In the field of complex percutaneous coronary interventions (PCI), chronic total occlusions (CTO) pose the highest degree of complexity. The success rates of CTO-PCI are the lowest and complication rates rank as the highest among all lesion types. Nonetheless, advances in technical and operator skills have led to progressively better success rates1.
Among CTO, in-stent CTO are particular challenging. Although re-permeabilisation rates are comparable to de novo CTO, in-stent CTO are associated with more stent-related adverse events during follow-up2,3.
IVUS may play a key role as a highly valuable tool for guiding and optimising PCI in CTO. Randomised trials have demonstrated that IVUS improves outcomes, probably due to better stent optimisation4,5. However, the benefit of IVUS in these procedures goes beyond optimising stent expansion, since IVUS is definitely helpful for monitoring different steps of wire crossing during both antegrade and retrograde procedures, thus potentially increasing re-permeabilisation rates.
The manuscript published in this issue of EuroIntervention by Yoon et al6 explores the value of stenting optimisation by IVUS in PCI procedures for in-stent CTO as compared to de novo CTO.
The study consists of a large observational registry including 1,516 consecutive patients who underwent PCI for 147 in-stent CTO and 1,439 de novo CTO between 2007 and 2018 in the Asan Medical Center. IVUS was used to guide stenting in over 90% of patients in both groups. The technical success and in-hospital adverse event rates were similar between CTO types. Among those who received drug-eluting stents, the five-year target vessel failure and target vessel revascularisation (TVR) rates were also comparable. In conclusion, the authors report similar high success rates and very good long-term outcomes for both types of CTO.
One of the unique features about this study is that IVUS was used in more than 90% of cases. This could be the main potential explanation for the excellent outcomes, in agreement with the previously mentioned trials4,5. Nonetheless, several aspects in this remarkable study deserve further discussion.
Patient selection, procedures and clinical outcomes
In this study, CTO located in vessels with a reference lumen diameter <2.5 mm were excluded. Implantation of drug-eluting stents in small vessels is not uncommon, and these portend the highest risk for restenosis. This selection bias along with a lower-risk clinical profile could favour the prognosis of the in-stent CTO group.
Being aware of the baseline differences between CTO groups, the investigators performed a propensity score matching which resulted in being consistent with the Cox analysis. Needless to say, this matched analysis was unable to eliminate the effect of all the potential confounders. In addition, the absolute number of patients in the in-stent CTO group was relatively small, limiting the statistical power for comparison.
As previously indicated, the observed TVR rate was very low, which is particularly remarkable for the in-stent CTO group. In fact, outcomes in this group appear quite different to those reported in other in-stent CTO registries2,3. Nonetheless, several significant differences between registries may account for this discrepancy (Table 1). Of note, in the TORO registry, 40.6% of successful cases had angiographic evaluation at follow-up and one third of them underwent target lesion revascularisation (TLR). In previous registries the use of IVUS was low (9.9-16.7%). In the TORO registry, the PCI success rate in those with IVUS use was 89.7% and 80.1% in those without (p=0.2), and the incidence of TLR was 11.2% and 13.9%, respectively (p=0.6).
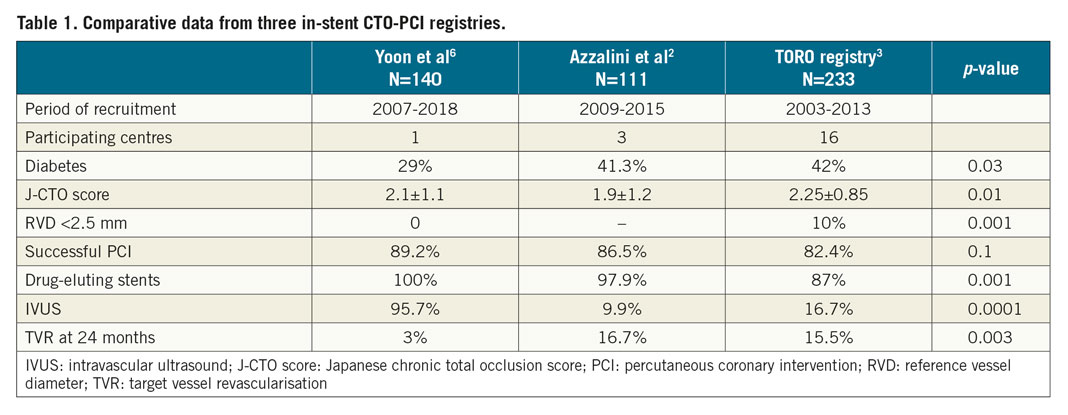
It is reasonable to assume that the large difference in the use of IVUS between registries could to some extent explain the different outcomes, but the highlighted differences between registries should be taken into consideration as contributors to the observed discrepancy (Table 1).
IVUS-guided optimisation
It is well known that baseline examination with IVUS, or even better with optical coherence tomography (OCT), helps to identify the underlying mechanisms for stent failure, leading to a more adequate PCI strategy. In this study, among the in-stent CTO, underexpansion was detected in 22.5% of cases.
As a matter of fact, the use of IVUS to optimise stenting implies an advantage in terms of acute procedural results, but this strategy needs to be informed by a set of criteria and optimisation targets to be pursued. The adequacy of these targets and adherence to them by the operators could make a difference to clinical outcomes7.
In this study, stent sizing was carried out considering the external elastic lamina diameters of the distal reference as well as the ability of the particular stent platform to accommodate expansion to the proximal reference diameter. The resulting stent sizing appeared to be somewhat aggressive with a mean stent diameter of 3.2 mm, given ~3 mm and ~2 mm for proximal and distal reference lumen diameters, respectively. Generally, the most commonly recommended approach to stent sizing is according to the distal reference lumen, to avoid stent distal edge problems. Thus, in cases with very long CTO lesions associated with marked tapering, stent platforms that tolerate larger overexpansion should be used. Alternatively, as in this study, stent sizing considering the external elastic lamina diameters of the distal reference site could be considered.
The selection of a 5.5 mm2 target for the minimum stent area seems appropriate as the best predictive cut-off value for restenosis, but it has a limited case-based value since reaching that cut-off area requires a very different degree of stent expansion according to the size of the reference vessel. Of note, in this study nearly half of the patients showed final stent underexpansion, similar in both groups whichever definition was considered. It is clear that CTO have several features that often make it challenging to achieve optimal stent expansion even under IVUS guidance (large and extensive plaque burden, frequent calcification and constrictive remodelling of the distal vessel). In this regard, IVUS could help to select the best plaque preparation approach. In the study by Yoon et al, the more aggressive stent sizing appears to have compensated for the limited degrees of stent expansion attained.
It is highly plausible, though speculative, that in-stent CTO-PCI guided solely by angiography would have led to a selection of smaller stents associated with a lower degree of stent expansion, and ultimately to a higher rate of stent-related events during follow-up.
The consistent demonstration of clinical benefits provided by intravascular imaging guidance during PCI, particularly in complex settings such as in-stent CTO, makes the inertia of its low penetration in European countries unjustifiable.
We know why, when and how, so let’s perform imaging in complex PCI.
Conflict of interest statement
J.M. de la Torre Hernandez is in receipt of grants/research support from Abbott Medical, Biosensors, Bristol Myers Squibb, and Amgen, and honoraria or consultation fees from Boston Scientific, Medtronic, Biotronik, AstraZeneca, and Daiichi Sankyo.
Supplementary data
To read the full content of this article, please download the PDF.

