significantly lower than the 13.7% in the angiography-guided group (p=0.021).
Clinical follow-up was available in all the patients (Table 3). There were no significant differences in the composite MACE and in the individual component of clinical adverse events between the two study groups. Notably, the rate of definite and/or probable ST at two-year follow-up was 0.9% in the IVUS-guided group, lower than the 6.1% in the angiography-guided group (p=0.043) (Figure 4).
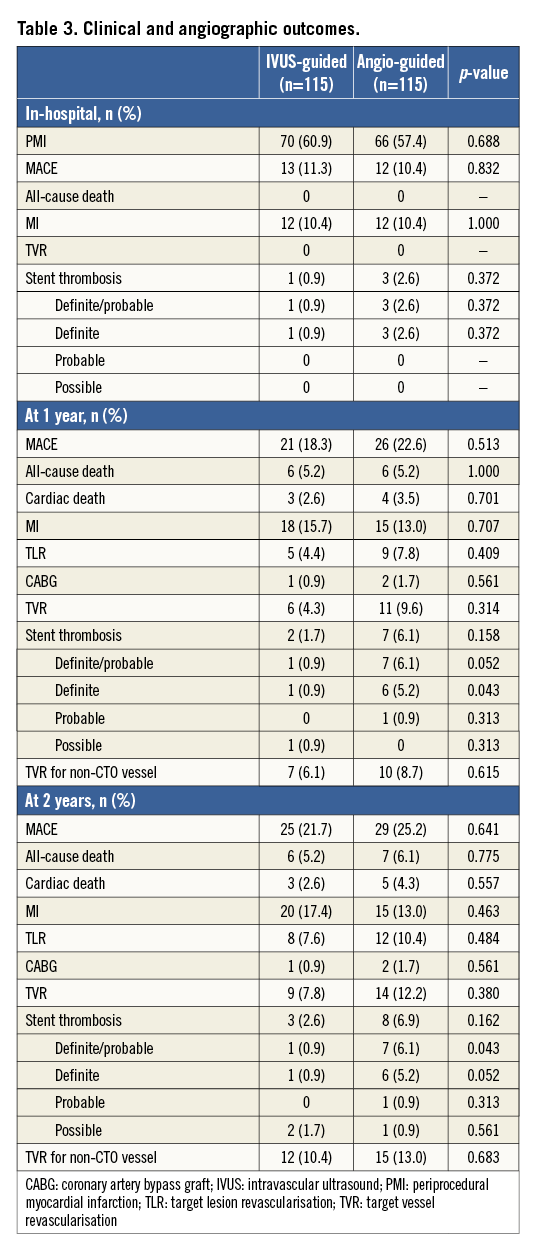
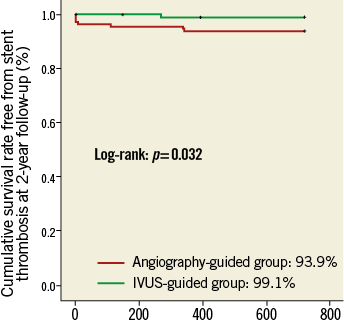
Figure 4. Kaplan-Meier curves of definite/probable ST. Cumulative rate free from definite/probable ST was 93.9% in the angiography- guided group and 99.1% in the IVUS-guided group (p=0.032). IVUS: intravascular ultrasound; ST: stent thrombosis
Discussion
To the best of our knowledge, the present study provides the first report of a reduction in LLL when a CTO-DES procedure was guided by IVUS, leading to less frequent restenosis, and when a DES was deployed in the true lumen, compared to angiography guidance. We found that the distal non-stented vessel diameter grew significantly, especially in the IVUS-guided group. Most importantly, we also found that IVUS-guided CTO-PCI was associated with a lower rate of ST.
Recently, the rate of successful CTO-PCI has improved significantly by using novel techniques9,18 and new devices, even for CTO lesions with unfavourable angiographic features19,20, such as calcification, the greater length of CTO lesions, poorly developed collateral, and bridging collaterals which primarily lead to the inability to pass a guidewire into the distal lumen. This rate was achieved overall in 86% of CTO lesions from our results. Previous studies have reported the advantage of IVUS-guided over angiography-guided PCI for the entire cohort of patients with obstructive coronary disease treated by implantation of a DES10-13. However, controversies exist, as it is hard to establish statistical differences in ST because of a relatively small sample size and very low incidence of ST especially in the new-generation DES era17. More recently, a meta-analysis by Jang et al demonstrated the improved clinical outcomes after PCI with DES guided by IVUS21. In particular, Hong et al22 have demonstrated that IVUS-guided CTO-PCI was associated with a reduction of ST and MI compared with angiography-guided CTO-PCI. However, the use of IVUS was determined at the discretion of the operator, and no specific IVUS guidance criteria were described. Unfortunately, there is a lack of randomised clinical data showing the superiority of IVUS guidance to angiography guidance for CTO lesions, a lesion subset having the most severe plaque burden (totally occluded), multiple CTO lesions, and multivessel diseases. IVUS-guided CTO-PCI can be used for the following purposes: to identify exactly the entry point for a CTO lesion with a non-tapered stump and a proximal side branch in which an IVUS catheter had been inserted21,23; to ensure a wire in the true lumen during full wire passage and to help direct wire manipulation into the true lumen12; to guide the reverse CART technique24; to guide the selection of predilation and stent size12,21,23; and to optimise acute results, including stent expansion, edge dissection and minimal stent area12. We found that an IVUS-guided penetrating entry point from a side branch at the occlusion site was used in approximately 9% of CTO lesions, which indicated that the presence of a side branch was no longer considered to be an independent factor of CTO-PCI procedure failure, and that it served as a guide for identifying the entry sites. In the present study, the IVUS guidance failed to direct all the wires into the true lumen, because there were multiple false lumens during full passage in 14 of 16 lesions, which suggested the importance of avoiding the creation of more or longer false lumens from the antegrade approach12,23, as evidenced by the relatively high rates of restenosis of stents in the false lumen.
The correlation of minimal stent area with subsequent restenosis and clinical events has been reported in previous studies25,26, even in studies of non-CTO lesions. Our results showed that IVUS guidance was associated with larger minimal stent CSA and good stent expansion because larger stents and repeated post-dilation were used to optimise stent expansion according to the IVUS criteria, leading to larger post-stenting MLD, which was inconsistent with the findings of the AVIO trial14. Most importantly, the current study achieved its primary endpoint, i.e., LLL in favour of the IVUS-guided group, a finding shared by the AVIO trial results14. As shown in Figure 3, both post-stenting MLD and MSA were negatively correlated with LLL; therefore, IVUS guidance was associated with less frequent restenosis. Until now, there have been no clear data showing the incidence of ISR if a stent was deployed in the false lumen. We found this incidence tended to be high in the IVUS-guided group, although without significant difference. The likely explanation would be the aggressive vessel damage which was induced by the IVUS guidance for negotiating the true lumen. However, the ISR for stents in the true lumen was significantly lower in the IVUS-guided group, which supported the routine use of the IVUS-guided CTO-PCI procedure10-12,14,25,26. Another important finding was the growth in the distal vessel segment sustained through one-year follow-up, from which reduction of plaque distal to the stent could not be ruled out.
Several previous studies have reported that the IVUS-guided PCI procedure was beneficial to patients with coronary artery disease10-13 after implantation of a DES. However, the AVIO trial, a first randomised study, did not find a predictive value of IVUS guidance for clinical adverse events14. In this first randomised study of stenting CTO lesions, we found that the improvement in LLL using IVUS guidance did not translate into a reduction of composite MACE. The likely reasons could include the following: the primary endpoint of the current study was LLL, which would be underpowered for the comparison of clinical solid endpoint(s); myocardial viability was not measured (leading to the disassociation of ISR and TLR or TVR which tended to be high in the angiography-guided group); and clinical follow-up was only for two years. In particular, we found that “in-false-lumen” stenting was associated with a high rate of ISR in the two groups, similar to a study by Valenti et al27 who reported that subintimal tracking was independently related to the risk of reocclusion. In addition, we found that IVUS-guided stenting of CTO lesions had a lower rate of ST at two years, a similar finding to the retrospective, registry study by Hong et al28. However, it was underpowered to provide any conclusive statement on this rare endpoint. As a result, we believe that optimal stent expansion will improve long-term outcomes in CTO lesions23.
Limitations
Selection bias was likely because CTO lesions in which recanalisation failed were excluded from this study. Because IVUS could not be used for CTO-PCI failure, we believe that the superiority of IVUS guidance over angiography guidance was fully compared. The second limitation was that myocardial viability was not measured, which would mask the actual requirement of revascularisation. Third, a high proportion of retrograde approach was used in the angiography group, which might have contributed to the lower risk of restenosis compared with the IVUS group29. Fourth, the procedures were performed by operators with a lot of experience of IVUS guidance for CTO-PCI. Thus, the degree of efficacy may be higher when it is introduced to a centre that routinely does very little IVUS in this setting. Fifth, this study was conducted in two participating centres in which three experienced operators (with a high volume of yearly PCI cases and IVUS-guided procedures) performed all of the procedures. Consequently, translating the results from our study into practice should be interpreted with caution. Sixth, LLL might not be a reliable parameter to predict the requirement of revascularisation, as it overestimated the incidence of ISR28, especially in the modern DES era using a second generation of cobalt-chromium everolimus-eluting stents which have a lower rate of ST17. However, our results provided the information that IVUS-guided stenting of CTO lesions reduced LLL and ST. If confirmed in a future randomised trial, this would represent a paradigm shift. Seventh, the different generations of DES have an impact on the primary endpoint of in-stent LLL, but less than 30% of patients received new-generation DES in the present study. Finally, it is hard to provide any definitive conclusions on clinical outcomes due to the limited sample size in this study.
Conclusions
The present study is the first to report that IVUS guidance CTO-PCI procedures are associated with a reduced LLL, ISR, and ST when stenting occurred within the true lumen. Additional studies with larger patient populations are required to identify the clinical benefits of IVUS guidance for CTO lesions.
| Impact on daily practice Chronic total occlusion (CTO) is the most challenging lesion subset and associated with a poor clinical prognosis. Intravascular ultrasound (IVUS) guidance facilitates crossing the CTO lesion with a wire, evaluating lesion severity, optimising the procedure, and subsequently improves angiographic and clinical outcomes in patients with CTO. Thus, in daily practice, it is reasonable to recommend the use of IVUS as a conventional strategy in patients with CTO undergoing drug-eluting stent implantation. |
Acknowledgements
We appreciate the assistance of Ms Ling Lin, Ms Hai-Mei Xu, Ms Yin-Yin Zhao, and Ms Ling-Ling Liu for their remote monitoring and data collection. We thank Ms Jing Kan for her statistical analyses.
Funding
This study was partially funded by the Jiangsu Provincial Clinical Medical Centre Program (JPCMCP-2011).
Conflict of interest statement
The authors have no potential conflicts of interest to declare.
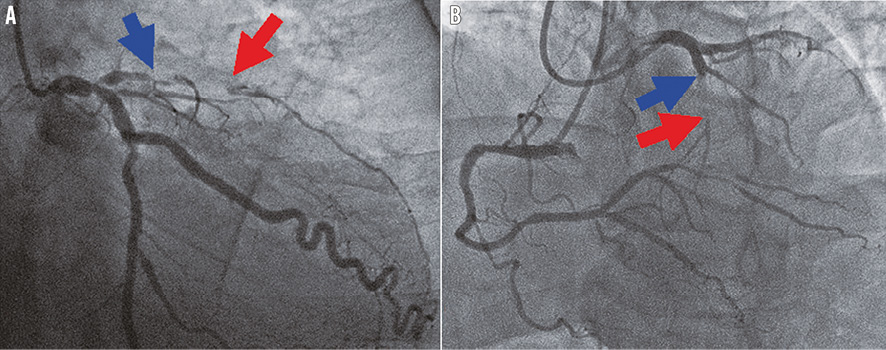
Online Figure 1. Assessment of CTO length. A chronic total occlusion in the LAD is shown. Collaterals from LCX (A) or RCA (B) clearly identified the distal CTO (red arrow), and the proximal edge of the CTO was evident by the antegrade injection (blue arrow). The distance between the two arrows was defined as the CTO length. CTO: chronic total occlusion; LAD: left anterior descending; LCX: left circumflex; RCA: right coronary artery
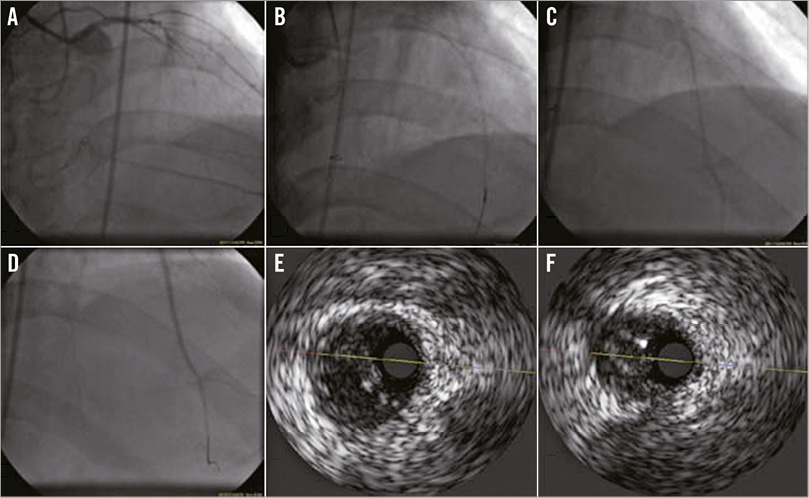
Online Figure 2. Identification of the true lumen using IVUS. A chronic total occlusion in the LAD is shown. A PROGRESS® 120 wire (Abbott Vascular, Santa Clara, CA, USA) was at the distal true lumen (A); the wire body was in the false lumen (B). Under IVUS guidance, the wire was directed to and passed into the true lumen (C), as confirmed by an injection of contrast material via the central lumen of a microcatheter (D). IVUS recorded at the mid-LAD demonstrated that a false lumen was localised on the left side of the true lumen (E) and that a second wire had penetrated into the true lumen (F). IVUS: intravascular ultrasound; LAD: left anterior descending