
Abstract
Aims: The goal of this study was to describe the procedural characteristics, strategy selection and associated technical and efficiency outcomes for chronic total occlusion (CTO) percutaneous coronary intervention (PCI) of the right coronary artery (RCA).
Methods and results: We examined the clinical and angiographic characteristics of patients who underwent RCA CTO PCI between 2012 and 2015 at 11 centres in the USA. The RCA was the CTO target vessel in 739 of 1,308 CTO PCIs (56%). Overall technical and procedural success rates were 90% and 88%, respectively. A major adverse cardiovascular event (MACE) occurred in 19 patients (2.6%). Technical success was most frequently achieved using antegrade wire escalation (38% of successful procedures) followed by retrograde (36%) and antegrade dissection/re-entry (26%). Technical success was similar between various locations of RCA CTOs (p=0.11). Compared with antegrade-only procedures, utilisation of any retrograde approach was associated with lower technical (85% vs. 95%, p<0.001) and procedural (82% vs. 94%, p<0.001) success and a higher MACE rate (3.8% vs. 1.4%, p=0.037).
Conclusions: RCA CTOs represent the majority of CTO target lesions, can be treated with high success and acceptable complication rates, and require frequent use of the retrograde approach and antegrade dissection/re-entry.
Introduction
The right coronary artery (RCA) is the most frequently attempted target vessel for chronic total occlusion (CTO) percutaneous coronary intervention (PCI)1-3. This is probably related to several factors: (a) the RCA is the most common culprit vessel for acute myocardial infarction4; (b) use of the left internal mammary artery (LIMA) graft to the left anterior descending artery (LAD) may improve survival5; and (c) bypass grafts to the RCA may have poor long-term patency, often making reintervention of the native vessel necessary6. Such RCA lesions can be attempted with various crossing strategies, including an antegrade or retrograde and intimal or subintimal approach. The goal of the present study was to assess the frequency of utilisation of different techniques for RCA CTO PCI, and their associated procedural characteristics and outcomes.
Methods
STUDY POPULATION
We examined the clinical and angiographic records of patients who underwent CTO PCI between May 2012 and November 2015 by experienced, high-volume operators at 11 CTO PCI centres in the USA (Appendix). Data collection was performed prospectively and retrospectively and recorded in a CTO database (PROGRESS-CTO, ClinicalTrials.gov Identifier: NCT02061436). Some centres only enrolled patients during part of the study period due to participation in other studies. The study was approved by the institutional review board of each site.
DEFINITIONS
Coronary CTOs were defined as coronary lesions with Thrombolysis In Myocardial Infarction (TIMI) grade 0 flow of at least three months’ duration. Estimation of the occlusion duration was based on first onset of anginal symptoms, prior history of myocardial infarction in the target vessel territory, or comparison with a prior angiogram. Calcification was assessed by angiography as mild (spots), moderate (involving ≤50% of the reference lesion diameter) and severe (involving >50% of the reference lesion diameter). Moderate proximal vessel tortuosity was defined as the presence of at least two bends >70° or one bend >90°, and severe tortuosity as two bends >90° or one bend >120° in the CTO vessel. Interventional collaterals were defined as collaterals deemed by the operator to be amenable to crossing by a guidewire and a microcatheter. A procedure was defined as “retrograde” if an attempt was made to cross the lesion through a collateral vessel supplying the target vessel distal to the lesion; if not, the procedure was classified as “antegrade-only”. Antegrade dissection/re-entry (or antegrade subintimal) was defined as antegrade PCI during which a guidewire was intentionally introduced into the subintimal space proximal to the lesion, or re-entry into the distal true lumen was attempted following intentional or inadvertent subintimal guidewire crossing. Technical success of CTO PCI was defined as successful CTO revascularisation with achievement of <30% residual diameter stenosis within the treated segment and restoration of TIMI grade 3 antegrade flow. Procedural success was defined as achievement of technical success with no in-hospital major adverse cardiac events (MACE). In-hospital MACE included any of the following adverse events prior to hospital discharge: death, myocardial infarction (MI), recurrent symptoms requiring urgent repeat target vessel revascularisation with PCI or coronary artery bypass graft surgery (CABG), tamponade requiring either pericardiocentesis or surgery, and stroke. Periprocedural and late in-hospital myocardial infarction were defined according to the Third Universal Definition of Myocardial Infarction7. The J-CTO score was calculated as described by Morino et al8 and the PROGRESS-CTO score as described by Christopoulos et al9.
STATISTICAL ANALYSIS
Categorical variables were expressed as percentages and compared using Pearson’s chi-square test or Fisher’s exact test. Continuous variables were presented as mean±standard deviation or median (interquartile range), and were compared using the t-test, analysis of variance, Wilcoxon rank-sum test or Kruskal-Wallis test. Logistic regression analysis was performed to identify clinical and angiographic parameters associated with technical failure for RCA CTO PCI. Variables with p<0.10 on univariate analysis (age, smoking, stump morphology, prior CABG, presence of a side branch at the proximal cap, distal cap location at a bifurcation, degree of calcification and proximal vessel tortuosity and absence of appropriate collaterals) were included in a multivariate model, along with variables shown by prior studies to be associated with PCI complexity (diabetes mellitus and prior MI). Receiver operating characteristic analysis was performed to assess the predictive capacity of the J-CTO and PROGRESS-CTO scores for technical outcome. All statistical analyses were performed with JMP 12.0 (SAS Institute, Cary, NC, USA) and SPSS, Version 22 (IBM Corp., Armonk, NY, USA). Two-sided p-values of 0.05 were considered statistically significant.
Results
CLINICAL AND ANGIOGRAPHIC CHARACTERISTICS AND CROSSING STRATEGIES
Of 1,308 CTO PCIs performed at participating centres during the study period, 739 (56%) were performed on the RCA and were included in the present analysis. A second CTO was treated in 16 of these procedures (2%), and a non-CTO lesion in 178 (24%).
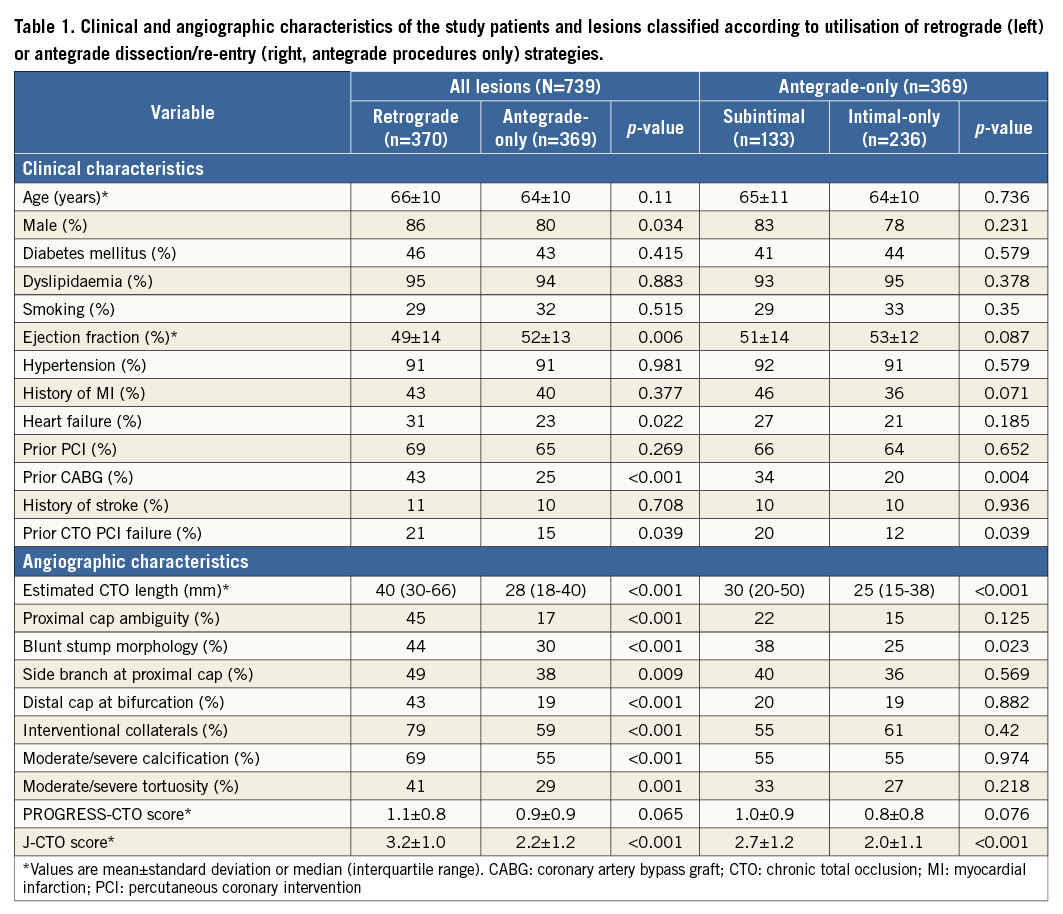
The strategy attempted most frequently was antegrade wire escalation (AWE, 66%), followed by retrograde (50%) and antegrade dissection/re-entry (ADR, 40%) (Figure 1). The most common final successful crossing strategy was AWE (38% of technical success), followed by retrograde (36%) and ADR (26%). Primary retrograde attempts were successful 62% of the time (vs. 51% for both primary AWE and primary ADR, p=0.042) (Figure 2). As compared with antegrade-only cases, cases in which a retrograde approach was utilised had higher clinical and angiographic complexity (Table 1, left panel). When comparing antegrade-only intimal and subintimal cases, lesions for which subintimal crossing was utilised were significantly longer (30 mm vs. 25 mm, p<0.001) and more likely to have a blunt proximal stump (38% vs. 25%, p=0.023) (Table 1, right panel).
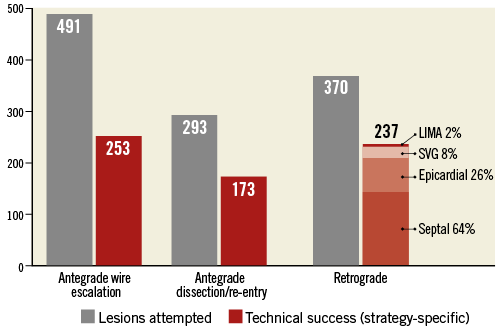
Figure 1. Number of lesions attempted and lesions in which technical success was achieved with each crossing strategy. The crossing collateral is displayed for successful retrograde PCIs. LIMA: left internal mammary artery; SVG: saphenous vein graft
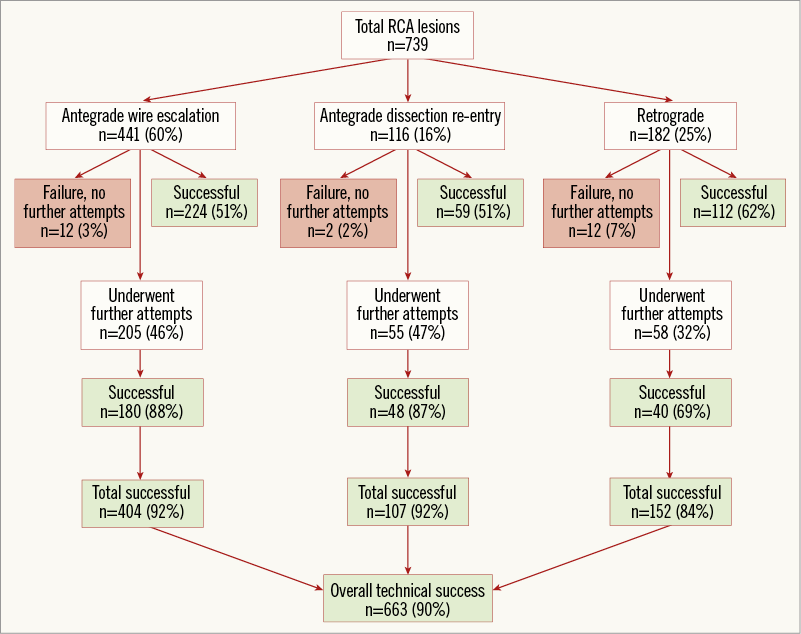
Figure 2. Flow chart depicting the crossing strategies utilised for recanalisation of the right coronary artery chronic total occlusions in PROGRESS-CTO.
LESION OUTCOMES
Technical and procedural success was achieved in 663 (90%) and 649 (88%) RCA CTO PCIs, respectively. On multivariate analysis, patient age more than 70 years, smoking, history of MI, blunt lesion stump morphology, lack of interventional collaterals, moderate or severe calcification and distal cap location at a bifurcation site were factors independently associated with technical failure for RCA CTO PCI (Figure 3). The J-CTO and PROGRESS-CTO scores both showed moderate predictive capacity for technical success in this cohort, with better performance for antegrade-only procedures (Figure 4). In-hospital MACE occurred in 19 patients (2.6%).
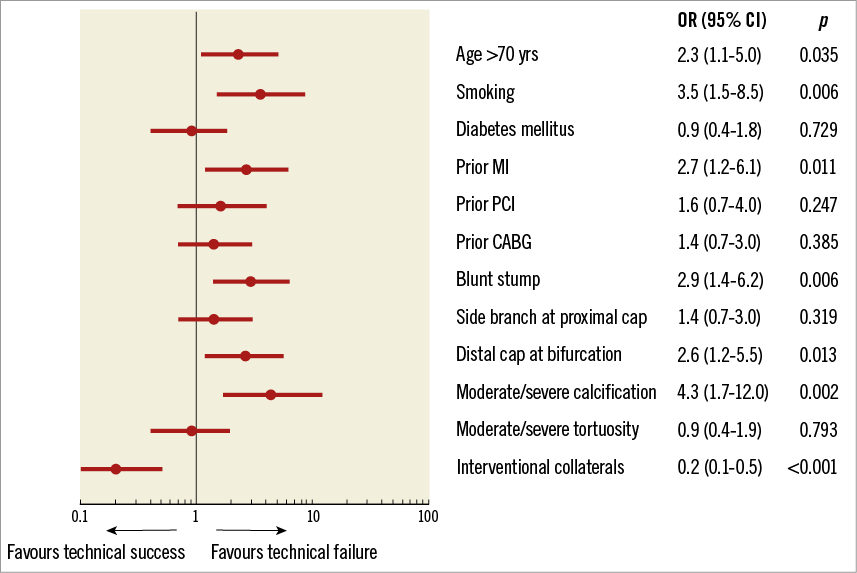
Figure 3. Predictors of technical failure of right coronary artery chronic total occlusion PCI. CABG: coronary artery bypass graft; MI: myocardial infarction; PCI: percutaneous coronary intervention
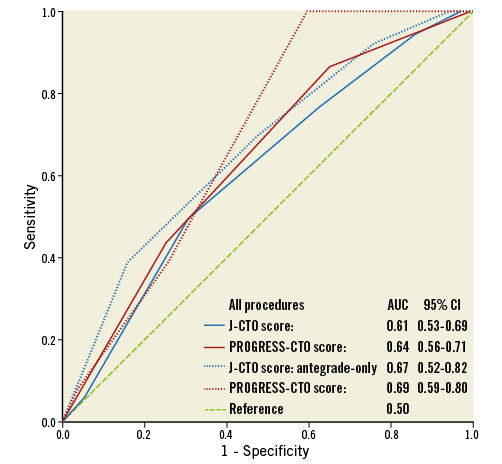
Figure 4. Receiver operating characteristic curves showing the predictive capacity of the J-CTO (blue) and PROGRESS-CTO (maroon) scores for technical outcome, in all (solid) and antegrade-only (dotted) procedures. AUC: area under the curve; CI: confidence interval
RETROGRADE APPROACHES
Procedures completed via a retrograde approach were most frequently completed via a septal collateral (64%), followed by epicardial collaterals (26%), saphenous vein grafts (8%) and left internal mammary artery (LIMA, 2%) grafts. Reverse controlled antegrade and retrograde tracking (rCART) was the most common retrograde technique leading to lesion recanalisation (73%), followed by retrograde wire true lumen puncture (19%) and CART (3%). Reasons for failure of retrograde crossing included failure to cross the collateral with a guidewire in 86 (23% of all retrograde attempts) and a microcatheter in 13 (4%) retrograde attempts; in 26 cases (7%) the CTO could not be crossed despite successful collateral negotiation; in eight cases (2%) the reason for retrograde failure was not specified. Utilisation of a retrograde approach was associated with higher utilisation of contrast, radiation, time, haemodynamic support devices and stents (Table 2), and resulted in lower technical (85% vs. 95%, p<0.001) and procedural (82% vs. 94%, p<0.001) success and a higher rate of MACE (3.8% vs. 1.4%, p=0.037) and coronary perforation (6.2% vs. 2.7%, p=0.021).
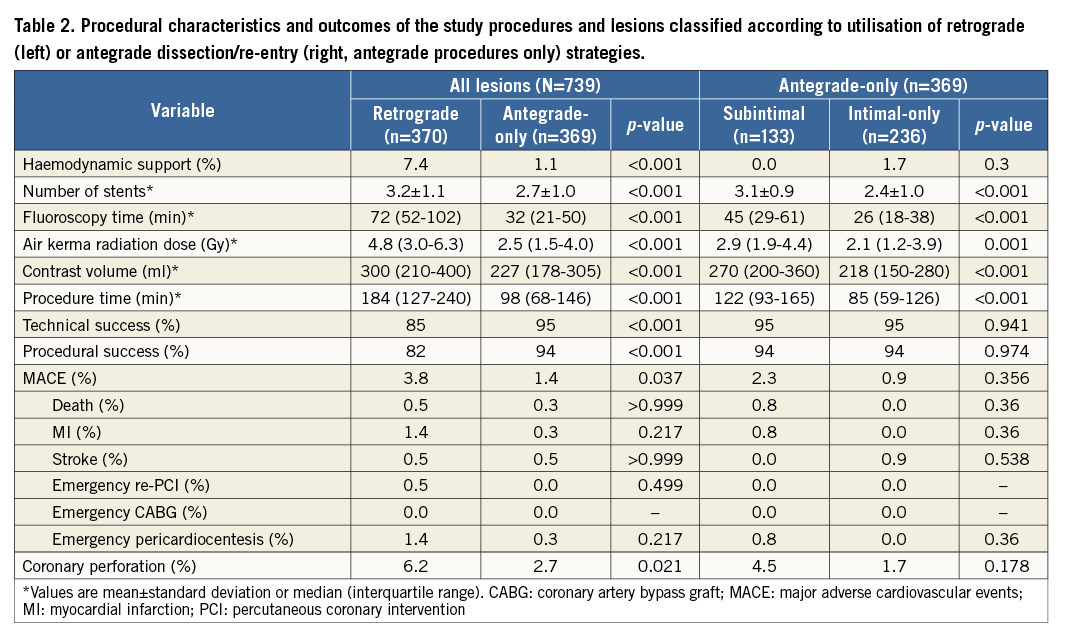
ANTEGRADE DISSECTION/RE-ENTRY
Entry into the subintimal space was intentional in 95% of ADR attempts. Successful subintimal lesion crossing was most frequently achieved using the CrossBoss™ microcatheter (Boston Scientific, Marlborough, MA, USA) (53%) or a knuckled wire (41%), and successful true lumen re-entry was most frequently achieved using a Stingray™ balloon (Boston Scientific) (77%) or wire-based subintimal tracking and re-entry (STAR)10 (14%). There was no significant difference between technical and procedural success or MACE rates between antegrade-only intimal and subintimal procedures, but the latter were associated with lower procedural efficiency (more contrast, radiation and time and larger number of stents per lesion) (Table 2, right panel).
LESION LOCATION
More than half (52%) of the study lesions were located within the proximal RCA segment, followed by the mid RCA (34%), distal RCA (10%) and right posterior atrioventricular segment, posterior descending artery and posterolateral segments (4% combined) (Table 3). When compared to all other RCA lesions combined, proximal lesions were more commonly at least moderately calcified (67% vs. 56%, p=0.039), had more proximal cap ambiguity (39% vs. 25%, p=0.001) and were longer (median length [IQR]: 40 [25-70] vs. 30 [18-40] mm, p<0.001), but were more likely to have suitable collaterals (74% vs. 66%, p=0.042). There was a significant tendency towards more frequent utilisation of AWE and less frequent utilisation of ADR within more distal RCA segments (Figure 5A). This was mirrored in the distribution of the final successful crossing strategy (Figure 5B): proximal lesions were crossed successfully using all three strategies with similar frequency, and were notably crossed with a retrograde approach 39% of the time (AWE: 32%, ADR: 29%). In contrast, more than half (57%) of the technical success in distal lesions was achieved using AWE, with antegrade subintimal crossing contributing only 17%. There was no significant difference in technical success, procedural success or the incidence of MACE between different RCA segments.
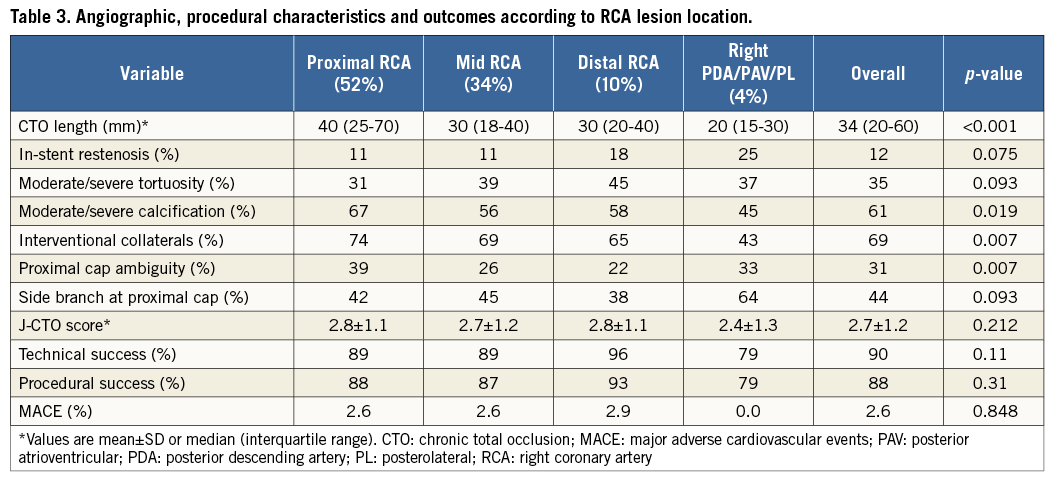
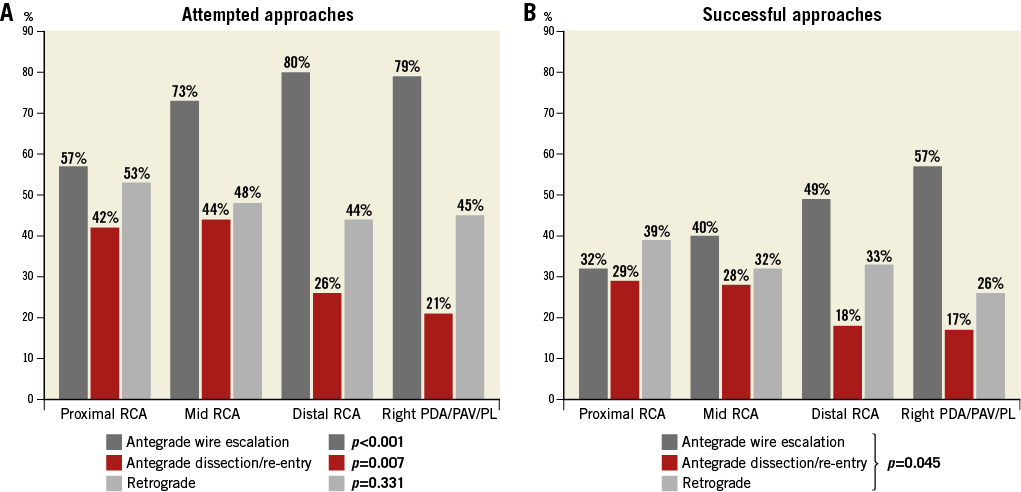
Figure 5. Distribution of attempted and successful right coronary artery (RCA) chronic total occlusion (CTO) crossing approaches, classified according to lesion location within the RCA. A) Attempted RCA CTO crossing approaches. B) Successful RCA CTO crossing approaches.
Discussion
The main findings of our study are: (a) the RCA is the most common vessel for CTO intervention; (b) AWE is the most commonly utilised and most commonly successful strategy for RCA CTO recanalisation; (c) the retrograde approach is frequently utilised for RCA CTO cases with high clinical and angiographic complexity, and is associated with lower success rates, lower procedural efficiency and higher in-hospital MACE rates; (d) antegrade dissection/re-entry for RCA CTOs is used for longer lesions, and appears non-inferior to antegrade wire escalation in terms of technical and in-hospital outcomes, but is associated with lower procedural efficiency; (e) lesion location within the RCA is not significantly associated with outcomes; and (f) more proximal lesions are frequently attempted and recanalised with a retrograde approach, whereas more distal lesions are more frequently successfully crossed with antegrade wiring.
In our registry, the RCA was the artery most commonly intervened on, as in previous reports11-14. RCA CTOs differ from left coronary artery CTOs as follows: (a) RCA CTOs are more likely to have well-developed “interventional” collaterals, and (b) RCA CTOs tend to have longer occlusion lengths, which may be due to the pathogenesis of CTOs (thrombus growing proximally until a side branch is encountered, at which point the lesion stops expanding and the proximal cap becomes blunt)15,16. Therefore, when facing a challenging RCA occlusion, the operator may frequently be forced to explore all available crossing strategies: antegrade wire escalation, retrograde through septal collaterals, retrograde through epicardial contralateral/ipsilateral bridging collaterals, and antegrade dissection/re-entry. As a result, RCA CTOs were more likely than other vessel CTOs to undergo retrograde and ADR crossing attempts in our registry17.
Expert groups from around the world have shared their CTO PCI experience. In 2011, Galassi et al published the first results from the European Registry of Chronic Total Occlusions (ERCTO)11. Similar to our findings, RCA CTOs represented the majority of the 1,983 lesions (n=993, 50.1%). Compared with antegrade procedures, utilisation of a retrograde approach was also associated with a lower success rate (64.5% vs. 83.2%, p<0.001), lower procedural efficiency (median procedure time: 156.9 min vs. 98.2 min, p<0.001), a higher incidence of coronary perforation (4.7% vs. 2.1%, p=0.04) and a trend for non-Q-wave MI (2.1% vs. 1.0%, p=0.08); similar to our findings, blunt stump and severe calcification were identified as independent predictors of technical failure, along with CTO length. In 2015, the European CTO Club expanded upon their retrograde experience with results from 1,582 retrograde CTO PCIs (RCA: 70.4%): septal collaterals were most frequently used (62.7%) and retrograde true lumen crossing was the most frequent crossing strategy (31.2%), whereas reverse CART was the most common strategy in PROGRESS-CTO18. Morino et al reported results from the J-CTO registry (multicentre CTO registry in Japan), with a high success rate (86.6%) over 528 lesions (RCA: 44.3%)13. A retrograde approach was employed in 25.7% of cases, with more than half (56.6%) being primary retrograde; as in ERCTO and PROGRESS-CTO, coronary perforation was higher with utilisation of retrograde techniques (13.6%). In the multicentre Korean CTO (K-CTO) registry (2,568 lesions, 80% success rate), patients with failed CTO PCI were significantly more likely to have an RCA occlusion compared to patients with successful intervention (47.8% vs. 38.1%, p<0.001)12.
In accordance with previous reports, the retrograde approach was more likely to be used in more complex patients and lesions in our study, and had lower efficiency and success rates as compared with occlusions approached with antegrade crossing only11,19. The importance of the retrograde approach, however, is highlighted by the principal role it played in revascularisation of proximal RCA lesions in our study (39% of all technical success for this segment). While one would expect lower technical success in proximal lesions (less guide support, uncertainty about distal vessel course), we observed no difference in technical and procedural success or MACE between proximal and distal RCA occlusions, a finding probably attributable to the success of retrograde approaches for proximal RCA lesions. Overall, the J-CTO and PROGRESS-CTO scores displayed moderate predictive capacity for technical failure in this cohort (AUC: 0.61 and 0.64, respectively). Similar to recent findings by Wilson et al, both scores performed better in antegrade-only procedures (AUC: 0.67 and 0.69, respectively)20. While these findings may imply that hybrid CTO PCI can overcome angiographic complexity as defined by CTO scoring systems, such scores remain useful due to their validated ability to predict procedural efficiency and the need for advanced crossing technique utilisation.
For longer (>20 mm) RCA occlusions and lesions with ambiguous proximal caps, antegrade dissection and re-entry is a valuable tool, especially in the absence of suitable collaterals21,22. Notably, outcomes with use of ADR were comparable to those with simple wire escalation in our study, albeit with a drop in procedural efficiency. This may be explained by the use of ADR primarily after failure of antegrade escalation or retrograde attempts (as occurred in the majority of ADR cases in our study). However, ADR is less likely to be a viable option for more distal lesions with a smaller diameter artery available for re-entry, and may also be less attractive nearing the PDA/PLV bifurcation, given the potential for side branch loss.
Limitations
Limitations of our study include the observational design, subject to selection bias. The study included procedures performed by highly skilled and experienced CTO operators, and thus our results may not apply to less experienced operators. Some of the participating centres enrolled patients during parts of the study period. There was no core laboratory adjudication of the angiograms or centralised clinical event adjudication. Also, long-term follow-up information was not available.
Conclusions
The right coronary artery is the most common target vessel for CTO PCI intervention, and numerous approaches can be used for lesions in the RCA. While antegrade wire escalation remains the most common strategy utilised for recanalisation of RCA CTOs, retrograde approaches contribute significantly to technical success, especially for more proximal lesions. However, utilisation of the retrograde approach is associated with lower overall technical and procedural success, and a higher MACE rate.
| Impact on daily practice The right coronary artery (RCA) represents the most common vessel targeted for chronic total occlusion (CTO) percutaneous revascularisation, and such occlusions can be approached with a range of crossing strategies, including antegrade or retrograde, and intimal or subintimal. While high success rates can be achieved in CTOs located in all RCA segments, distal lesions can be recanalised using wire escalation in the majority of cases, whereas proximal lesions are more likely to require a retrograde approach or dissection and re-entry into the true lumen. Retrograde techniques are important for achieving high success rates, but must be used judiciously due to a higher potential for complications. |
Appendix. Participating centres
Appleton Cardiology, Appleton, WI, USA; Columbia University, New York, NY, USA; Henry Ford Hospital, Detroit, MI, USA; Massachusetts General Hospital, Boston, MA, USA; Medical Center of the Rockies, Loveland, CO, USA; Piedmont Heart Institute, Atlanta, GA, USA; PeaceHealth St. Joseph Medical Center, Bellingham, WA, USA; St. Luke’s Health System’s Mid-America Heart Institute, Kansas City, MO, USA; Torrance Memorial Center, Torrance, CA, USA; VA North Texas Health Care System, Dallas, TX, USA, and VA San Diego Healthcare System, San Diego, CA, USA.
Acknowledgements
Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at the University of Texas Southwestern Medical Center, Dallas, TX, USA23. REDCap is a secure, web-based application designed to support data capture for research studies, providing: 1) an intuitive interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for importing data from external sources.
Funding
Research reported in this publication was supported by the Clinical and Translational Science Awards Program of the National Institutes of Health (Bethesda, MD, USA) under grant number UL1-RR024982. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of interest statement
D. Karmpaliotis is on the speakers’ bureau of Abbott Vascular, Medtronic, and Boston Scientific, and is a consultant to BridgePoint Medical. K. Alaswad reports receiving consulting fees from Terumo, Asahi Intecc and Boston Scientific and is a consultant (no financial support) for Abbott Laboratories. F. Jaffer is a consultant to Boston Scientific, Siemens, and Merck, and has received non-financial research support from Abbott Vascular and a research grant from the National Institutes of Health (HL-R01-108229), Siemens and Kowa. R. Yeh has received a Career Development Award (1K23HL118138) from the National Heart, Lung, and Blood Institute, is a consultant for Abbott Vascular, Gilead Sciences and Boston Scientific, is also on the advisory board of Abbott Vascular, and has received salary support from Harvard Clinical Research Institute. M. Patel is on the speakers’ bureau for AstraZeneca. M. Wyman has received honoraria/consulting/speaking fees from Boston Scientific, Abbott Vascular, and Asahi. W. Lombardi has equity with BridgePoint Medical and is a consultant to Boston Scientific, Abiomed and Abbott Vascular. A. Grantham has received speaking fees, consulting fees, and honoraria from Abbott Vascular, Boston Scientific and Asahi Intecc, research grants from Boston Scientific, Asahi Intecc, Abbott Vascular and Medtronic, is a member of Boston Scientific Executive Physician Leadership Committee, is on the advisory board for BSCI, and is on the CTO advisory board for Abbott Vascular. D. Kandzari has received research/grant support and consulting honoraria from Boston Scientific and Medtronic Cardiovascular, and research/grant support from Abbott. N. Lembo is on the speakers’ bureau of Medtronic and on the advisory board of Abbott Vascular and Medtronic. J. Moses is a consultant to Boston Scientific and Abiomed. A. Kirtane has received institutional research grants to Columbia University from Boston Scientific, Medtronic, Abbott Vascular, Abiomed, St. Jude Medical, Vascular Dynamics, Glaxo SmithKline, and Eli Lilly. S. Garcia has received consulting fees from Medtronic and SurModics. C. Thompson is an employee of Boston Scientific. S. Banerjee has received research grants from Gilead and The Medicines Company, is a consultant for and has received speaker honoraria from Covidien, Merck and Medtronic, declares ownership in MDCARE Global (spouse), has intellectual property in HygeiaTel, and declares an educational grant from Boston Scientific (spouse). E. Brilakis has received consulting/speaker honoraria from Abbott Vascular, Asahi, Cardinal Health, Elsevier, GE Healthcare and St. Jude Medical, research support from Boston Scientific and Infraredx, and his spouse is an employee of Medtronic. The other authors have no conflicts of interest to declare.

