
Abstract
Aims: The retrograde approach is critical for achieving high success rates in chronic total occlusion (CTO) percutaneous coronary intervention (PCI), but has been associated with higher risk of complications. We examined the contemporary outcomes of the retrograde approach to CTO PCI aiming to identify areas in need of improvement.
Methods and results: We compared the technical and procedural outcomes of retrograde (n=1,515) and antegrade-only CTO PCIs (n=2,686) in a contemporary multicentre CTO registry. The mean age of patients undergoing retrograde PCI was 65±10 years and 86% were men, with high prevalence of prior myocardial infarction (51%), prior PCI (71%), and coronary artery bypass graft surgery (45%). The mean J-CTO score (3±1 vs 2±1, p<0.001) was higher in retrograde PCIs. The most commonly used collateral channels were septals (65%), epicardials (32%), saphenous venous grafts (14%) and left internal mammary artery grafts (2%). Overall technical (79% vs 91%, p<0.001) and procedural (75% vs 90%, p<0.001) success rates were lower with the retrograde approach, and these patients had a higher rate of in-hospital major complications than antegrade-only PCI patients (5.1% vs 0.8%, p<0.001), due to higher mortality (1.1% vs 0.1%, p<0.001), acute myocardial infarction (1.9% vs 0.2%, p<0.001), repeat PCI (0.7% vs 0.1%, p=0.001), and pericardiocentesis (1.7% vs 0.3%, p<0.001).
Conclusions: In summary, the retrograde approach to CTO PCI is performed in higher complexity lesions and is associated with lower success rates and a higher rate of major complications. Clinical Trial Registration: NCT02061436, Prospective Global Registry for the Study of Chronic Total Occlusion Intervention (PROGRESS-CTO)
Introduction
Since its introduction more than a decade ago1,2, the retrograde approach has become an essential tool for chronic total occlusion (CTO) percutaneous coronary intervention (PCI), and has been instrumental in improving technical success to ~85-90% from ~80% achieved with antegrade-only interventions3,4. The retrograde approach usually requires two guide catheters and has four key technical steps: (a) advancing a guidewire and microcatheter through a collateral vessel or a bypass graft distal to the occlusion, (b) crossing the occlusion, (c) wire externalisation, and (d) balloon angioplasty with stent implantation5. In the hybrid approach6, upfront retrograde crossing in CTOs with proximal cap ambiguity and/or poor distal target as well as after failure of antegrade crossing, if there are interventional collaterals, is recommended. We examined the contemporary outcomes of the retrograde approach to CTO PCI aiming to identify areas in need of improvement.
Material and methods
We analysed the clinical, angiographic, and procedural characteristics of 4,201 CTO PCIs performed in 4,108 patients enrolled in the PROGRESS-CTO (Prospective Global Registry for the Study of Chronic Total Occlusion Intervention; NCT02061436) registry between May 2012 and November 2018 at 21 US, one European, and one Russian centres (Supplementary Appendix 1). Some centres only enrolled patients during part of the study period due to participation in other studies. The study was approved by the institutional review board of each centre.
Coronary CTO was defined as a coronary lesion with Thrombolysis In Myocardial Infarction (TIMI) grade 0 flow of at least three months duration. Estimation of the duration of occlusion was clinical, based on the first onset of angina, prior history of myocardial infarction (MI) in the target vessel territory, or comparison with a prior angiogram. Calcification was assessed by angiography as mild (spots), moderate (involving ≤50% of the reference lesion diameter) and severe (involving >50% of the reference lesion diameter). Moderate proximal vessel tortuosity was defined as the presence of at least two bends >70° or one bend >90° and severe tortuosity as two bends >90° or one bend >120° in the CTO vessel. Blunt or no stump was defined as lack of tapering or lack of a funnel shape at the proximal cap. Interventional collaterals were defined as collaterals considered amenable to crossing by a guidewire and a microcatheter by the operator. Werner classification was used for collateral channel assessment7. Additional angiographic definitions are described in Supplementary Appendix 2.
A procedure was defined as “retrograde” if an attempt was made to cross the lesion through a collateral vessel or bypass graft supplying the target vessel distal to the lesion; if not, the procedure was classified as “antegrade-only”. Antegrade dissection/re-entry was defined as antegrade PCI during which a guidewire was intentionally introduced into the subintimal space proximal to the lesion, or re-entry into the distal true lumen was attempted following intentional or inadvertent subintimal guidewire crossing.
Technical success was defined as successful CTO revascularisation with achievement of <30% residual diameter stenosis within the treated segment and restoration of TIMI grade 3 antegrade flow. Procedural success was defined as the achievement of technical success without any in-hospital complications. In-hospital major adverse cardiac events (MACE) included any of the following adverse events prior to hospital discharge: death, MI, recurrent symptoms requiring urgent repeat target vessel revascularisation with PCI or coronary artery bypass graft surgery (CABG), tamponade requiring either pericardiocentesis or surgery, and stroke. MI was defined using the third universal definition of myocardial infarction (type 4a MI). Major bleeding was defined as bleeding causing reduction in haemoglobin >3 g/dl or bleeding requiring transfusion or surgical intervention. The J-CTO score was calculated as described by Morino et al8, the CASTLE score as described by Szijgyarto et al9, the PROGRESS CTO score as described by Christopoulos et al10, and the PROGRESS-CTO Complications score as described by Danek et al11.
STATISTICAL ANALYSIS
Categorical variables are expressed as percentages and were compared using Pearson’s chi-square test or Fisher’s exact test. Continuous variables are presented as mean±standard deviation or median (interquartile range [IQR]) unless otherwise specified and were compared using the t-test and one-way analysis of variance (ANOVA) for normally distributed variables; the Wilcoxon rank-sum test and the Kruskal-Wallis test were applied for non-parametric continuous variables, as appropriate. Multivariable logistic regression was used to examine the association between use of the retrograde approach and in-hospital MACE, as well as to identify variables associated with retrograde technical failure. Variables with significant univariable association (p<0.1) were entered into the models. All statistical analyses were performed with JMP 13.0 (SAS Institute, Cary, NC, USA). A two-sided p-value of 0.05 was considered statistically significant.
Results
Retrograde CTO PCI was attempted in 1,505 patients (36.7%) undergoing 1,515 interventions that were compared to 2,686 antegrade-only CTO PCIs performed in 2,603 patients (63.3%).
Patients undergoing retrograde CTO PCI were older and had higher prevalence of coronary risk factors, prior MI, history of prior PCI and CABG (Table 1).
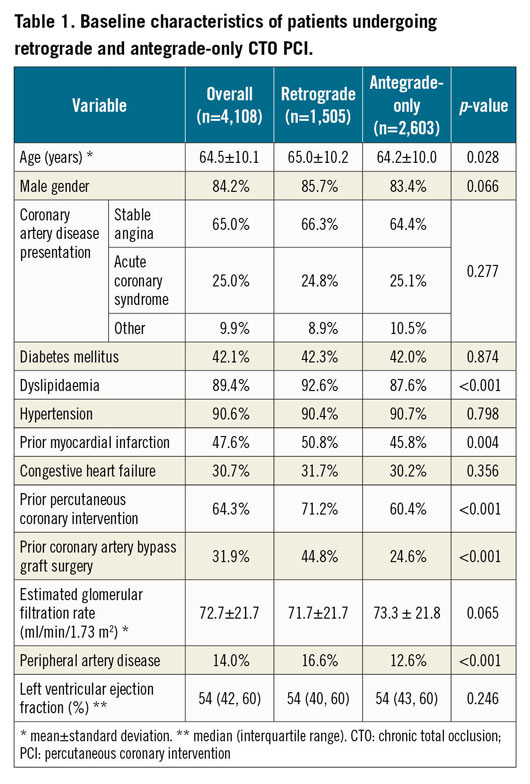
In the retrograde group, lesions were significantly more complex (Table 2). Retrograde cases had better developed collaterals (Werner Class 1: 58.1% vs 49.0%; Werner Class 2: 31.8% vs 20.7%, both p<0.001).
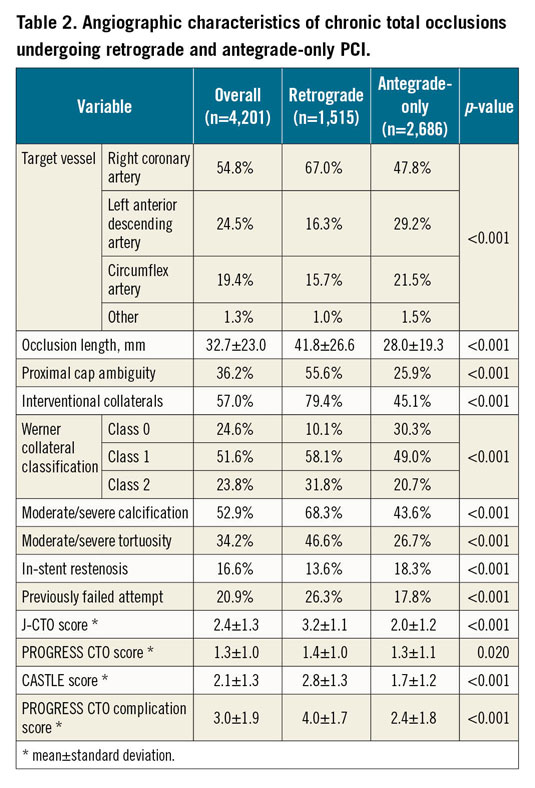
The main technical characteristics are summarised in Table 3. In primary retrograde cases the main indications for selection of the retrograde approach were the following: long occlusion length (36.6%), ostial occlusions (27.5%), side branch at proximal cap (25.6%), poor distal target vessel (24.6%), bifurcation at distal cap (22.1%), proximal cap ambiguity (15.8%), and tortuosity (14.2%). The retrograde approach was more successfully applied as the crossing strategy in more complex lesions (Figure 1).
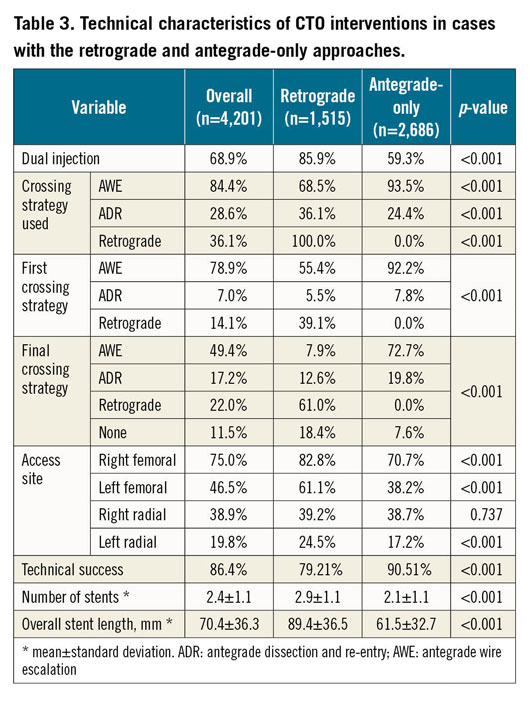
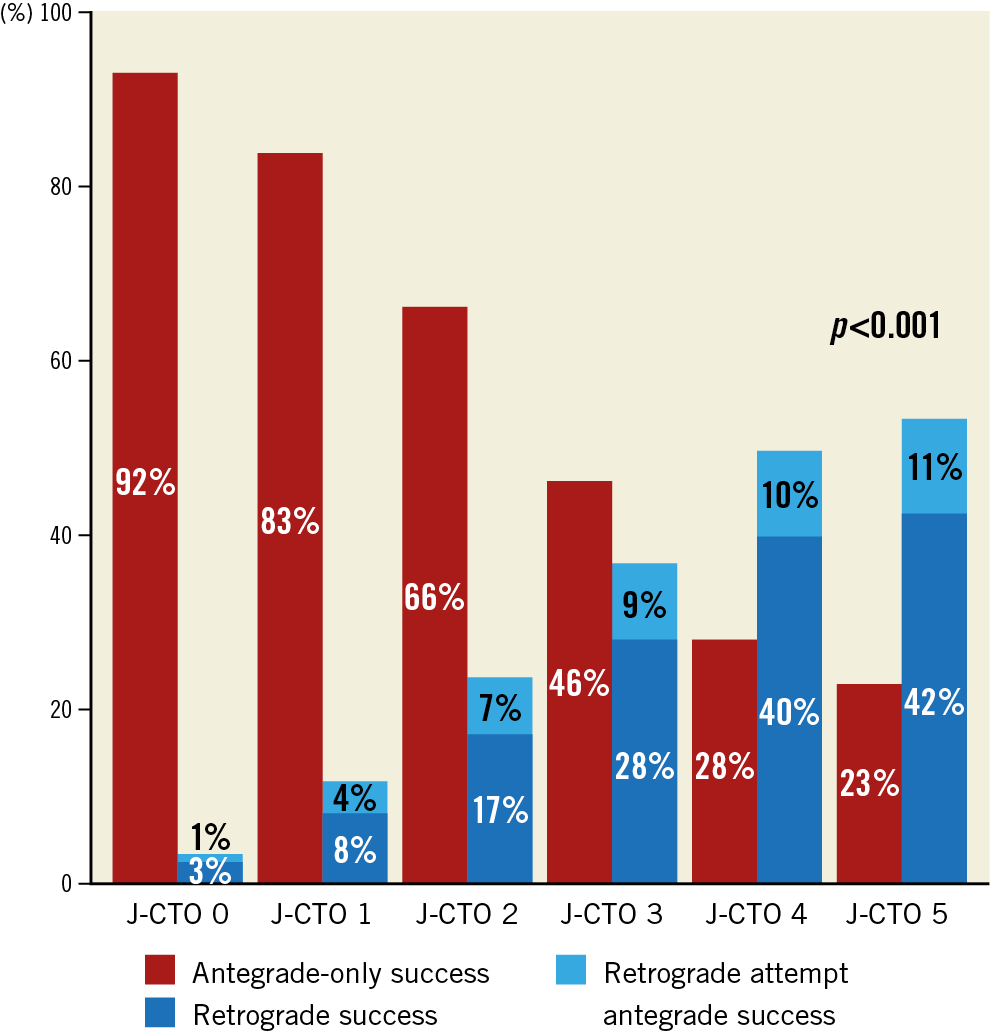
Figure 1. Technical success of CTO interventions with the retrograde approach (n=1,515) compared to antegrade-only interventions (n=2,686) stratified by the J-CTO score.
Overall technical and procedural success in the retrograde group was 79.2% and 75.4%, respectively, and was significantly lower compared with antegrade-only cases (90.5% and 90.0%, both p<0.001). In cases with successful retrograde CTO crossing, technical success was 98.4%, whereas in CTOs with retrograde wire crossing failure successful CTO revascularisation was achieved in 49.4% (Supplementary Table 1). The following collateral pathways were used: septal collaterals (62.0%), contralateral epicardial collaterals (29.9%), and saphenous vein grafts (13.5%) (Figure 2A). Successful collateral channel crossing was achieved in 75.3% (Figure 2B). Epicardial collaterals were more likely to be attempted as a second or third collateral, and low-profile microcatheters were used more frequently (Figure 3). The most commonly used crossing techniques are summarised in Figure 2C. In lesions with successful retrograde attempt, the most commonly used crossing technique was the reverse controlled antegrade and retrograde tracking (CART) (53.2%) (Figure 2D). In eight cases retrograde wire crossing was achieved with various retrograde techniques (marker wire technique [n=4]; CART [n=2]; true-to-true wiring [n=1]; reverse CART [n=1]); however, externalisation failed leading to technical failure. The predictors of retrograde technical failure are summarised in Supplementary Table 2. Retrograde cases required longer procedure and fluoroscopy times, higher volume of contrast and air kerma radiation dose compared with antegrade-only procedures (Figure 4).
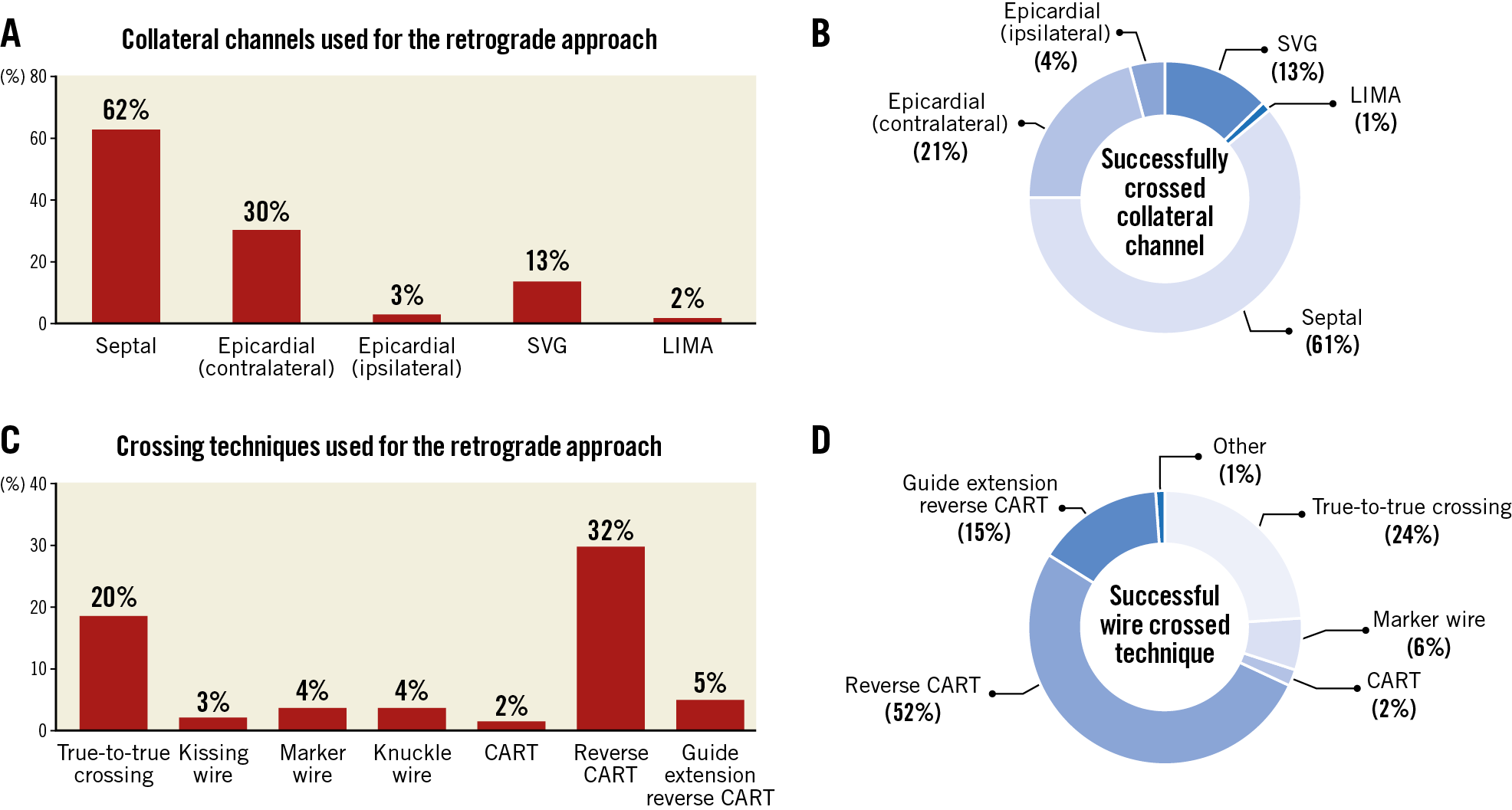
Figure 2. Technical outcomes of all the retrograde CTO interventions in terms of collateral crossing (A & B) and wire crossing techniques (C & D*). *Eight cases included with successful wire crossing, but retrograde failure due to failed wire externalisation.
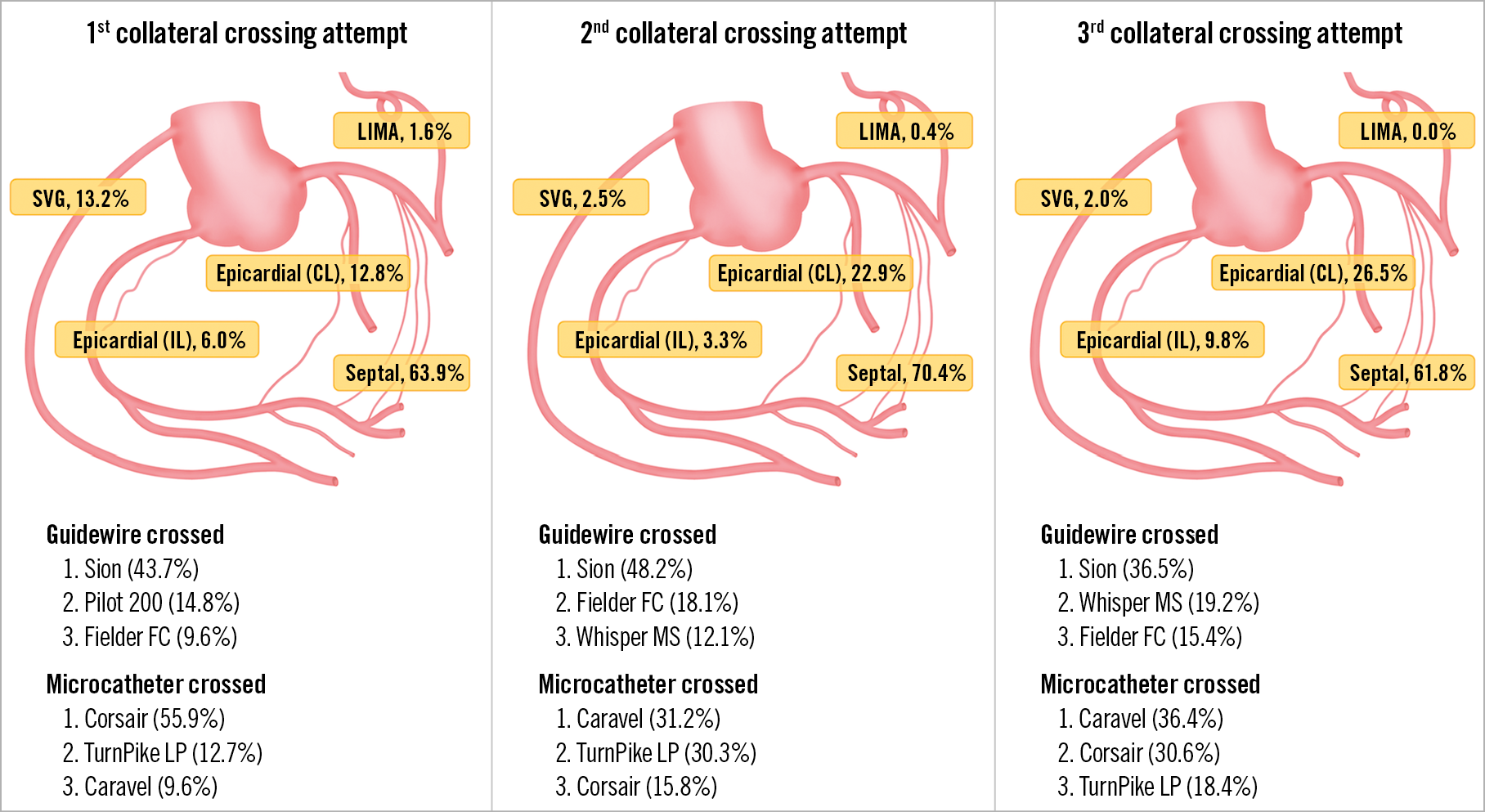
Figure 3. Technical characteristics of collateral channel crossing attempts during the retrograde approach in terms of collaterals used, successfully crossed guidewires and microcatheters. Manufacturer details: Asahi® Sion™, Caravel, Corsair, Fielder FC™, all Asahi Intecc, Aichi, Japan; HI-TORQUE PILOT 200 and WHISPER® MS, Abbott Vascular, Santa Clara, CA, USA; Turnpike® LP, Teleflex Medical, Wayne, PA, USA/Vascular Solutions, Minneapolis, MN, USA.
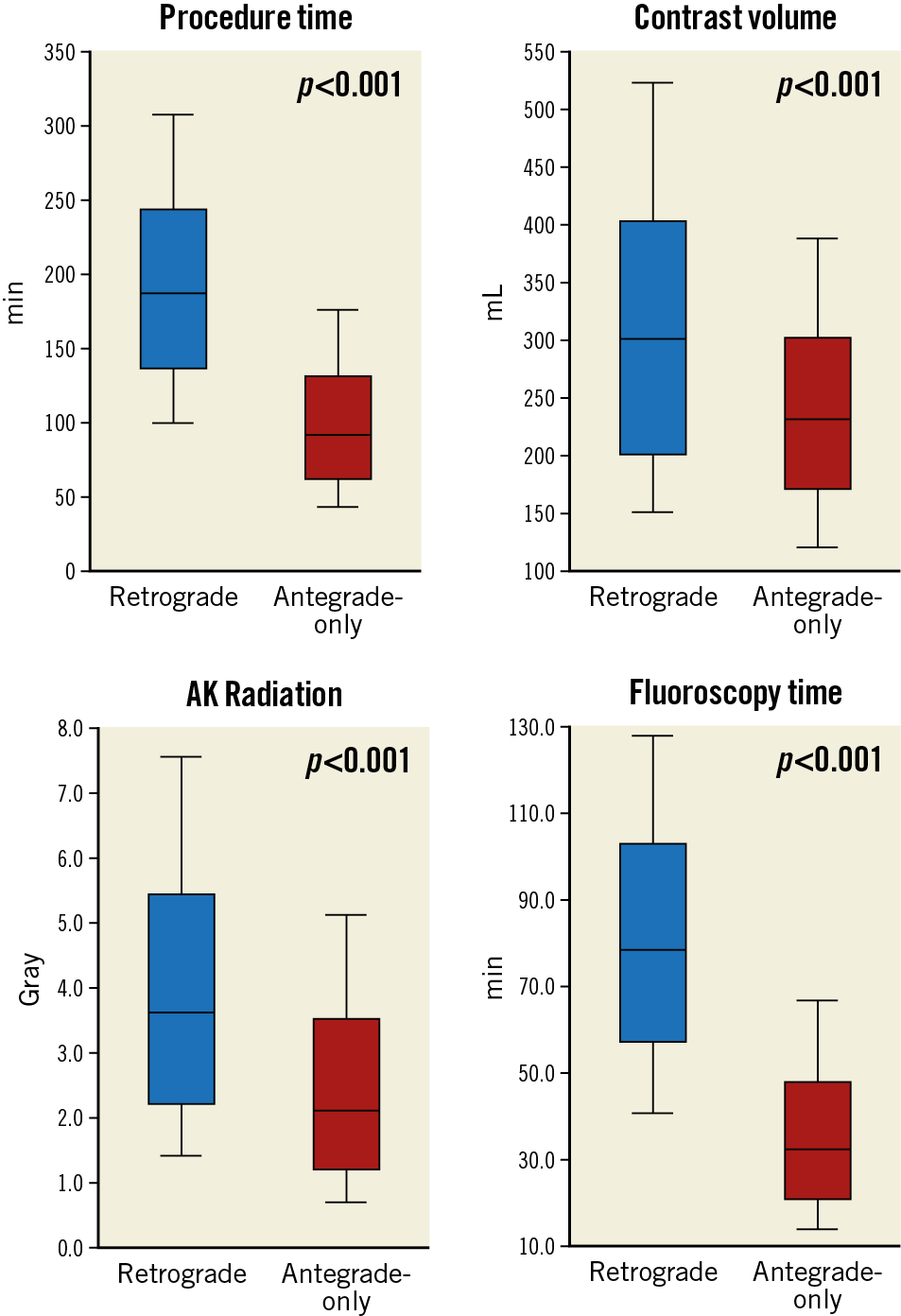
Figure 4. Procedural characteristics compared between retrograde CTO PCI and antegrade-only interventions.
The overall in-hospital MACE rate of retrograde CTO PCI was 5.1%, and any procedure-related complication occurred in 18.9% (Figure 5A, Figure 5B, Table 4). The incidence of coronary perforation was significantly higher in retrograde cases (8.6% vs 2.2%, p<0.001), and perforations were more severe (Ellis Class III or Class III-cavity spilling 41.3% vs 22.9%, p=0.133). The incidence of tamponade requiring pericardiocentesis, however, was caused similarly by antegrade wiring (n=12, 46.2%) and retrograde wiring (n=14, 53.8%) attempts during retrograde interventions, and it occurred more frequently if the retrograde approach was the successful crossing technique (66.7% vs 33.3%, p=0.225). Covered stents were used in 10.0% (n=10) of all perforations during the retrograde approach (n=140), and in 11.5% (n=3) of all cases when pericardiocentesis was required (n=26). On multivariable logistic regression (Supplementary Table 3), the retrograde approach was independently associated with increased risk of in-hospital MACE (OR 3.94, 95% CI: 2.01-8.20, p<0.001). In-hospital MACE and periprocedural complication rates were similar in cases with retrograde success compared with retrograde attempt failure (Figure 6, Figure 7).
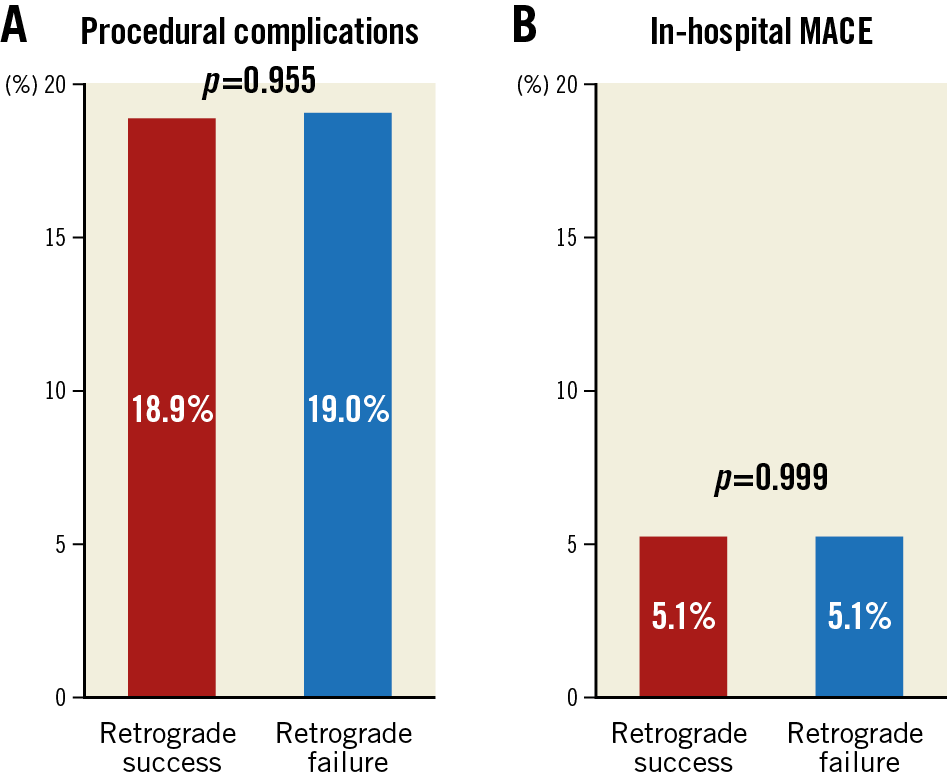
Figure 5. Distribution of periprocedural complications (A) and in-hospital major complications (B) of successful and failed retrograde CTO PCIs.
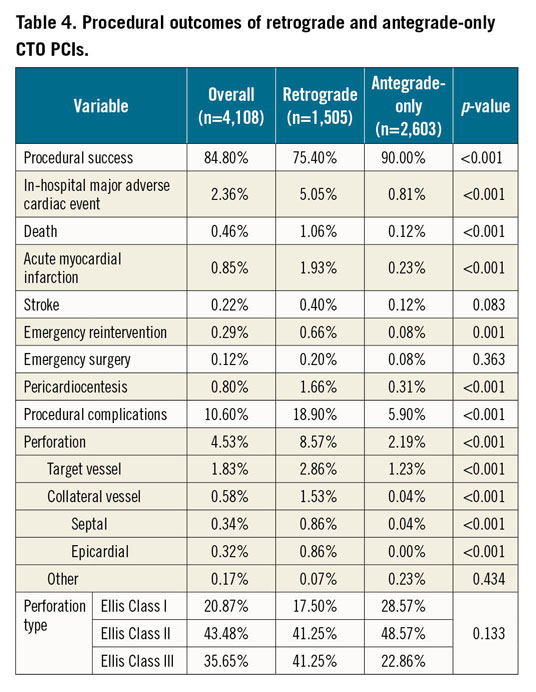
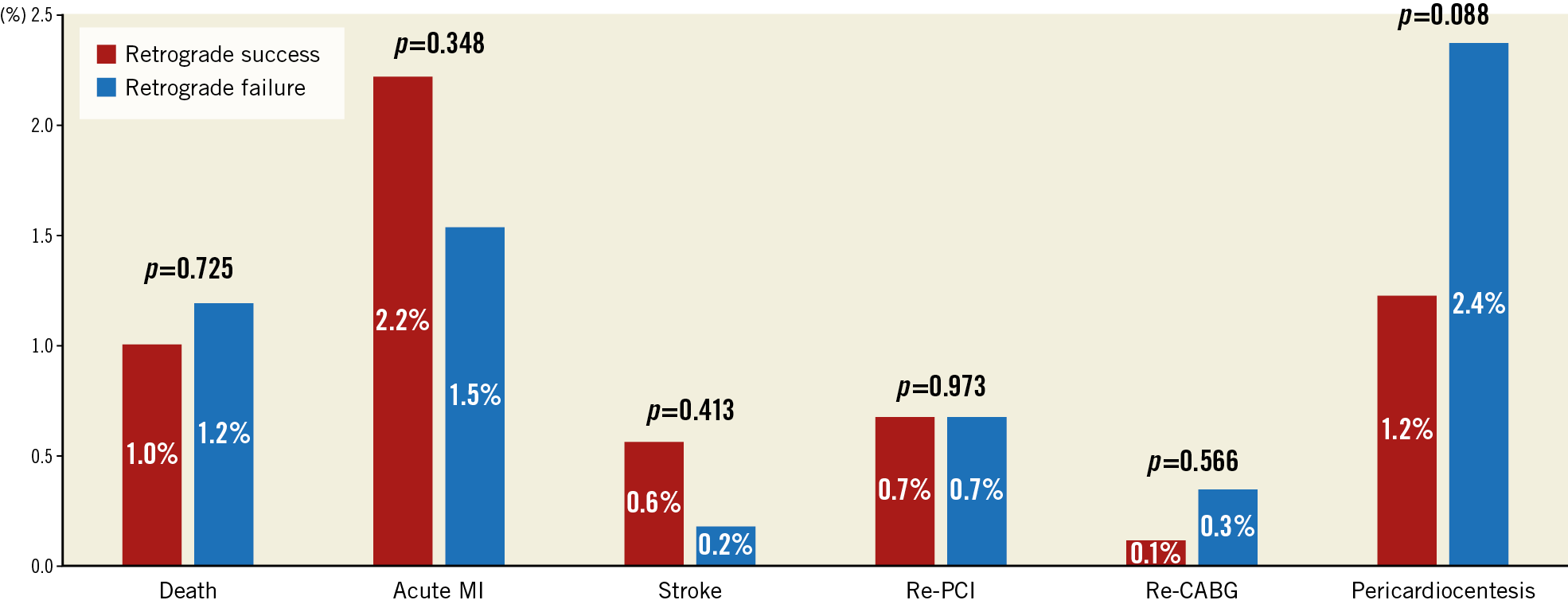
Figure 6. In-hospital major adverse cardiac events in failed and successful retrograde CTO PCIs.
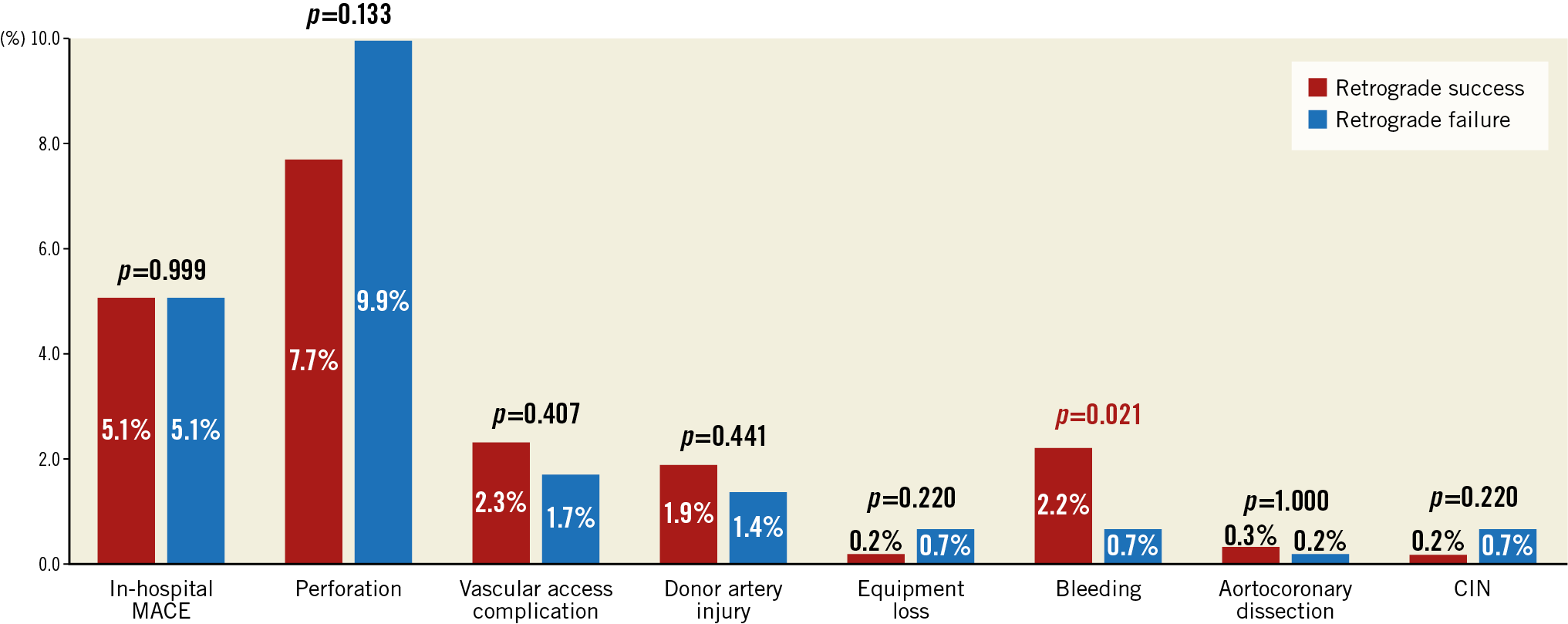
Figure 7. Periprocedural complications of failed and successful retrograde CTO PCIs.
The temporal trends of retrograde CTO PCI outcomes are presented in Supplementary Table 4. Additional procedural and patient characteristics are given in Supplementary Table 5.
Discussion
Our study is one of the largest published to date examining the technical outcomes of retrograde CTO PCI. The major findings are as follows: (a) retrograde CTO PCI remains essential for CTO PCI, especially in complex lesions; (b) retrograde interventions are associated with higher in-hospital and periprocedural complications compared with antegrade-only interventions; (c) collateral crossing is successful in three quarters of cases, limiting retrograde CTO crossing success; (d) approximately half of the perforations requiring pericardiocentesis during retrograde CTO PCI were due to antegrade wiring attempts.
Antegrade wire escalation remains the most frequently used and most often successful CTO crossing strategy. The retrograde approach, however, remains essential for CTO PCI success, especially in complex occlusions when the antegrade approach fails or is not feasible3. The decreasing use of the retrograde approach in recent years may reflect improvements in antegrade wiring techniques and devices (especially novel, highly torquable guidewires).
In our study, the overall success rate of retrograde interventions was 79.2% with procedural success of 75.4%, which is similar to those of the European CTO Registry (n=1,582 CTO PCI, clinical success of 75.3%4). The slight decrease over time in technical and procedural success is probably explained by increasing CTO complexity (Supplementary Table 4) and a high prevalence of prior CABG.
Septal surfing and contrast-guided techniques (also known as distal tip injection technique) are currently used for collateral crossing12. Dautov et al showed that septal collateral surfing was successful in 81% in 240 CTO PCIs, even if collaterals are invisible (Werner Class CC 0)13. Septal surfing was the most common collateral crossing strategy in cases with retrograde success (75.5%) in our study, whereas microcatheter tip injection guidance was used in 24.5%. The overall collateral channel crossing (by guidewire and microcatheter) success rate was 75.3%.
Once the guidewire and microcatheter cross the collateral channel, CTO crossing can also be challenging and is usually achieved using the reverse CART technique1. In our cohort, reverse CART was the most commonly attempted and most commonly successful crossing strategy.
Retrograde CTO PCI has been reported to have a higher risk of complications in numerous cohorts compared with antegrade approaches (both antegrade wiring and/or antegrade dissection re-entry techniques). Similarly, in our study in-hospital MACE was 5.1% in the retrograde group (overall procedural complications 18.9%) and was significantly higher than in antegrade-only interventions. However, approximately half of the complications (46.2%) were caused by antegrade wiring, suggesting that a significant proportion of the higher retrograde CTO PCI risk is related to the complexity of the treated lesions. Furthermore, although coronary perforations were observed in 8.6% of the procedures treated with the retrograde approach, only 1.7% required pericardiocentesis, with the majority being treated conservatively.
Performing retrograde CTO PCI over the years has undergone significant changes with decreasing use of radiation and contrast despite increased lesion complexity, as reflected in the increasing J-CTO scores (Supplementary Table 4). Such improvements could be attributed to optimised imaging, use of contrast-saving equipment and techniques (such as the DyeVert™ system [Osprey Medical Inc., Minnetonka, MN, USA], use of IVUS) and continuous education. Although the retrograde approach carries increased risk of complications, our study shows that some of the complications attributed to the retrograde approach were actually due to antegrade crossing attempts during the same procedure and were probably related to high lesion complexity.
Limitations
Limitations of our study include the lack of core laboratory assessment of the study angiograms, lack of independent clinical events adjudication, and limited availability of follow-up for the study patients. Furthermore, the procedures were performed in dedicated, high-volume CTO centres by experienced operators, limiting the extrapolation to less experienced operators and lower-volume centres. The selection of crossing strategy was made by each operator, probably reflecting local expertise and operator/patient preferences. The novel classification of the reverse CART techniques was not used in our current study; however, it was only introduced recently14. Procedure-related MI events are site reported without systematic post-procedural biomarker assessment, hence the rate of such complications may be underestimated.
Conclusions
The retrograde approach remains critical for the success of CTO PCI, especially in more complex occlusions. The retrograde approach should be performed by experienced operators and centres given its association with higher risk for complications.
|
Impact on daily practice The retrograde approach is an important technique for crossing coronary chronic total occlusions, especially for more complex lesions. However, the retrograde approach carries increased risk of complications, especially perforation, even though some of the complications are related to antegrade crossing attempts during the same procedure. As a result, appropriate case selection, early recognition and, if necessary, timely treatment of coronary perforation are critical for reducing the rate of complications during retrograde chronic total occlusion interventions. |
Appendix. Study collaborators
Robert W. Yeh, MD, MBA; Hector Tamez, MD; Beth Israel Deaconess Medical Center, Boston, MA, USA. Ehtisham Mahmud, MD; VA San Diego Healthcare System and University of California San Diego, La Jolla, CA, USA. James W. Choi, MD; Baylor Heart and Vascular Hospital, Dallas, TX, USA. Dmitrii Khelimskii, MD; Meshalkin Siberian Federal Biomedical Research Center, Ministry of Health of Russian Federation, Novosibirsk, Russian Federation. Jaikirshan J. Khatri, MD; Cleveland Clinic, Cleveland, OH, USA. Ioannis Tsiafoutis, MD; Korgialeneio-Benakeio Hellenic Red Cross General Hospital of Athens, Athens, Greece. Anthony H. Doing, MD; Phil Dattilo, MD; Medical Center of the Rockies, Loveland, CO, USA. Catalin Toma, MD; University of Pittsburgh Medical Center, Pittsburgh, PA, USA. Barry F. Uretsky, MD; VA Central Arkansas Healthcare System, Little Rock, AR, USA. Habib Samady, MD; Emory University, Atlanta, GA, USA. Brian Jefferson, MD; Taral Patel, MD; Tristar Centennial Medical Center, Nashville, TN, USA. Srinivasa Potluri, MD; The Heart Hospital Baylor Plano, Plano, TX, USA. David Kandzari, MD; Piedmont Heart Institute, Atlanta, GA, USA. R. Michael Wyman, MD; Torrance Memorial Medical Center, Torrance, CA, USA. Shuaib Abdullah, MD; Subhash Banerjee, MD; VA North Texas Health Care System and University of Texas Southwestern Medical Center, Dallas, TX, USA. Jeffrey Moses, MD; Nicholas Lembo, MD; Manish Parikh, MD; Ajay Kirtane, MD; Ziad A. Ali, MD; Juan J. Russo, MD; Emad Hakemi, MD; Columbia University, New York, NY, USA. Bavana Rangan, BDS, MPH; Minneapolis Heart Institute, Abbott Northwestern Hospital, Minneapolis, MN, USA. Imre Ungi, MD, PhD; University of Szeged, Division of Invasive Cardiology, Second Department of Internal Medicine and Cardiology Center, Szeged, Hungary.
Acknowledgements
Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at the Minneapolis Heart Institute Foundation, Minneapolis, MN, USA. REDCap is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
Funding
The PROGRESS-CTO registry has received support from the Abbott Northwestern Hospital Foundation, Minneapolis, MN, USA and a gift from the Joseph F. and Mary M. Fleischhacker Foundation.
Conflict of interest statement
D. Karmpaliotis reports speaker honoraria from Abbott Vascular, Boston Scientific, Medtronic, and Vascular Solutions. K. Alaswad reports consulting fees from Terumo, and Boston Scientific, and being a consultant (non-financial) for Abbott Laboratories. F.A. Jaffer reports being a consultant for Abbott Vascular, Boston Scientific, Siemens, and Philips, and research grants from Canon, Siemens, and National Institutes of Health. R.W. Yeh reports receiving a Career Development Award (1K23HL118138) from the National Heart, Lung, and Blood Institute. M. Patel reports being on the speakers’ bureau of AstraZeneca. E. Mahmud reports consulting fees from Medtronic and Corindus, speaker’s fees from Medtronic, Corindus, and Abbott Vascular, educational programme fees from Abbott Vascular and clinical events committee fees from St. Jude. M.N. Burke reports consulting/speaking honoraria from Abbott Vascular and Boston Scientific. R.M. Wyman reports honoraria/consulting/speaking fees from Boston Scientific, Abbott Vascular, and Asahi. D. Kandzari reports research grant/consulting honoraria from Boston Scientific, and Medtronic Cardiovascular, and a research grant from Abbott. S. Garcia reports consulting fees from Medtronic. J. Khatri reports a research grant from Asahi Intecc, and consultant/speaker honoraria from Abbott Vascular, Philips, and Abiomed. J. Moses reports being a consultant for Boston Scientific and Abiomed. N. Lembo reports being on the speaker bureau of Medtronic and the advisory board of Abbott Vascular and Medtronic. M. Parikh reports being on the speaker bureau of Abbott Vascular, Medtronic, CSI, BSC, and Trireme, and on the advisory boards of Medtronic, Abbott Vascular, and Philips. A. Kirtane reports receiving institutional research grants to Columbia University from Boston Scientific, Medtronic, Abbott Vascular, Abiomed, St. Jude Medical, Vascular Dynamics, Glaxo SmithKline, and Eli Lilly. Z. Ali reports consultant fees/honoraria from St. Jude Medical, and AstraZeneca Pharmaceuticals, ownership interest/partnership/principal in Shockwave Medical, and VitaBx Inc, and research grants from Medtronic and St. Jude Medical. B. Rangan reports research grants from Infraredx, Inc., and The Spectranetics Corporation. S. Banerjee reports research grants from Gilead, and The Medicines Company, consultant/speaker honoraria from Covidien, and Medtronic, ownership of MDCARE Global (spouse), and intellectual property in HygeiaTel. E.S. Brilakis reports consulting/speaker honoraria from Abbott Vascular, American Heart Association (associate editor Circulation), Boston Scientific, Cardiovascular Innovations Foundation (Board of Directors), CSI, Elsevier, GE Healthcare, Infraredx, and Medtronic, research support from Regeneron, and Siemens, and being a shareholder in MHI Ventures. The other authors/study collaborators have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

