Introduction
Chronic total occlusion (CTO) percutaneous coronary intervention (PCI) can be highly challenging, with continually evolving equipment and techniques12. A standardisation of the definitions, techniques and the guiding principles of CTO PCI has recently been achieved through global collaboration23. We examined the evolution of the techniques, outcomes and temporal trends of CTO PCI in a diverse group of patients operators from around the world.
Methods
We analysed the baseline clinical and angiographic characteristics and procedural outcomes of 10,249 CTO PCIs performed in 10,019 patients between 2012 and 2022 at 40 US and non-US centres. Data collection was recorded in a dedicated online database (PROGRESS-CTO: Prospective Global Registry for the Study of Chronic Total Occlusion Intervention; ClinicalTrials.gov: NCT02061436).
Technical success was defined as a successful CTO revascularisation with the achievement of <30% residual diameter stenosis within the treated segment and restoration of Thrombolysis in Myocardial Infarction (TIMI) grade 3 antegrade flow. Procedural success was defined as the achievement of technical success without any in-hospital major adverse cardiac events (MACE). In-hospital MACE included any of the following adverse events prior to hospital discharge: death, myocardial infarction (MI), recurrent symptoms requiring urgent repeat target vessel revascularisation with PCI or coronary artery bypass graft (CABG) surgery, tamponade requiring either pericardiocentesis or surgery, and stroke.
Results
The mean age was 64±10 years and 81% of the patients were men. The prevalence of prior PCI (62%), prior CABG (29%) and diabetes mellitus (43%) was high. The most common CTO target vessel was the right coronary artery (53%), followed by the left anterior descending artery (LAD; 26%), and the left circumflex (19%). Failed CTO PCI was associated with a longer lesion length and higher prevalence of unfavourable characteristics, such as proximal cap ambiguity, distal cap at bifurcation, moderate to severe calcification, and moderate to severe proximal tortuosity. The target CTOs were highly complex with a mean J-CTO score of 2.4±1.3 and PROGRESS-CTO score of 1.3±1.0 that did not change over time.
Use of the bifemoral approach decreased from 46% in 2016 to 29% in 2021 (p for trend <0.001), with a significant increase in femoral-radial access from 24% to 38% (p for trend <0.001) during the same time period. Over time, the rate of antegrade wiring as the final successful crossing strategy increased, whereas antegrade dissection and re-entry decreased, and there was no significant change in the retrograde approach (Figure 1A). This increase in antegrade wiring may reflect the improvement in guidewire and microcatheter technology as well as increasing operator expertise.
Septal (64%) and epicardial (26%) collaterals were the most commonly used collaterals for retrograde crossing, with a decreasing trend for epicardial collaterals over time (Figure 1B). In experienced hands, epicardial collaterals may represent an efficient option for retrograde crossing of a CTO lesion; however, their use was associated with a higher risk of collateral perforation and tamponade, which may explain the decrease in utilisation over time4. Intravascular ultrasound was used in 47% of the cases overall, with a significant increase from 37% in 2016 to 60% in 2021 (p for trend <0.001).
Technical success increased over time, from 82% in 2016 to 88% in 2021 (p for trend <0.001), with an increase in procedural success rates during the same time period (Figure 1C). From 2016 to 2021, a significant decrease was observed in contrast volume, air kerma radiation dose, fluoroscopy time, and procedure time. Potential explanations for the decrease in radiation dose and contrast volume over time include the increased use of newer X-ray systems, increased use of intravascular imaging, increasing operator expertise, and improvements in equipment and techniques. The incidence of in-hospital MACE was 2.1% overall and was higher in technical failure cases (5.4% vs 1.6%; p<0.001). Overall MACE was 3.1% in 2016 and 1.7% in 2021 (p for trend=0.194) (Figure 1D).
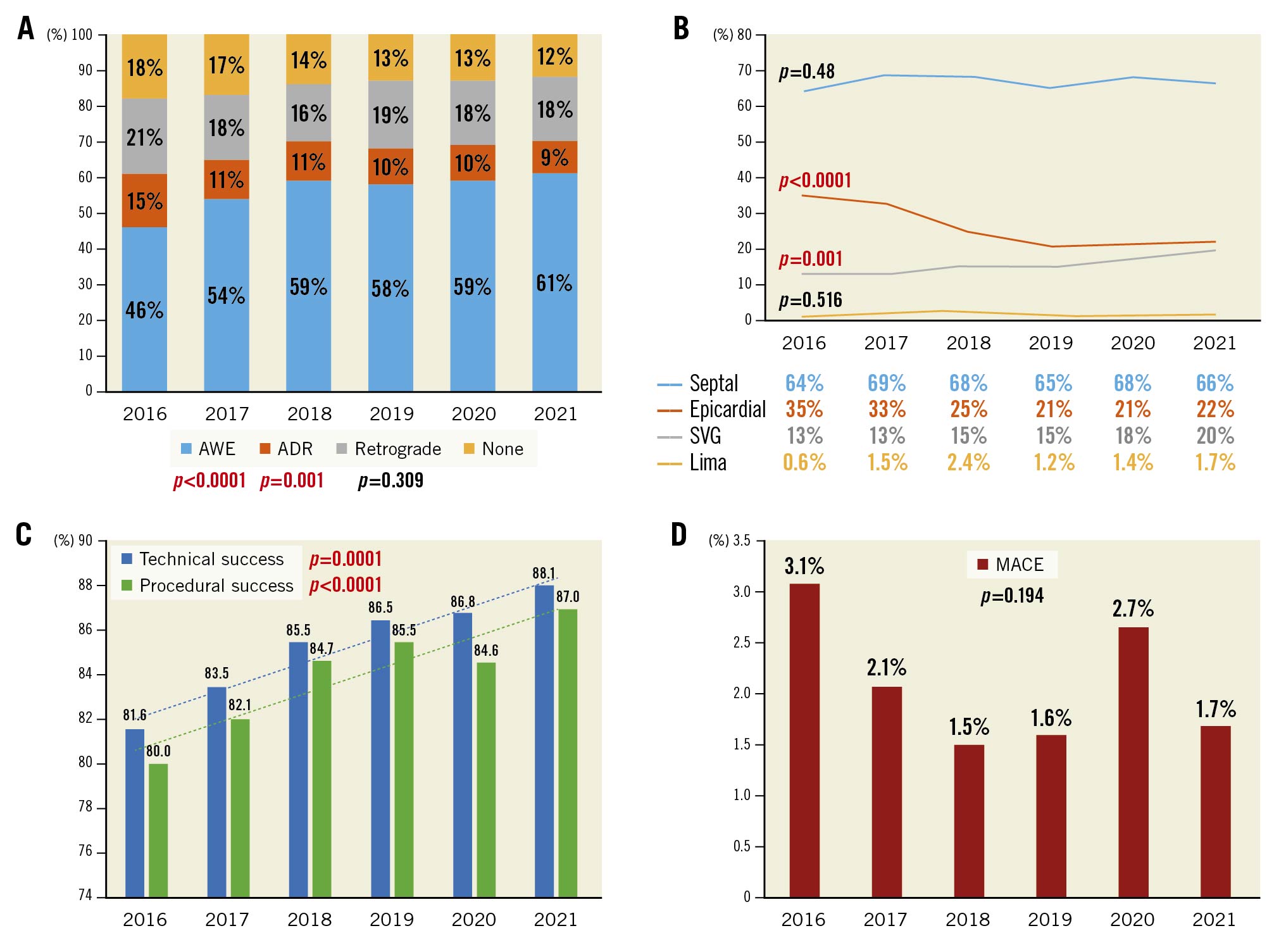
Figure 1. Temporal trends of percutaneous coronary intervention for chronic total occlusion. Temporal trends on: A) final crossing strategy, B) collaterals used for retrograde crossing, C) technical and procedural success, and D) major adverse cardiovascular events. ADR: antegrade dissection and re-entry; AWE: antegrade wiring escalation; LIMA: left internal mammary artery; MACE: major adverse cardiac events; SVG: saphenous vein graft
Discussion
In the present study, we found that technical and procedural success remained high over time with reasonably low complication rates. Previous studies have provided similarly encouraging results5. The development of advanced CTO PCI equipment and techniques during the study period can potentially explain the increasing technical success rate and the overall procedural efficiency. Furthermore, the performance of CTO PCI by increasingly skilled interventionalists and teams likely contributed to the decrease in periprocedural complications.
Limitations
Our study has limitations. First, PROGRESS-CTO is an observational study with limited long-term follow-up. Second, there was no core laboratory assessment of the study angiograms or clinical event adjudication, and there was no routine measurement of cardiac biomarkers at most centres. Third, the procedures were performed at dedicated, high-volume CTO centres by experienced operators, limiting the generalisability of our findings to centres with limited CTO PCI experience.
Conclusions
In conclusion, the success and efficiency of CTO PCI has increased in recent years without concomitant increase in the incidence of complications and with increasing success of antegrade wiring.
Funding
The authors are grateful for the generosity of our many philanthropy partners, including our anonymous donors, Drs Mary Ann and Donald A. Sens, Ms Dianne and Dr Cline Hickok, Ms Charlotte and Mr Jerry Golinvaux Family Fund, the Roehl Family Foundation, and the Joseph Durda Foundation, Ms Wilma and Mr Dale Johnson, for making this work possible at the Minneapolis Heart Institute Foundation's Science Center for Coronary Artery Disease (CCAD).
Conflict of interest statement
K. Alaswad has been a consultant and speaker for Boston Scientific, Abbott Cardiovascular, Teleflex, and CSI. D. Karmpaliotis has received honoraria from Boston Scientific and Abbott Vascular; and has equity in Saranas, SoundBite Medical, and Traverse Vascular. F. Jaffer has done sponsored research for Canon, Siemens, Shockwave, Teleflex, Mercator, and Boston Scientific; and has been a consultant for Boston Scientific, Siemens, Magenta Medical, IMDS, Asahi Intecc, Biotronik, Philips, and Intravascular Imaging Inc. He has equity interest in Intravascular Imaging Inc. and DurVena;and the right to receive royalties throughMassachusetts General Hospital licensing arrangements with Terumo, Canon and Spectrawave. J. Khatri has received personal honoraria for proctoring and speaking from Abbott Vascular, Asahi Intecc, Terumo, and Boston Scientific. P. Poommipanit has been a consultant for Asahi Intecc, and Abbott Vascular. J. Choi is on the Medtronic advisory board. W.A. Jaber has received fees from Medtronic and proctoring fees from Abbott. S. Rinfret has received fees from Abbott Vascular, Abiomed, Boston Scientific, and SoundBite Medical and has been a consultant for Teleflex. W. Nicholson has been a proctor for and on the speakers bureau and advisory boards for Abbott Vascular, Boston Scientific, and Asahi Intecc; and he reports intellectual property with Vascular Solutions. M. Patel has received consulting honoraria from Abbott, Medtronic, Terumo, and Cardiovascular Systems. E. Mahmud has been a consultant for Abiomed, Medtronic, and Boston Scientific, and chairs multiple Data, Safety and Monitoring Boards. A.M. ElGuindy has received consulting honoraria from Medtronic, Boston Scientific, Asahi Intecc, and Abbott; has received proctorship fees from Medtronic, Boston Scientific, Asahi Intecc, and Terumo; and has received educational grants from Medtronic. J. Wollmuth has been a proctor/consultant for Abbott Vascular, Boston Scientific, and Asahi Intecc. R. Riley has been a consultant and speaker for Boston Scientific, Medtronic, Asahi Intecc, and Shockwave Medical. R.E. Davies has received honoraria from Asahi Intec, Boston Scientific, Medtronic, and Siemens Healthineers; in addition to being a member of an advisory board for Shockwave Medical. R.W. Yeh has received grants and personal fees from Abbott Vascular, AstraZeneca, Medtronic, and Boston Scientific. N. Abi Rafehhas been a proctor and received speaker honoraria from Boston Scientific and Abbott Vascular. S. Garcia has been a consultant for Edwards Lifesciences, Medtronic and BSCI, as well as a proctor for Edwards Lifesciences. N. Burke has done consulting and received speaker honoraria from Abbott Vascular and Boston Scientific. E. Brilakis has done consulting and received speaker honoraria from Abbott Vascular, American Heart Association (Associate Editor,Circulation), Amgen, Asahi Intecc, Biotronik, Boston Scientific, Cardiovascular Innovations Foundation (Board of Directors), ControlRad, CSI, Elsevier, GE Healthcare, IMDS, Infraredx,Medicure, Medtronic, Opsens, Siemens, and Teleflex; has had research support from Boston Scientific, and GE Healthcare; is the owner of Hippocrates LLC; and is a shareholder in MHI Ventures, Cleerly Health, and Stallion Medical. The other authors have no conflicts of interest to declare.

