Abstract
Background: In-stent chronic total occlusion (CTO) presents various occlusion patterns, which complicate percutaneous coronary intervention (PCI).
Aims: The aim of the study was to investigate the initial outcome and strategy of PCI for in-stent CTO according to the angiographic occlusion patterns.
Methods: This study assessed 791 in-stent CTOs from the Japanese CTO-PCI Expert Registry from 2015 to 2018. They were divided into four patterns: pattern A (n=419), CTO within the stent segment; pattern B (n=196), CTO beyond the distal edge; pattern C (n=85), CTO beyond the proximal edge; and pattern D (n=69) CTO beyond both the proximal and distal edges.
Results: There were significant differences in the technical success rates (96.2%, 86.2%, 92.9%, and 75.4% for patterns A–D, respectively; p<0.001), guidewire crossing times (22 [interquartile range: 10-46], 52 [24-102], 40 [20-78], and 86 [45-127] min, respectively; p<0.001), and the rates of antegrade approach alone (90.9%, 61.2%, 67.1%, and 31.9%, respectively; p<0.001).
Conclusions: PCI for CTO within the stent segment was associated with excellent initial outcomes with the antegrade approach. However, PCI for CTO beyond both the proximal and distal edges was associated with the poorest outcomes, even with the bidirectional approach.
Introduction
The initial outcome of percutaneous coronary intervention (PCI) for chronic total occlusion (CTO) has been improved by the widespread use of the retrograde approach and antegrade dissection re-entry technique1,2. In-stent CTOs represent 5%-15% of all CTO-PCIs, and recent studies have reported good results of PCI for in-stent CTO3,4,5. However, in a real clinical setting, in-stent CTO presents various occlusion patterns, which often complicate the PCI procedure6. In the present study, we assessed the impact of occlusion patterns on the initial outcomes and strategies of the PCIs performed for in-stent CTOs by certified operators.
Materials and methods
POPULATION AND EVALUATION OF OUTCOMES
The Japanese Board of CTO Interventional Specialists was established in 2013 and arranged for the development of a prospective non-randomised registry of patients undergoing CTO-PCIs performed by highly experienced Japanese specialists. The design of this registry has been reported in detail previously7. The study protocol was approved by the ethics committees of each participating centre. Written informed consent was obtained from all patients in accordance with the study protocol.
We assessed the PCIs for in-stent CTOs performed by 49 highly experienced Japanese specialists from January 2015 to December 2018. All in-stent CTOs were classified into four patterns according to the geographical distribution of the target occlusion in relation to the previously implanted stent from the angiogram prior to the PCI procedure. The classified patterns were as follows (Central illustration):
– Pattern A: CTO within the stent segment. An occlusion positioned within the previously implanted stent segment.
– Pattern B: CTO beyond the distal edge. An occlusion extending beyond the distal edge of the previously implanted stent.
– Pattern C: CTO beyond the proximal edge. An occlusion extending beyond the proximal edge of the previously implanted stent.
– Pattern D: CTO beyond both the proximal and distal edges. An occlusion extending beyond the proximal and distal edges of the previously implanted stent.
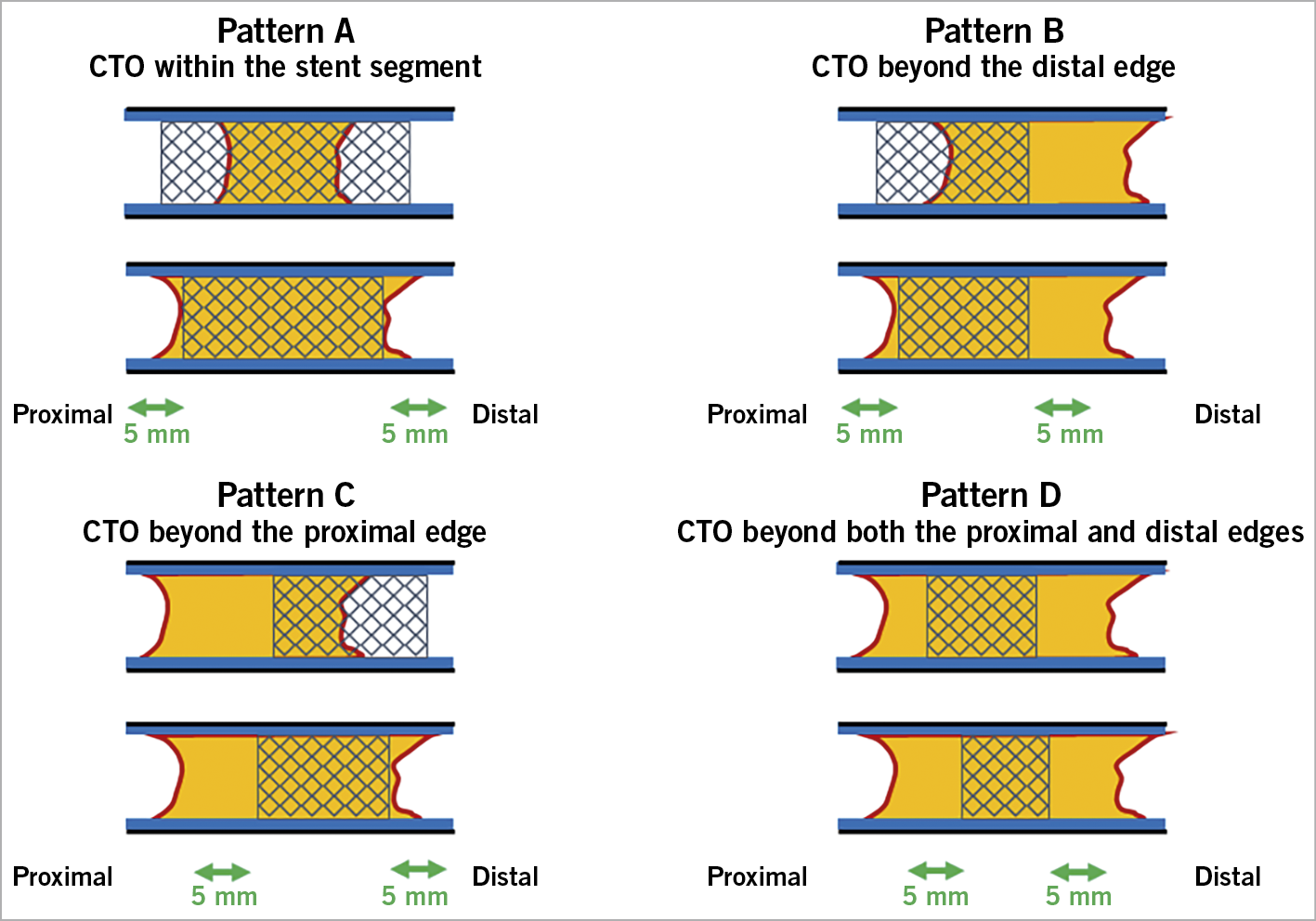
Central illustration. Occlusion patterns of in-stent chronic total occlusions. According to the occlusion patterns, in-stent CTOs were divided into the following groups. A) CTO within the stent segment. B) CTO beyond the distal edge. C) CTO beyond the proximal edge. D) CTO beyond both the proximal and distal edges. An edge was defined as an area 5 mm from the stent edge. The stent segment included the in-stent region as well as the proximal and distal edges.
An edge was defined as an area 5 mm from the stent edge. The stent segment included the in-stent region, as well as the proximal and distal edges. The selection of the CTO-PCI strategy was at the operator’s discretion. In each occlusion pattern, the procedural strategies were classified into an antegrade approach alone, rescue bidirectional approach, and primary bidirectional approach. The procedural strategy, guidewire success, technical success, procedural success, procedural time, contrast volume, fluoroscopic time, in-hospital major adverse cardiac and cerebrovascular events (MACCE), and other complications were compared among the four occlusion patterns. The guidewire crossing time was also compared to the guidewire success.
STUDY DEFINITIONS
CTO was defined as the presence of Thrombolysis In Myocardial Infarction (TIMI) flow grade 0 within an occluded arterial segment for more than three months. The CTO-PCI procedure was defined as a bidirectional approach when an attempt was made to cross the collateral channel supplying the vessel distal to the target CTO lesion for retrograde revascularisation. The collateral connection grade was classified as previously reported8. The J-CTO (Multicenter CTO Registry in Japan) score is useful for classifying the lesion difficulty of a de novo CTO, as previously reported9. In this study, the assumed J-CTO score was applied to assess the difficulty of the in-stent CTO lesions. In-hospital MACCE were defined as death, myocardial infarction, stroke, or revascularisation during the same admission. Myocardial infarction was defined as both continuous changes on the electrocardiogram and increasing peak serum creatine kinase value >3 times the upper reference within 48 hours after CTO-PCI. Contrast-induced nephropathy was defined as either a 25% increase in serum creatinine from baseline, or a 0.5 mg/dl increase in absolute serum creatinine value within 72 hours after CTO-PCI10. The procedural time was defined as the time from the initial insertion of the guidewire into the coronary lumen to the final angiography of the CTO lesion. Guidewire success was determined when a guidewire crossed a CTO lesion from either the antegrade or retrograde direction. The guidewire crossing time was defined as the time required to cross the CTO lesion. Technical success was achieved when both a guidewire and balloon crossed the CTO lesion, the occluded artery was successfully dilated, and the antegrade flow was restored (TIMI flow grade of 3) with less than 50% residual stenosis on final angiography. Procedure success was defined as technical success without any in-hospital MACCE or major side branch occlusion7.
STATISTICAL ANALYSIS
Continuous variables are presented as mean±standard deviation (normally distributed data) or median (interquartile range [IQR]: non-normally distributed data) and were compared using one-way ANOVA or the Kruskal-Wallis test. Additional repeated measures of post hoc analysis were performed using Tukey’s test or the Steel-Dwass test. Categorical variables are expressed as frequencies or percentages and were compared using Fisher’s exact test and the chi-square test, as appropriate. Additional repeated measures of post hoc analysis were examined using the Bonferroni test. All reported p-values were two-sided, and p-values <0.05 were considered statistically significant. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). EZR is a modified version of R Commander which was designed to add statistical functions that are frequently used in biostatistics11.
Results
Figure 1 shows a flow chart of the present study. Within the study period, 6,365 CTO-PCIs were enrolled into the registry; of these, 145 were excluded by the registry secretariat and independent core laboratory owing to missing data or inappropriate angiographic assessment of the target lesion. In total, 791 (12.7%) of the 6,220 analysable CTO-PCIs were performed for in-stent CTOs and were classified into four patterns according to their coronary angiograms (Central illustration). Of the 791 in-stent CTOs, 53% (n=419) were pattern A (CTO within the stent segment), 24.8% (n=196) were pattern B (CTO beyond the distal edge), 10.8% (n=85) were pattern C (CTO beyond the proximal edge), and 8.7% (n=69) were pattern D (CTO beyond both the proximal and distal edges). Twenty-two CTO-PCIs were impossible to judge from their angiograms prior to PCI procedures.
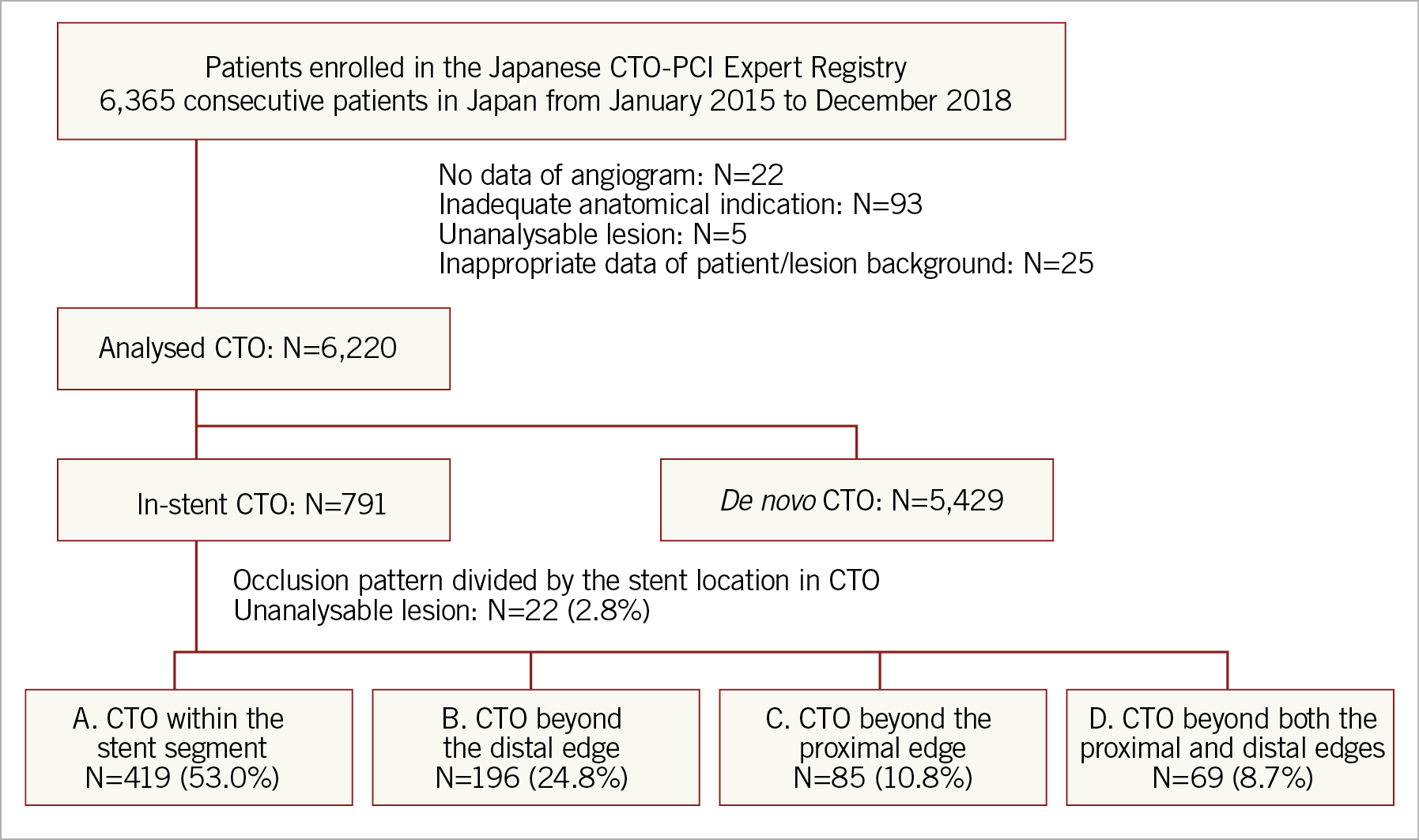
Figure 1. Flow chart of the study.
Table 1 shows the baseline patient characteristics. Among the four patterns, there were significant differences in the mean ages (pattern A, 68.7±10.0 years; pattern B, 67.0±10.8 years; pattern C, 67.4±11.5 years; and pattern D, 64.5±11.5 years; p=0.008), the frequency of males (80.4%, 80.1%, 83.5%, and 94.2%, respectively, p=0.024), the rates of prior myocardial infarction (51.8%, 65.3%, 64.7%, and 72.5%, respectively, p<0.001), and the distributions of the target vessel (p<0.001).
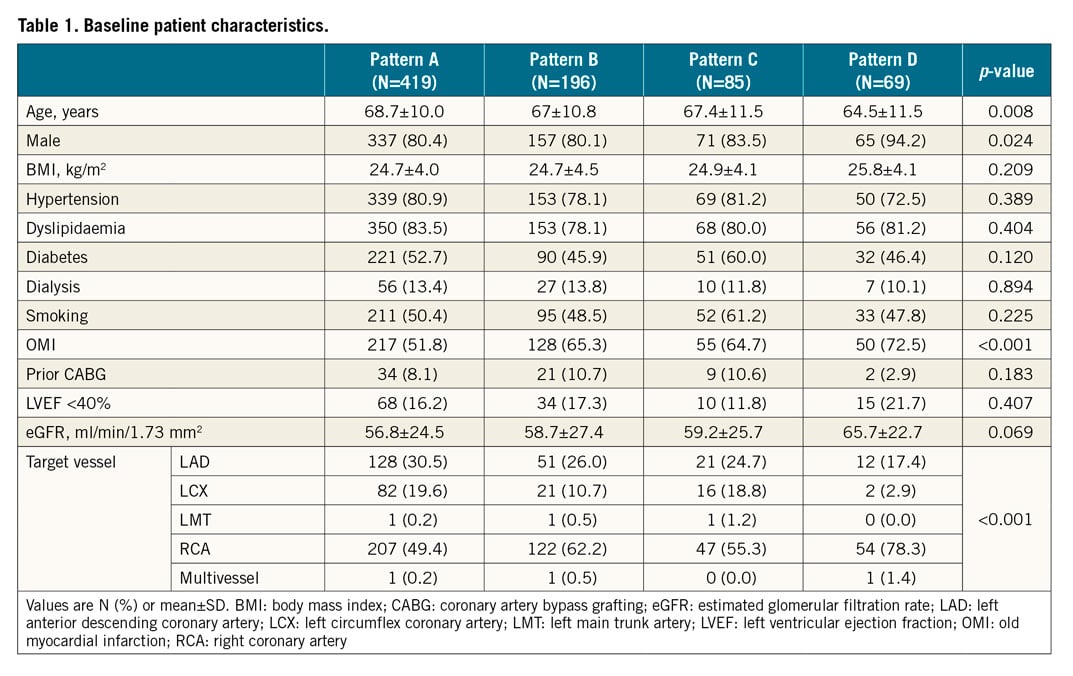
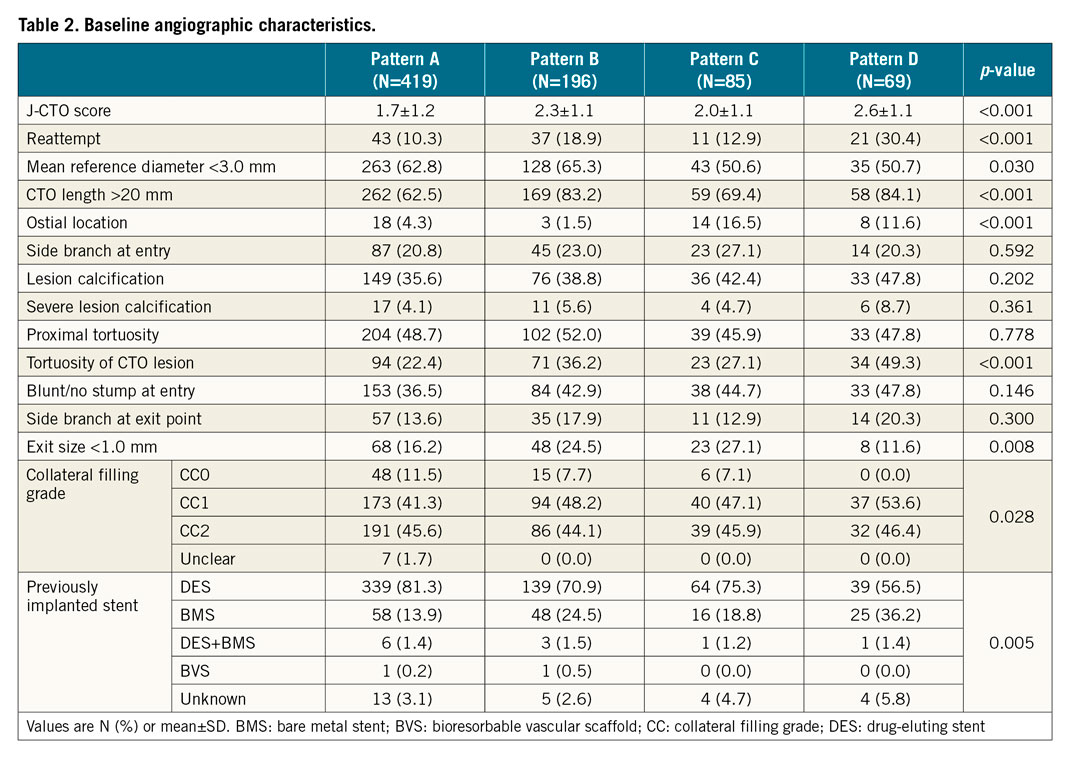
Table 2 shows the baseline angiographic characteristics. Among the four patterns there were significant differences in the mean assumed J-CTO scores (1.7±1.2, 2.3±1.1, 2.0±1.1, and 2.6±1.1, respectively, p<0.001), the rates of reattempt (10.3%, 18.9%, 12.9%, and 30.4%, respectively, p<0.001), the rates of CTO length ≥20 mm (62.5%, 83.2%, 69.4%, and 84.2%, respectively, p<0.001), the rates of ostial lesion (4.3%, 1.5%, 16.5%, and 11.6%, respectively, p<0.001), the rates of tortuosity of the CTO lesion (22.4%, 36.2%, 27.1%, and 49.3%, respectively, p<0.001), and the types of previously implanted stents (p=0.005).
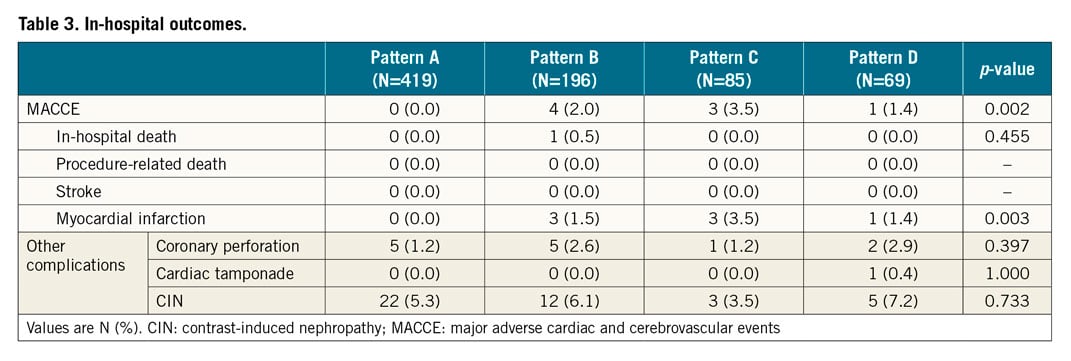
Supplementary Table 1 shows the technical and clinical results. The rates of technical success were significantly different among the four patterns (96.2%, 86.2%, 92.9%, and 75.4%, respectively, p<0.001). The rate of technical success of pattern A was significantly higher than that of patterns B and D, while the rate of technical success of pattern C was higher than that of pattern D with additional repeated measures of post hoc analysis (Figure 2). Among the four patterns, there were significant differences in the rates of guidewire success (97.6%, 89.8%, 92.9%, and 81.2%, respectively, p<0.001), the rates of procedural success (95.9%, 84.2%, 89.4%, and 75.4%, respectively, p<0.001), the procedural time (103 [IQR: 70-145] minutes, 145 [IQR: 100-205] minutes, 138 [IQR: 97-201] minutes, and 180 [IQR: 145-254] minutes, respectively, p<0.001), the contrast volumes (150 [IQR: 110-200] ml, 175 [IQR: 131-260] ml, 195 [IQR: 141-260] ml, and 190 [IQR: 141-300] ml, respectively, p<0.001), and the fluoroscopic times (41 [IQR: 27-64] minutes, 69 [IQR: 46-116] minutes, 63 [IQR: 43-91] minutes, and 82 [IQR: 56-121] minutes, respectively, p<0.001) (Figure 3). The guidewire crossing times of the procedures with guidewire success were also significantly different among the four patterns (22 [IQR: 10-46] minutes, 52 [IQR: 24-102] minutes, 40 [IQR: 20-78] minutes, and 86 [IQR: 45-127] minutes, respectively, p<0.001). The mean guidewire crossing time of pattern D was significantly longer than that of patterns A, B, and C, while that of pattern B was significantly longer than that of pattern A with additional repeated measures of post hoc analysis (Figure 4). The distribution of procedural strategy was significantly different among the four patterns (p<0.001). The rate of antegrade approach alone in pattern A was significantly higher than that in patterns B, C, and D (90.9%, 61.2%, 67.1%, and 31.9%, respectively), while that in pattern D was significantly lower than those in patterns B and C with additional repeated measures of post hoc analysis (Figure 5). Table 3 shows the in-hospital outcomes. The incidences of myocardial infarction (0%, 1.5%, 3.5%, and 1.4%, respectively, p=0.003) and MACCE (0%, 2.0%, 3.5%, and 1.4%, respectively, p=0.002) were also significantly different among the four patterns.
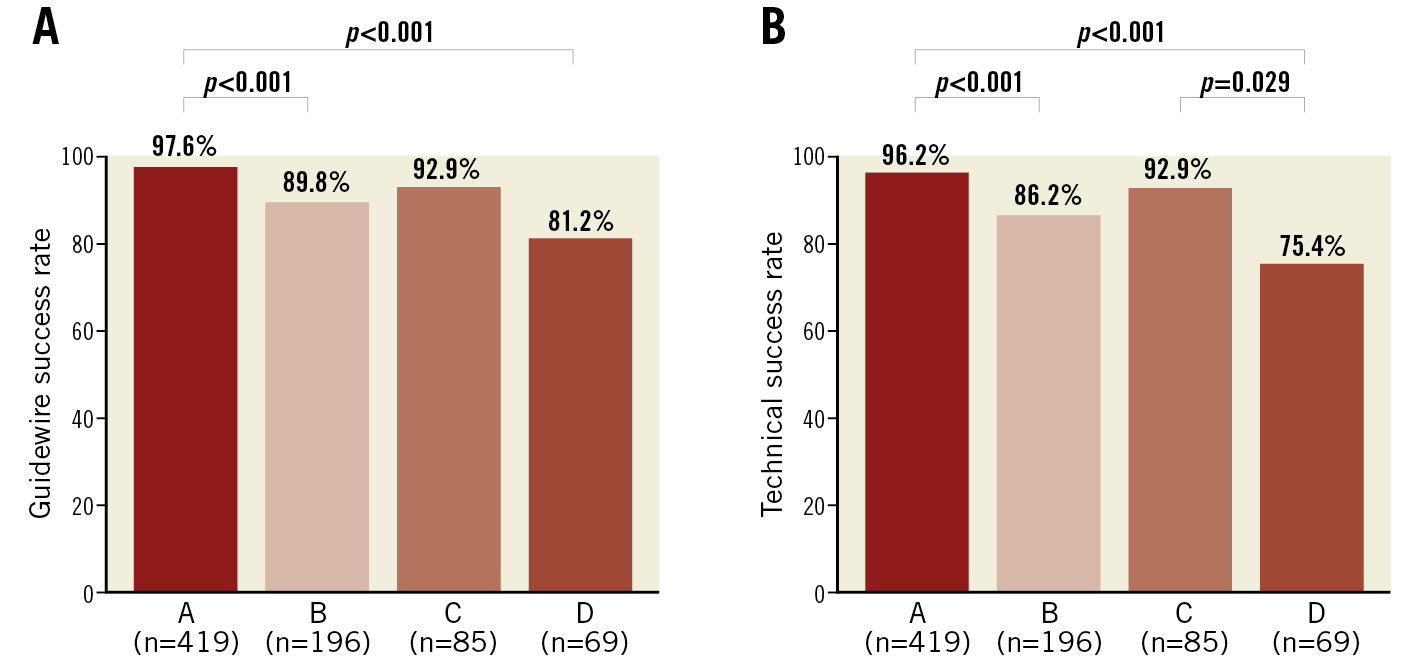
Figure 2. Guidewire and technical success rates for each occlusion pattern of in-stent chronic total occlusion. A) Guidewire success rates for each occlusion pattern. B) Technical success rates for each occlusion pattern.
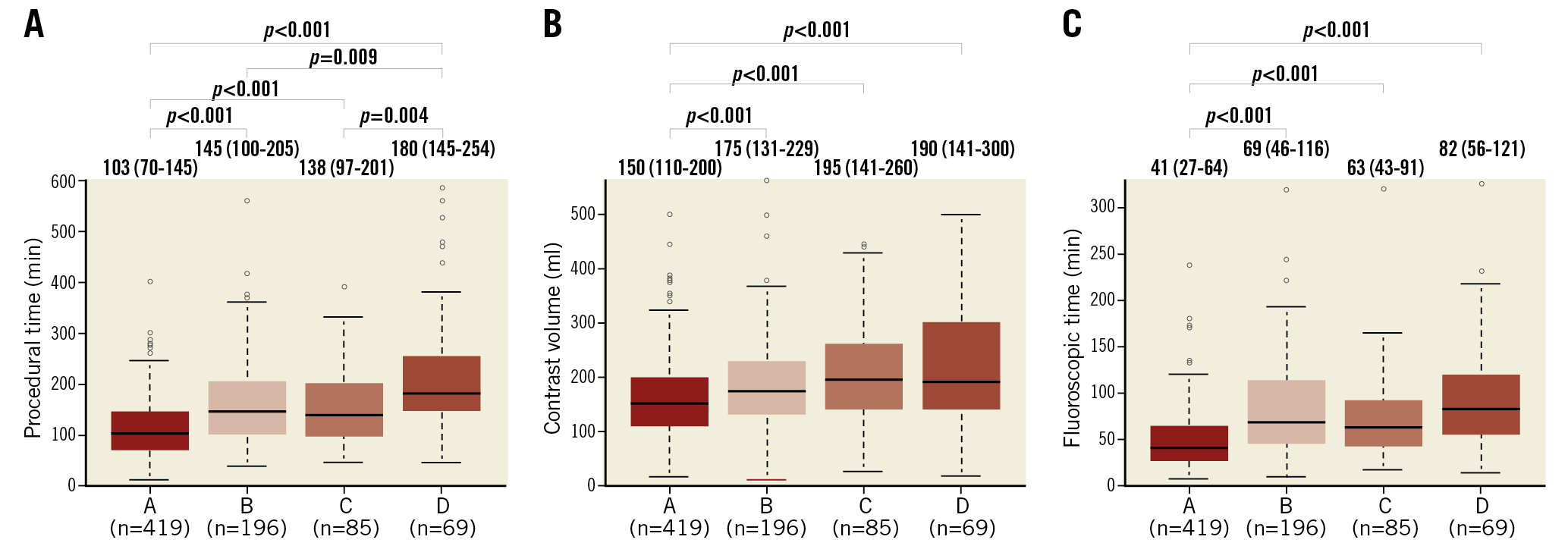
Figure 3. Procedural times, contrast volumes, and fluoroscopic times for each occlusion pattern of in-stent chronic total occlusion. A) Procedural times for each occlusion pattern. B) Contrast volumes for each occlusion pattern. C) Fluoroscopic times for each occlusion pattern. Values are median (interquartile range).
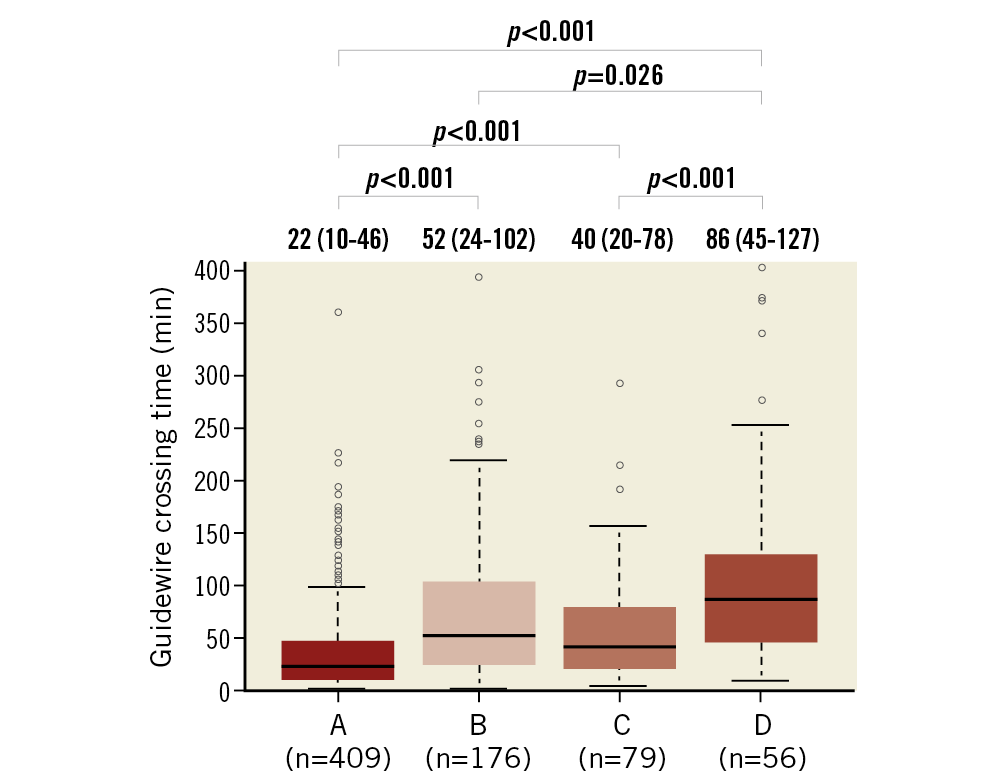
Figure 4. Guidewire crossing times for each occlusion pattern of in-stent chronic total occlusion. Values are median (interquartile range).
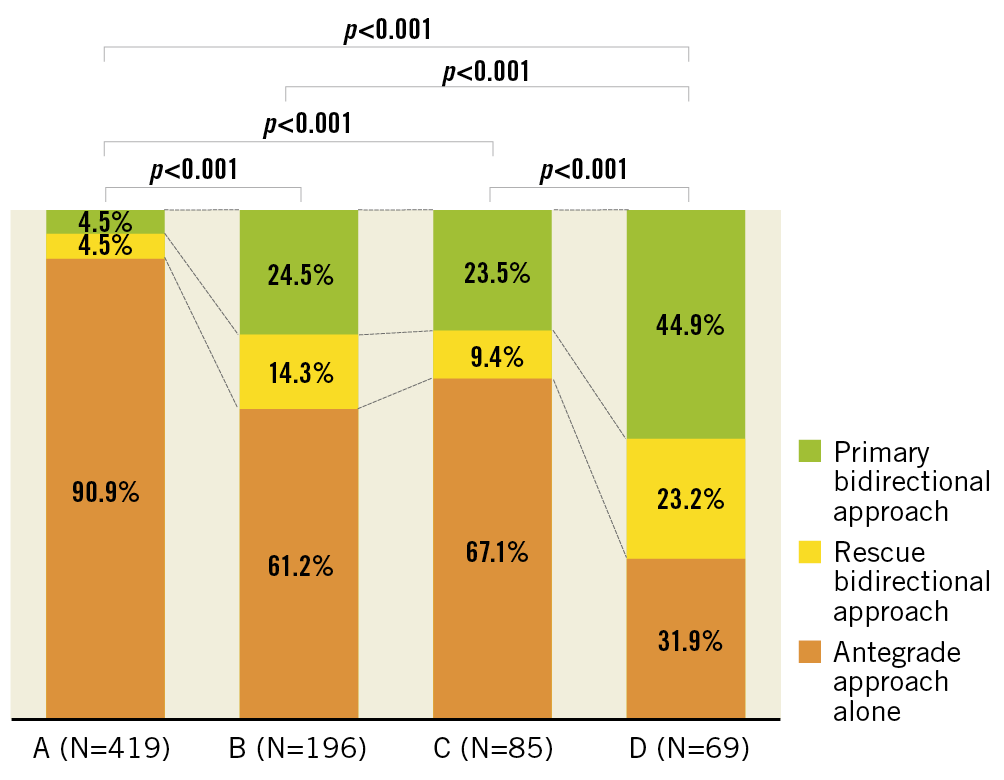
Figure 5. Actual strategies for each occlusion pattern of in-stent chronic total occlusion.
Discussion
The main findings of the present study are as follows: 1) PCI for CTO within the stent segment (pattern A) showed excellent initial outcomes, and most of these procedures were successful with only the antegrade approach technique; 2) PCI for two types of in-stent CTO lesions that extend to the distal edge of the previously implanted stent (patterns B and D) had a lower technical success rate; and 3) PCI for in-stent CTO lesions that extend to both the proximal and distal edges of the previously implanted stent (pattern D) showed the poorest initial outcomes, even with the bidirectional approach.
The present study shows excellent initial outcomes of PCI for CTO within the stent segments (pattern A), with a technical success rate of 96.2%, without any in-hospital MACCE. Several reasons for the excellent initial outcomes of PCI for CTO within the stent segments (pattern A) have been proposed. 1) As the proximal cap and the distal exit of the CTO lesion are positioned within the previously implanted stent, a guidewire can be advanced easily from the proximal cap into the intimal plaque of the CTO body, and from the distal exit into the distal true lumen in the antegrade approach technique. 2) The previously implanted stent acts as a road map for the target vessel, due to the newly developed coronary angiogram equipment, as well as the visible materials used in recent coronary stents. 3) The development of new devices, such as guidewires and microcatheters, makes it possible to perform the antegrade wiring and tracking of an in-stent CTO that can be visualised with the previously implanted stent.
PCI for CTO beyond the proximal edge (pattern C) had a longer procedural time and longer fluoroscopic time than for CTOs within the stent segment (pattern A). However, there was no significant difference in the technical success rates and the mean guidewire crossing times between these two patterns. The bidirectional approach technique was required more frequently in PCI for CTO beyond the proximal edge than for CTO within the stent segment. It is suggested that the bidirectional techniques led to guidewire success in case of a failure in the antegrade intentional intimal plaque tracking from the proximal cap to the proximal stent segment.
PCI for CTO beyond the distal edge (pattern B) showed a lower technical success rate, longer guidewire crossing time, longer procedural time, longer fluoroscopic time, and a higher contrast volume than CTO within the stent segment (pattern A). The bidirectional approach was required more frequently in PCI for CTO beyond the distal edge than CTO within the stent segment. It was to be expected that the bidirectional approach was required in case of a failure in the antegrade intentional intimal plaque tracking at the occluded lesion beyond the distal stent segment. However, the previously implanted stent and the longer and tortuous CTO lesions, as are characteristic of pattern B, could interfere with the bidirectional approach techniques12.
With regard to CTO beyond both the proximal and distal edges (pattern D), the technical success rate was 75.4%, which was lower than that of the overall CTO-PCIs in recent studies7. The bidirectional approach rate in pattern D was 68.1%, which seems extremely high. The guidewire crossing time was 86 (IQR: 45-127) minutes, which seems very long. In addition, the longer procedural time, longer fluoroscopic time, and higher contrast volume served as a reminder that PCI for CTOs beyond both the proximal and distal edges was more complicated, despite the certified expert operators. The longer and tortuous CTO lesions, as are characteristic of pattern D, could interfere with the entire PCI procedure. Furthermore, we suggest that guidewire crossing through a CTO beyond both the proximal and distal edges presents unique limitations. Indeed, the bidirectional approach technique is required in case of a guidewire failure in the antegrade intentional intimal plaque tracking at the transition from the proximal cap to the proximal stent segment, or from the distal stent segment to the distal exit. However, we need to achieve successful intentional intimal plaque tracking at the transition from the CTO lesion without stent into the previously implanted stent from the antegrade or retrograde direction. Suzuki et al reported that in-stent occlusion as well as lesion calcification and tortuosity were predictors of retrograde CTO-PCI failure after successful collateral crossing12. Guidewire failure and poor run-off tend to be the reasons for technical failure in patterns B and D. We suggest that a guidewire from the antegrade and retrograde directions can enter outside the previously implanted stent at the stent edge. A diffuse and long occlusion extending beyond the distal stent edge may relate to poor distal artery run-off. Recent studies have reported good results of PCI for in-stent CTO3,4,5. However, complex occlusion patterns, especially pattern D, were associated with poor initial outcomes and required skilful techniques and complex strategies. We recommend that an expert operator should perform the PCI for in-stent CTO with such complex occlusion patterns.
It has been reported that there are pathological differences in in-stent CTOs using bare metal stents (BMSs) and drug-eluting stents (DESs)13. Lee et al5 reported a possibility that the success rate of DES-CTO was higher than that of BMS-CTO. In the present study, BMS-CTOs were involved more frequently in patterns B, C, and D than in pattern A. Yin et al14 reported that in-stent CTO had four distinct mechanisms from the intravascular ultrasound (IVUS) images: in-stent neointimal hyperplasia, stent underexpansion, proximal new lesion progression at the stent edge, and neoatherosclerosis. This is reminiscent of the association of in-stent neointimal hyperplasia with pattern A and new lesion progression at the stent edge with patterns B, C and D. However, in the present study, the IVUS images were not analysed. The relationship between the occlusion mechanisms from IVUS images and our occlusion patterns of in-stent CTO was not evaluated. Therefore, there is a possibility that the pathological findings and the occlusion mechanisms from IVUS images of in-stent CTO influence the technical success of PCIs.
Limitations
This study has some limitations. First, this was a prospective, non-randomised, multicentre study, and therefore the treatment policy was determined by the individual operators. Second, the occlusion patterns of stent-related CTOs were classified by using the coronary angiogram before the PCI procedure only. The length, expansion, fracture, multiple implantations, and time since implantation of the previously implanted stent were not analysed.
Conclusions
In this analysis from the Japanese CTO Expert Registry, PCI for in-stent CTO showed differences in both the initial outcomes and the strategies according to occlusion patterns. PCI for CTO within the stent segment was associated with excellent initial outcomes. However, PCI for CTO beyond both the proximal and distal edges was associated with the poorest initial outcomes, even when the bidirectional approach was used.
|
Impact on daily practice This multicentre registry analysed the initial outcomes and strategies of in-stent CTOs according to their occlusion patterns. CTOs within the stent segment showed excellent initial outcomes with the use of the antegrade approach. However, CTOs beyond both the proximal and distal edges showed the poorest initial outcomes, even if the retrograde approach was used. Classification of occlusion patterns may be helpful when considering a clinical plan for patients with in-stent CTO. |
Funding
This study was funded by the Japan Chronic Total Occlusion Intervention Specialist Council, Asahi Intecc, Abbott Vascular Japan, Biosensors Japan, Boston Scientific, Daiichi Sankyo, Kaneka Medics, Medtronic Japan, Nipro, and Terumo.
Conflict of interest statement
M. Sekiguchi is a consultant for Kaneka Medics. E. Tsuchikane is a consultant for Abbott Vascular Japan, Boston Scientific Japan, Kaneka Medics, Nipro, and Asahi Intecc. S. Sumitsuji is a consultant for Terumo, Kaneka Medics, Nipro, Asahi Intecc, Abbott Vascular Japan, Boston Scientific Japan, Medtronic Japan, Biosensors, and OrbusNeich, has received grants from Terumo, Kaneka Medics, Nipro, and Asahi Intecc, and has received personal fees from Abbott Vascular Japan, Boston Scientific, Medtronic Japan, Biosensors, OrbusNeich, Amgen Astellas BioPharma, Daiichi Sankyo, Shionogi, Takeda Pharmaceutical, Heart Organization, Sumitomo Dainippon Pharma, Hokushin Medical, Canon Medical, Fujifilm Medical, Shimadzu, Bristol-Myers Squibb, Boehringer Ingelheim, Sanyo Chemical Industries, and Siemens Healthcare. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

