Abstract
Percutaneous treatment of coronary chronic total occlusions (CTO) has advanced greatly since its advent in the late 1970s through the development of dedicated wires and microcatheters, the improved skills of highly experienced operators and the adoption of new sophisticated strategies to guide procedural planning. The contemporary procedural success rate is 80-90% with a reduction in complications. Although there has been no improvement in prognosis in randomised trials to date, they, and other controlled registries of thousands of patients, confirm the pivotal role of CTO recanalisation in the treatment of angina and dyspnoea and an improvement in quality of life. Despite this evidence, CTO recanalisation is grossly underutilised. This review reports a detailed overview of the history, indications and treatment strategies for CTO recanalisation and hopes to increase interest among new, and especially young, operators in this demanding, rapidly evolving field of interventional cardiology.
Introduction
When primary angioplasty was introduced in the 1990s, many expected a dramatic fall in the incidence of chronic total occlusions (CTO), but the incidence of occluded coronary vessels among patients with significant coronary artery disease has remained at 16-18% in recent large series12. The ageing population may, in part, explain this persistently high percentage. Collateral recruitment avoids or limits fibrotic transformation, preserving viability and, in part, the function of the supplied myocardium. Andreas Gruentzig said that “… the total closure is a real problem, if we cannot solve the total closure problem we probably will never really address the question of multivessel dilatation”. This statement remains true. SYNTAX (SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery) II, the only percutaneous coronary intervention (PCI) registry reporting the long-term event rate of the surgical arm of SYNTAX, included centres able to achieve a success rate in CTO recanalisation of 87%3. In FAME (Fractional Flow Reserve versus Angiography for Multivessel Evaluation) 34, the only recent global PCI vs coronary artery bypass graft (CABG) trial using intracoronary physiology to guide lesion selection for PCI, outcomes following PCI were inferior to surgery at 12 months. In this trial, only single-vessel CTO was allowed, as the presence of more than 1 major CTO was an exclusion criterion. Unlike in the original SYNTAX trial, the FAME 3 protocol recommended the CTO recanalisation to be performed as a separate, staged procedure in line with the accepted current practice. The published results, however, do not specify how many of the 20.8% of CTOs in the PCI arm were recanalised compared to the 23.1% in the CABG group. The importance of not attempting or failing to recanalise CTOs in other PCI vs CABG trials, leading to incomplete revascularisation and increased event rates, has been confirmed in multiple substudies567. The improved success rate in CTO recanalisation and the ability to secure long-term patency with drug-eluting stents (DES) have been key positive developments in the field of PCI8910111213141516.
There are multiple, recently updated documents produced by the main groups working in CTO recanalisation171819202122232425262728. The aim of this review is not to repeat these comprehensive consensus articles but to provide the opinion of a group of experienced operators on contemporary indications for CTO recanalisation after the conflicting data of many randomised controlled trials (RCTs) and registries for both CTO recanalisation, in particular, and in chronic coronary syndromes (CCS), in general. We also provide a practical guide for operators new to this field to orient themselves in the variety of techniques (Central illustration) and algorithms.
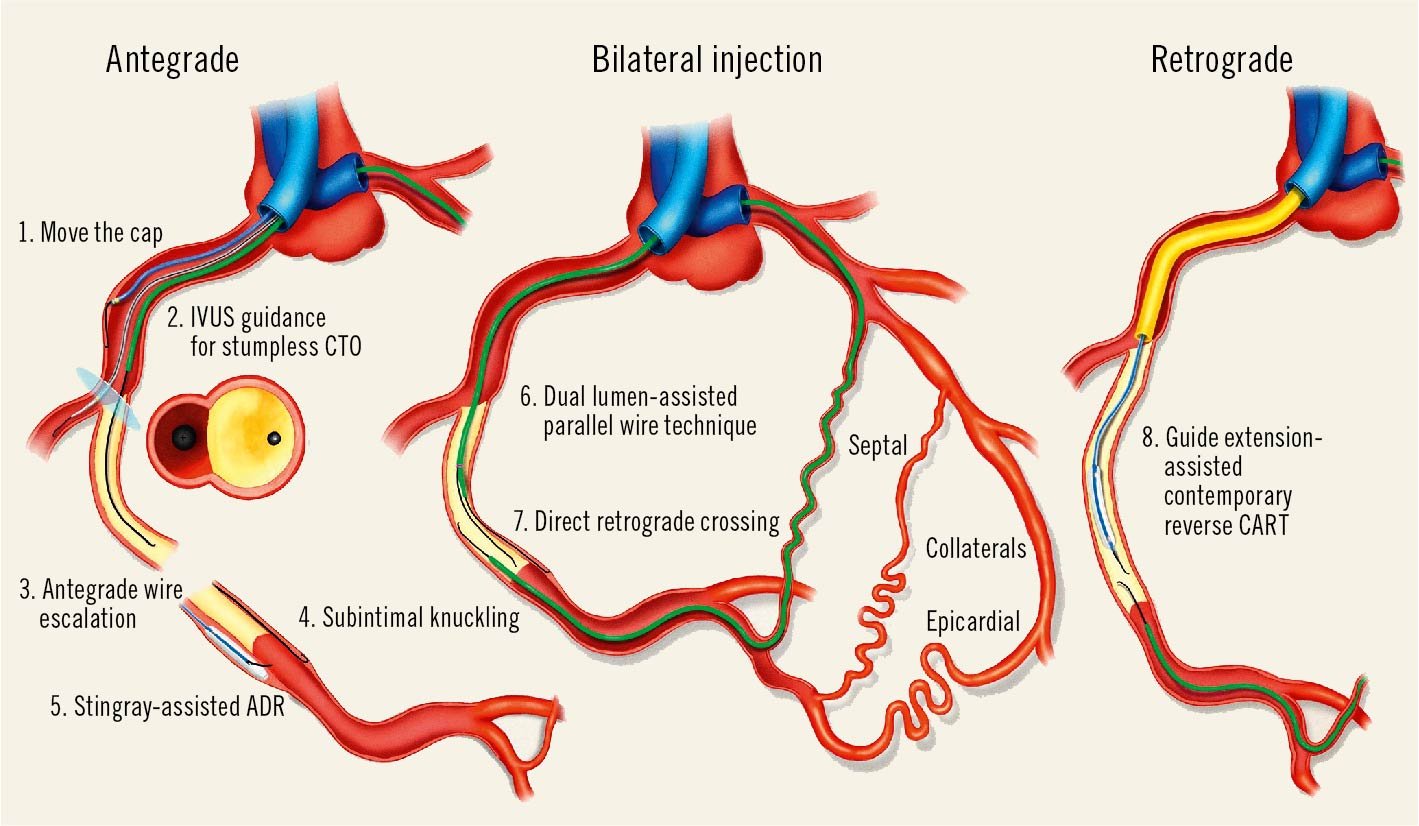
Central illustration. Different modalities of antegrade and retrograde CTO recanalisation. The upper left image describes various techniques of antegrade recanalisation, starting with the “conventional” microcatheter-assisted antegrade wire escalation (3). When the stump of the occluded vessel is not visible, IVUS guidance from a probe positioned in a side branch originating immediately proximal to the occlusion can monitor in real time the direction of the cap puncture (2). When the stump of the proximal occlusion cannot be penetrated normally because of heavy calcification, aggressive balloon dilatation of the proximal vessel may allow penetration of the wire subintimally, using a knuckled wire to advance around the impenetrable cap (1). The lower left image shows along the right wall a knuckled wire advanced subintimally around the occluded segment, a valid option when there is a long occluded segment and its path is ambiguous (4). While in the past some operators were forcing the knuckled wire into very distal branches causing long dissections (STAR), in contemporary practice, a controlled re-entry is preferred soon after the distal stump. Along the left wall, a dedicated device for antegrade dissection re-entry (Stingray) is used for re-entry (5). In the central image a modified version of the parallel wire technique is shown with the assistance of a dual lumen microcatheter, which is useful to steer a second wire inside the occlusion after the first wire enters the subintimal space (6). Below, a retrograde microcatheter has been advanced through a septal collateral up to the distal CTO cap. Multiple appropriately shaped retrograde wires can be advanced to cross the occlusion (7, retrograde wire escalation/de-escalation). The right image illustrates another modality to obtain a successful retrograde crossing. A small balloon inflated in the occluded segment is used as a target to advance the retrograde wire (8). Cannulation of the proximal artery with a guide extension facilitates the externalisation of the retrograde wire. ADR: antegrade dissection re-entry; CART: controlled antegrade and retrograde tracking; CTO: chronic total occlusion; IVUS: intravascular ultrasound; STAR: subintimal tracking and re-entry
A brief history of CTO PCI
CTO recanalisation is nearly as old as percutaneous transluminal coronary angioplasty (PTCA), with the first attempts made by Martin Kaltenbach in Frankfurt in the late 1970s and by Geoffrey Hartzler in Kansas City in the following decade29303132. Not surprisingly, their fellows, Nicolaus Reifart and Barry Rutherford, became the early leaders of CTO recanalisation in the West. The main challenges in the 1980s were the poor steerability of wires, the large profile of balloons and the absence of stents to hold open the grossly disrupted, previously occluded segments. In the 1990s, stents became a routine part of PCI, securing the immediate effect of balloon angioplasty. This renewed interest in lesions that were previously disregarded for percutaneous recanalisation in favour of surgery. Innovative Japanese operators (Osamu Katoh, Hideo Tamai, Kazuaki Mitsudo and others) partnered with local companies to develop dedicated wires and microcatheters and started specific demonstration courses. In the West, attempts to develop techniques to avoid the painfully slow process of wire progression within the occlusion using dedicated devices such as laser or ultrasound or mechanical energy (Magnum wire [Biontronik], laser wire, SafeCross guidewire [IntraLuminal Therapeutics], Rotacs catheter [Osypka], Frontrunner XP CTO catheter [Cordis]) failed but caught the attention of the interventional community33343536373839. Bilateral injection and guidance from orthogonal views became standard in selected centres. The third millennium saw the advent of the DES revolution, offering a drastic reduction in restenosis and reocclusion rates after CTO recanalisation8910111213141516. The retrograde approach, initially described for saphenous vein grafts (SVGs)40, was pioneered in Japan by Katoh41. He addressed the frequent failures and complications caused by crossing and dilating septal collaterals with small balloons by introducing microcatheters that could be advanced across septal and also epicardial collaterals. A more “aggressive” antegrade approach developed by Antonio Colombo involved knuckling a wire into an adventitial dissection up to the more distal branches to create a distal re-entry (subintimal tracking and re-entry [STAR])42. This was abandoned because of its unpredictability in shaving multiple side branches, causing distal slow flow and frequent reocclusion434445. The technique was resurrected with appropriate modifications by William Lombardi and a group of US colleagues (limited antegrade subintimal tracking [LAST])46 to avoid wire exits outside of the “vessel architecture” in long occluded segments with an undetermined course. Re-entry into the true lumen from the dissection plane created by a knuckled wire was achieved with reverse controlled antegrade and retrograde tracking (CART), while a dedicated flat balloon with lateral exit ports (Stingray; Boston Scientific) offered an elegant antegrade alternative47. An enthusiastic group of US operators developed the first dedicated (“hybrid”) algorithm for CTO recanalisation, stressing the need for rapid switching from one approach to the other without wasting time in “failure mode”48. In practice, the hybrid algorithm prioritised retrograde or antegrade dissection and re-entry (ADR) over antegrade wiring (AW). The introduction of more steerable tapered wires and of dual lumen microcatheters (DLMC) promoted a return to the antegrade approach, with the use of intravascular ultrasound (IVUS) in a side branch to solve the problem of stumpless occlusions, and of parallel wires to take advantage of an initial wire exit for better orientation of the second wire. This 40-year journey (Figure 1) saw the success rate immediately increase from 50-60% to more than 90%20495051 in the hands of experienced operators. Complex, long-standing, previously failed CTOs that nobody 20-30 years ago dreamt could be treatable with PCI are now routinely and mostly successfully approached.
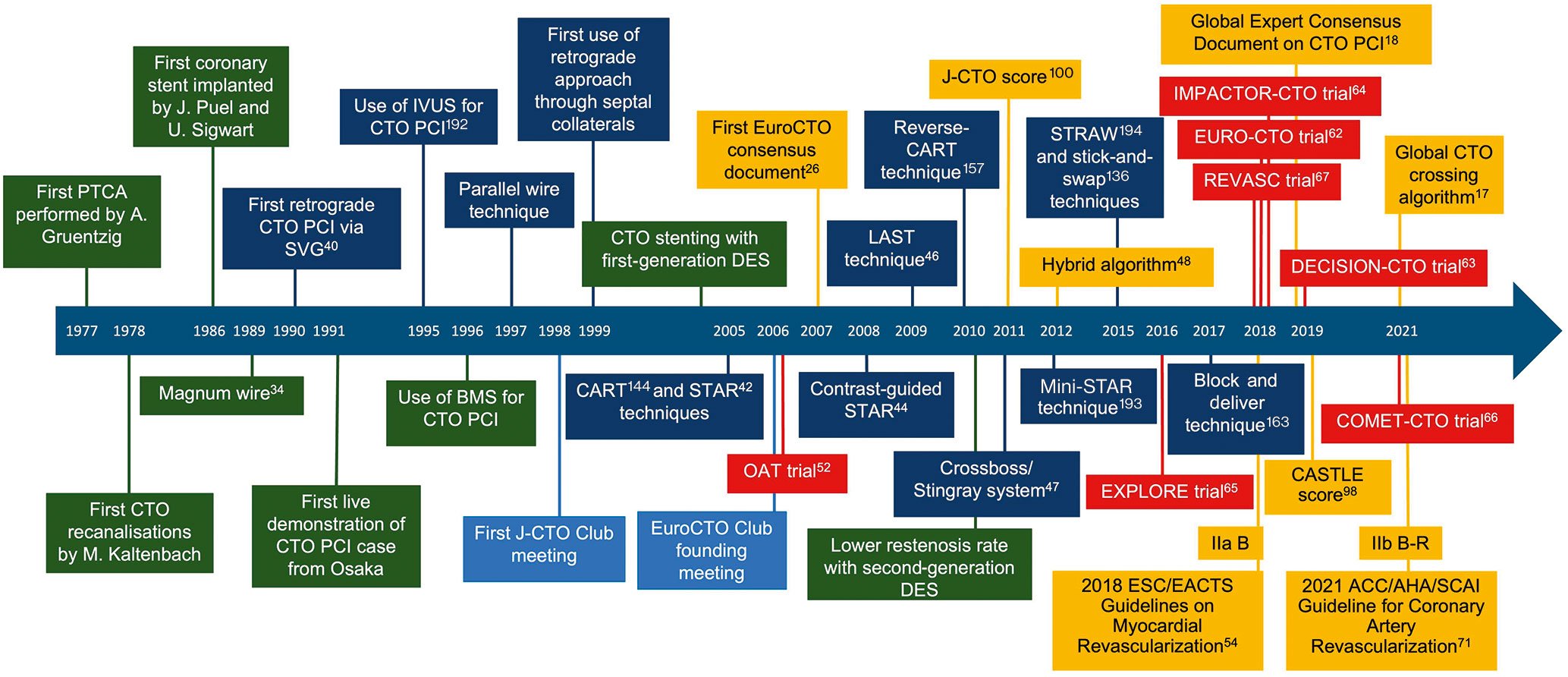
Figure 1. Main events in the history of CTO recanalisation. ACCF: American College of Cardiology Foundation; AHA: American Heart Association; BMS: bare metal stent; CART: controlled antegrade and retrograde tracking; CASTLE score: CABG, Age, Stump anatomy, Tortuosity, Length of CTO, Extent of calcification; COMET-CTO: Randomized Controlled Comparison of Optimal Medical Therapy with Percutaneous Recanalization of Chronic Total Occlusion; CTO: chronic total occlusion; DECISION-CTO: Drug-Eluting Stent Implantation Versus Optimal Medical Treatment in Patients With Chronic Total Occlusion; DES: drug-eluting stent; EACTS: European Association for Cardio-Thoracic Surgery; ESC: European Society of Cardiology; EXPLORE: Evaluating XIENCE and Left Ventricular Function in Percutaneous Coronary Intervention on Occlusions After ST-Segment Elevation Myocardial Infarction; IMPACTOR-CTO: Impact on Inducible Myocardial Ischemia of PercutAneous Coronary InTervention versus Optimal Medical TheRapy in Patients with Right Coronary Artery Chronic Total Occlusion; IVUS: intravascular ultrasound; J-CTO: Japanese Multicenter CTO Registry; LAST: limited antegrade subintimal tracking; OAT: Occluded Artery Trial; PCI: percutaneous coronary intervention; PTCA: percutaneous transluminal coronary angioplasty; REVASC: A Randomized Trial to Assess Regional Left Ventricular Function After Stent Implantation in Chronic Total Occlusion; SCAI: Society for Cardiovascular Angiography and Interventions; STAR: subintimal tracking and re-entry; STRAW: Subintimal TRAnscatheter Withdrawal; SVG: saphenous vein graft
Indications for CTO recanalisation
The reduction in mortality obtained with primary angioplasty for ST-segment elevation myocardial infarction (STEMI) and, in general, early revascularisation in acute coronary syndromes (ACS) could not be replicated in CCS. In the past, CTO revascularisation received punitive treatment in guidelines, starting from the first European Society of Cardiology (ESC) revascularisation guidelines, which inappropriately applied the negative results of the multicentre OAT (Occluded Artery Trial) trial in late (>48 hours but within 1 month) revascularisation of a total or subtotal occlusion of an infarct-related artery to CTO recanalisation5253. Fortunately, this unjustified discrimination has been removed in the most recent European guidelines54. The mortality benefit consistently observed in thousands of patients enrolled in controlled registries of successful CTO recanalisation versus a failed procedure (Table 1)55565758596061 raised hopes that this could be confirmed in randomised studies. Unfortunately, among the 1,892 patients enrolled across 6 RCTs626364656667, no mortality benefit of CTO PCI was shown compared to medical therapy. The randomised CTO trials were not designed to answer this question, forced to use surrogate (quality of life, angina relief, improvement in regional left ventricular function) or combined endpoints (including myocardial infarction [MI] and need for urgent revascularisation) because of the limited number of patients that they were able to recruit. The neutral results in the largest of them, the DECISION-CTO (Drug-Eluting Stent Implantation Versus Optimal Medical Treatment in Patients With Chronic Total Occlusion) trial63, might be, in part, explained by a considerable crossover between groups and by the treatment of all significant lesions in non-occluded arteries after randomisation.
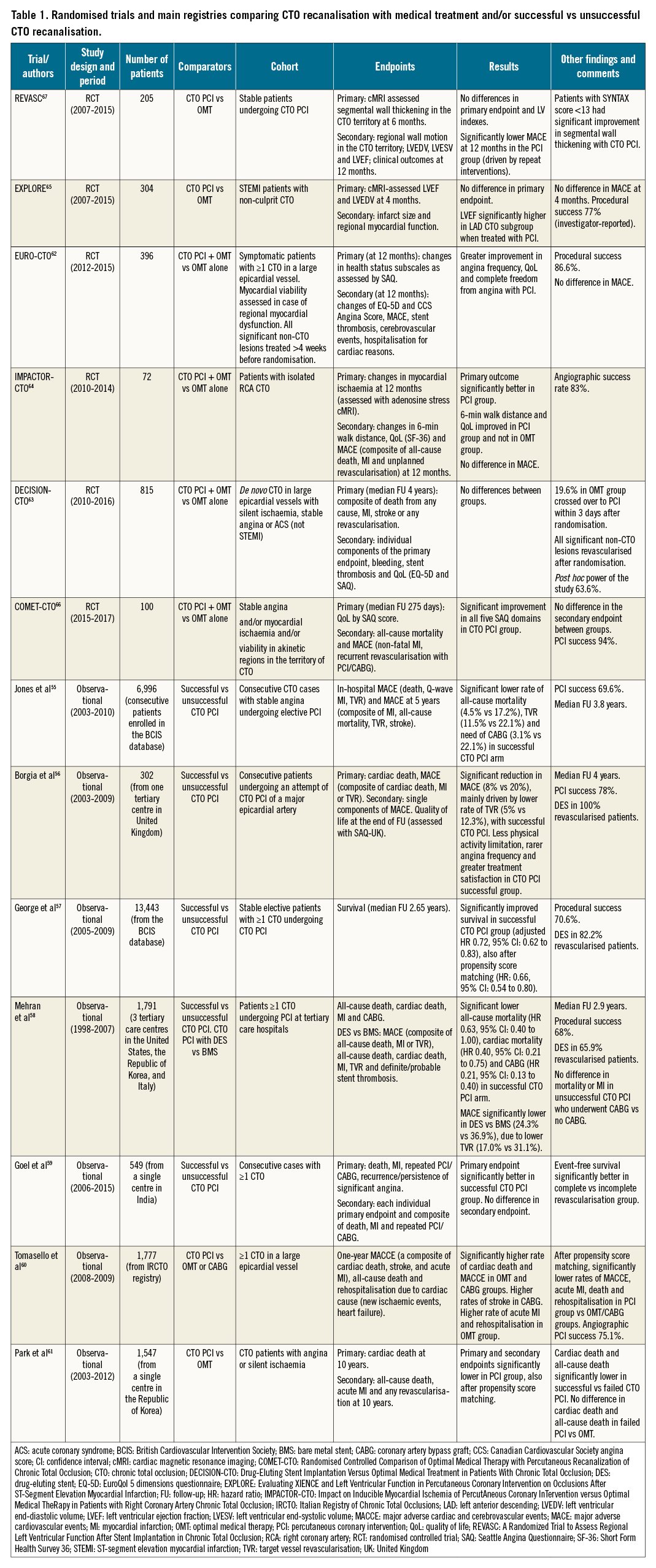
Furthermore, like most interventional trials with PCI compared to medical therapy, all CTO trials suffered from the bias of selecting patients with good left ventricular (LV) function and excluding highly symptomatic patients. Unlike the EXPLORE65 (Evaluating XIENCE and Left Ventricular Function in Percutaneous Coronary Intervention on Occlusions After ST-Segment Elevation Myocardial Infarction) and REVASC67 (A Randomized Trial to Assess Regional Left Ventricular Function After Stent Implantation in Chronic Total Occlusion) RCTs, multiple larger registries of CTO recanalisation have shown improvements in regional wall motion and overall LV ejection fraction after CTO success, with the greatest improvement in patients with lower LV function6869. A recent positron emission tomography imaging study has also demonstrated an improvement in absolute perfusion in the remote myocardium, mirroring the perfusion increase in the CTO territory and confirming the possibility of a beneficial effect of CTO recanalisation even on the myocardium subtended by the donor artery70.
This unsurmountable selection bias, funding issues and the practical difficulties of using a sham procedure in the control arm to avoid the placebo effect makes the feasibility of future randomised CTO trials challenging. The recent downgrade from a IIa to IIb indication for CTO recanalisation in the December 2021 American College of Cardiology/American Heart Association/Society for Cardiovascular Angiography and Interventions (ACC/AHA/SCAI) Guideline for Coronary Artery Revascularization71 reflects the disappointing RCT results.
These guidelines have also restricted the use of PCI in CCS in general, focusing instead on medical therapy for angina control. The recently published ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches) trial72 showed that patients with proven ischaemia randomised to revascularisation with PCI or CABG vs medical therapy had similar adverse events at 3.7 years, including mortality. Lack of complete revascularisation in the presence of CTOs may have played a role in this disappointing outcome. The ISCHEMIA CTO substudy, presented at the American Heart Association in 2020 but not yet published, showed that 1,470 patients (47.2%) had at least 1 CTO based on computed tomography (CT) angiography (total CTO lesions 1,797; mean per patient 1.22). Procedural success of CTO PCI in the trial has not been specifically reported, but patients with CTOs assigned to the invasive strategy more often underwent CABG than PCI when compared to the group without CTOs. CTO patients in the conservative strategy arm received more antianginal agents. At 4 years, patients with CTOs had higher cardiovascular death than patients without a CTO (5.2% vs 2.6%; p=0.003). As in the main trial, the invasive strategy in patients with CTOs was associated with more procedural MI (3.1% vs 1.2%) but a reduced risk of spontaneous MI (4.8% vs 8.6%) when compared to the conservative strategy. Quality of life, angina frequency and the Seattle Angina Questionnaire (SAQ)-7 summary score were improved in the invasive strategy regardless of the presence of a CTO.
The relatively short duration of follow-up might influence the neutral effect of CTO recanalisation on mortality737475. The STICH (Surgical Treatment for Ischemic Heart Failure) trial was negative for its primary endpoint at 5 years76 but showed a statistically significant benefit of revascularisation with CABG in patients with multivessel disease and reduced LV ejection fraction at 10 years77. Park et al similarly demonstrated the importance of long-term follow-up in CTO PCI, with no difference in all-cause death, cardiac death, acute MI or any revascularisation at 3 years in patients with successful CTO PCI compared to a propensity score-matched medical therapy arm but with a significantly better outcome in the CTO PCI group at 10-year follow-up61. The late mortality benefit of CTO recanalisation can be explained by disease progression and acute occlusion of the donor vessel impairing collateral flow to the CTO artery and compromising a larger territory (double jeopardy phenomenon)78. There are countless anecdotal reports of a recanalised occluded artery that protected and avoided infarction in the donor vessel, which may make CTO recanalisation prognostically more beneficial than angioplasty of an equally important subtotally occluded artery.
This potential benefit, however, did not translate into a favourable mortality trend in the randomised CTO trials, in ISCHEMIA or in other PCI vs medical therapy randomised trials. In their Editorial to The New England Journal of Medicine on the ISCHEMIA trial, Antman and Braunwald concluded that “patients with stable ischemic heart disease who fit the profile of those in ISCHEMIA and do not have unacceptable levels of angina can be treated with an initial conservative strategy. However, an invasive strategy, which more effectively relieves symptoms of angina, is a reasonable approach at any point in time for symptom relief”79. Since symptoms are the main drive for CTO recanalisation, it is important to know that they are often peculiar in patients with CTOs. Many patients with CTO come to medical observation because of the development of an acute syndrome in another artery or because of incidental findings, such as silent electrocardiogram (ECG) or echocardiographic abnormalities in routine examinations. Patients with CTO often deny symptoms altogether and you must dig deeply into the patient’s history to find activities that they could previously effortlessly perform and now avoid, attributing the deterioration to “old age” or “lack of training”, excuses that became more frequent after the repeated lockdowns of the COVID-19 era. Symptoms are described as classical angina in less than 1/3 of cases, while the patient is more likely to indicate chest discomfort, breathlessness or general fatigue as reasons to stop exercising80. Multiple studies have shown objective changes in exercise capacity confirmed by cardiorespiratory tests8182. Dyspnoea, present in 30-50% of CTO patients, improved in 70% and disappeared in 42% of patients after successful recanalisation in the controlled OPEN CTO (Outcomes, Patient Health Status, and Efficiency IN Chronic Total Occlusion Hybrid Procedures) registry83. The results of ISCHEMIA also drive scepticism on the usefulness of provocation imaging tests. Still, in the absence of necrotic changes (Q-waves or, better, >50% subendocardial late gadolinium enhancement [LGE] seen on cardiac magnetic resonance [CMR]), testing with provocation imaging tests (stress echocardiogram, CMR or nuclear scan) should be considered in patients with an impaired exercise capacity not explained by other comorbidities (lung disease, arthrosis, etc.). In principle, with success rates now above 80% even for the most complex occlusions and improved complication rates, patients with firm indications for recanalisation should be offered this therapy option. Figure 2 suggests general rules for patient screening for CTO recanalisation. Among the preliminary imaging tests, great emphasis is placed on CMR as the gold standard to detect fibrosis. The presence of full thickness (>50%) LGE is not an absolute contraindication to CTO recanalisation if the extension of the scar is obviously smaller than the supplied territory848586878889. Superimposed ischaemia (a perfusion defect seen only after injection of adenosine/regadenoson involving segments without full thickness infarction based on LGE) can help but interpretation remains subjective and, when symptoms appear convincing from history, other confirmatory provocative tests more sensitive for ischaemia detection may be required. Heart Teams must also give recommendations for surgery or PCI. While disease complexity in other territories should always be taken into account, the decision should not be solely influenced by the punitive score given in SYNTAX to occluded segments909192.
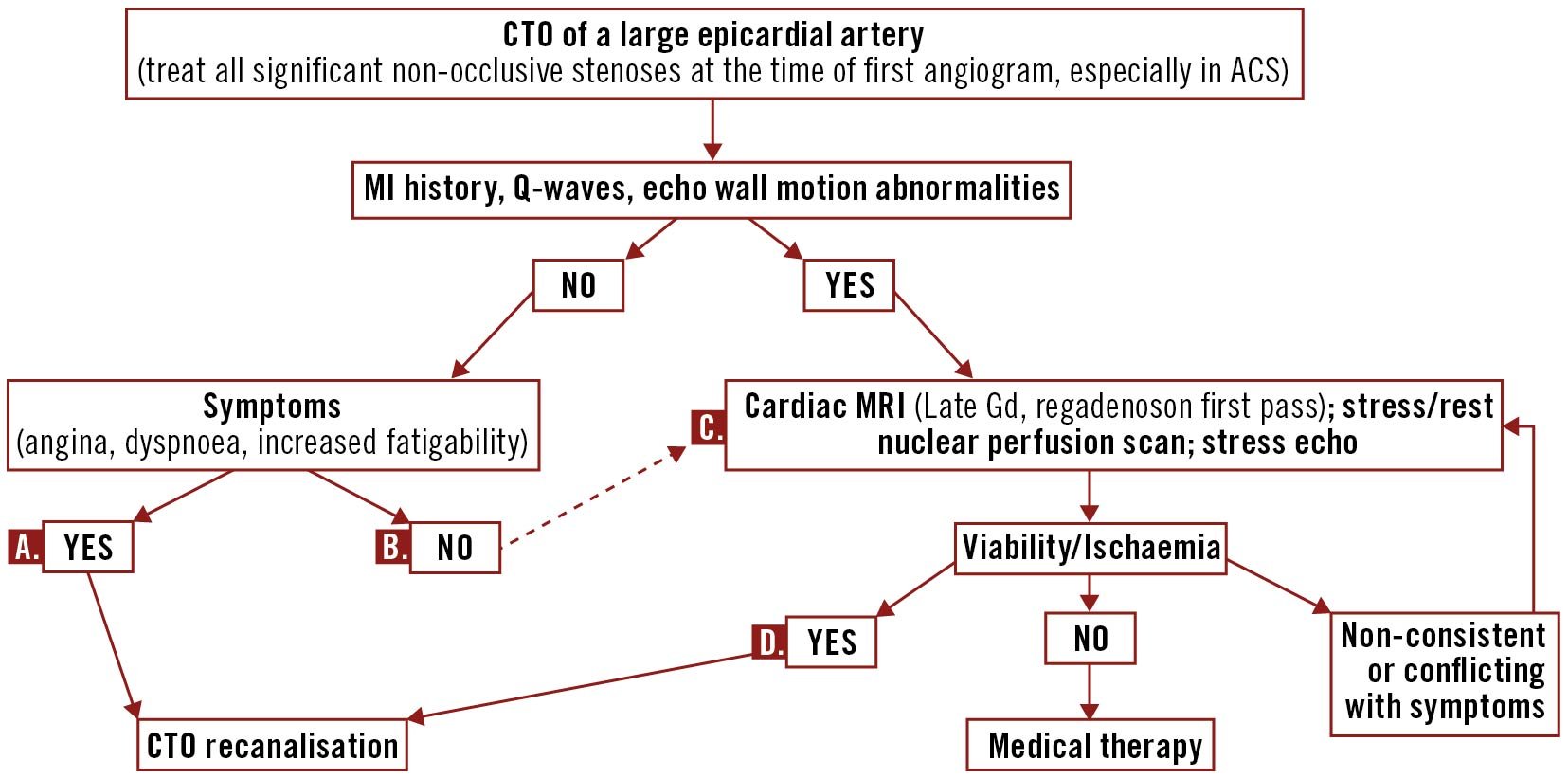
Figure 2. Indications for CTO recanalisation: a pragmatic stepwise approach to confirmatory non-invasive tests. A. If symptoms persist after maximally tolerated anti-anginal therapy. B. The negative results of the ISCHEMIA randomised trial have challenged this practice, supported by previous guidelines and results of large multicentre registries. C. Selection based on local availability and expertise. D. Consider possible prognostic role based on size of ischaemia. ACS: acute coronary syndromes; CTO: chronic total occlusion; Gd: gadolinium; ISCHEMIA: International Study of Comparative Health Effectiveness with Medical and Invasive Approaches; MI: myocardial infarction; MRI: magnetic resonance imaging
Preparing the patient for a CTO procedure
It is uncommon that patients receive a separate specific consent form for CTO recanalisation. CTO procedures require double arterial punctures in more than 70% of cases (always when collaterals arise from the contralateral vessel) and, with an average duration >90 minutes, are at least 3-4 times longer than most PCI procedures. Such procedures deserve a more detailed description than a generic PCI consent form. Renal function must be carefully checked, especially in diabetic patients. If the patient does not experience severe prolonged hypotension and appropriate hydration is maintained throughout the procedure, a total amount of contrast of less than 4 times the glomerular filtration rate in ml/min is unlikely to cause contrast-induced acute kidney injury, let alone permanent dialysis93949596. However, blood loss during a long procedure with 2 access sites is often unavoidable and may increase the risk of renal damage97.
Multiple scores have been developed to grade CTO complexity (Table 2, Supplementary Figure 1)9899100101102103104105106107108109110. There is convincing evidence that scores can predict procedure duration and possibly help in the selection of patients that should be handled only with support from a dedicated CTO operator. Their accuracy in the prediction of success and complications in individual patients is more questionable because the results, derived from the patient characteristics and operator skills in the database used to develop and validate the scores, may not be generalisable to CTO operators of different levels of experience. Furthermore, scores mostly attribute the same points to all the statistically significant predictive variables, irrespective of the magnitude. We encourage keeping generic percentages in the consent form, with percentages of success ranging between 70 and 95% and major complications between 0.1% and 5% 111112113114115116. This will allow the operator to adjust the estimates to the specific anatomy of the occlusion and his/her expectations of success in reopening the vessel. The general principle is that the patient should be made aware that, unlike for most PCI procedures that have a success rate close to 100%, failure is rare but still possible. A similar approach can be recommended for complications. The consent form (Supplementary Appendix 1) should list, in detail, the specific risks of CTO recanalisation. It will be up to the operator to fine-tune numbers, according to the likelihood that aggressive CTO techniques with higher complication rates (ADR, retrograde) will be required. With the increase in immediate success, “investment procedures” of partial recanalisation without stent placement in preparation for a second attempt are now less common but it is important to indicate this possibility to the patient at the outset. Puncture site complications remain among the most frequent types of complication. If the procedure complexity or the operator’s experience suggest that a bifemoral or a radial-femoral approach is required, the operator should apply all the available technical refinements learned from transcatheter aortic valve implantation (TAVI)117118119120121 to avoid complications (puncture after fluoroscopic screening of the femoral head and under ultrasound guidance, knowledge of anatomy based on CT angiography or contralateral injection from the radial using a 125 cm long catheter). It should be mentioned that for long procedures, which require high doses of heparin (activated clotting time >250 sec. for antegrade, >300 sec. for retrograde), a substantial risk of haematomas still remains. A final confirmation of successful haemostasis of the femoral puncture after the use of closure devices with contralateral injection takes advantage of the bilateral approach routinely required for CTOs. With modern X-ray systems the radiation dose for most CTO procedures is far below the 5 Gy threshold where minor skin damage can be expected122123124125126127. Still, the use of a low frame rate during fluoroscopy and angiography, avoiding too small field of views and too skewed projections, is helpful in reducing stochastic damage, especially for young and/or heavy patients, as well as the operator’s dose, a sensitive issue after the recent reduction in the maximal allowed eye dose (20 mSv/year) prompted by the new EU regulations. CTO recanalisation is, by definition, an elective procedure and starting a P2Y12 inhibitor before the procedure allows an effective concentration to be reached when stents are implanted. There are no specific randomised trials that suggest whether the intensity and duration of antiplatelet treatment should be modified for CTO procedures and results from existing observational studies are conflicting128129. An individualised approach is often applied based on a combination of existing scores of bleeding and recurrent ischaemia and specific procedural CTO characteristics (length of stented segment, long subintimal tracking, less than TIMI [Thrombolysis In Myocardial Infarction] 3 post-procedural flow)128130131132133.
Progress in antegrade recanalisation
Techniques of antegrade CTO PCI are divided into 2 groups: (I) antegrade wire escalation/de-escalation and (II) antegrade dissection and re-entry (Central illustration).
Antegrade wiring
To date, AW remains the most frequently used technique in contemporary CTO PCI49134. All major CTO algorithms currently used suggest AW as a first-line approach in the case of (i) a non-ambiguous proximal CTO cap, (ii) a good quality distal vessel, and (iii) a short occlusion length (<20 mm in the hybrid algorithm)172248. The presence of occluded stents indicating the arterial path also favours the antegrade option, irrespective of occlusion length20. In addition, when 1 or more of the above-mentioned conditions are not fulfilled, AW can be used for “proximal cap preparation” when there is a high likelihood that retrograde or dissection re-entry techniques will need to be used.
The use of retrograde injection of contrast medium is almost mandatory to visualise the coronary vessel distal to the occlusion, offering a roadmap during the recanalisation procedure (Figure 3). Lower complexity CTOs are generally crossed with AW and, considering that the rate of procedural complications is significantly lower with this technique, AW is suggested as a “first-step” approach, particularly for CTO operators at the beginning of their learning curve134. When CTO complexity increases, more complex techniques (dissection re-entry techniques and/or retrograde approaches) are usually required, which also demand greater operator experience.
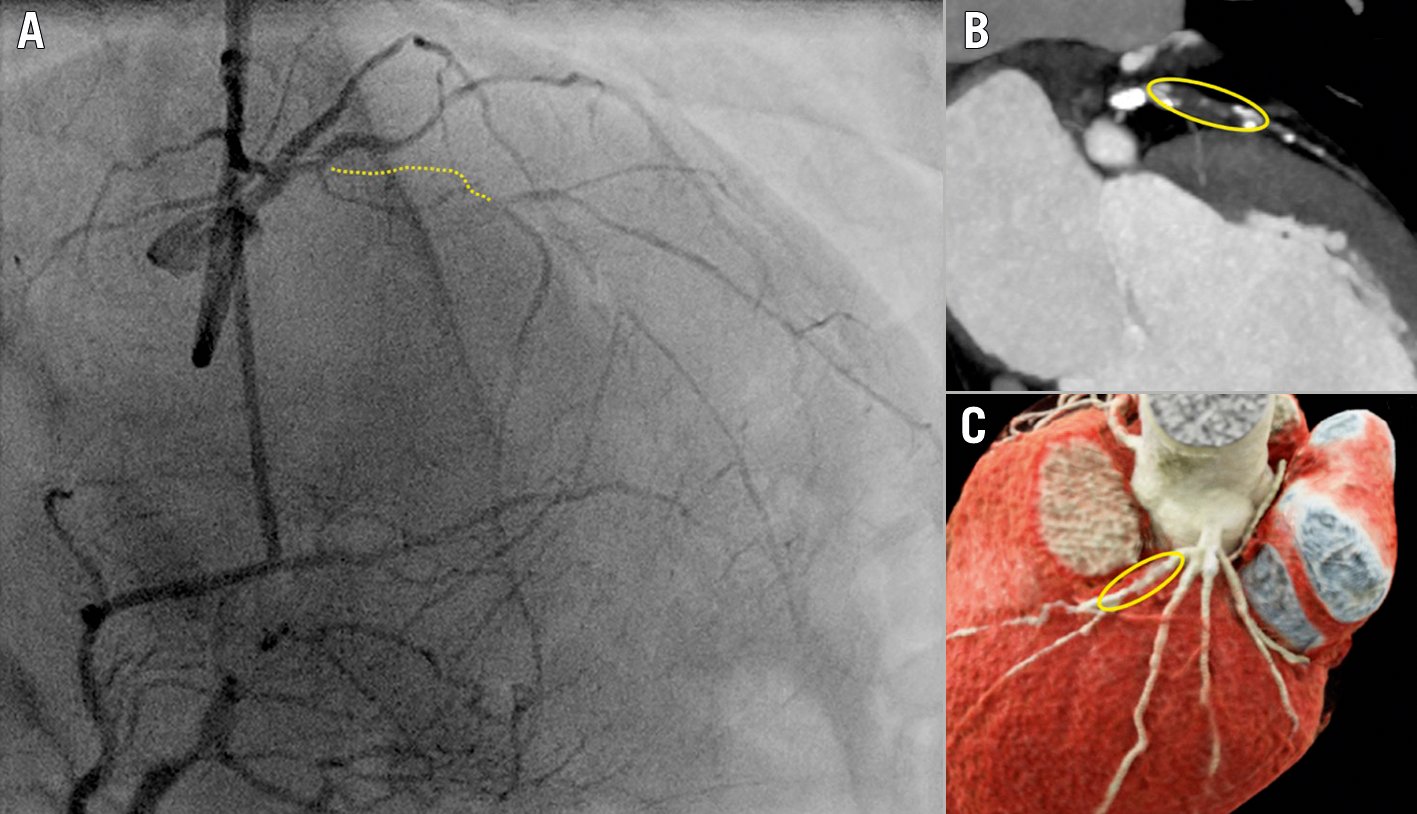
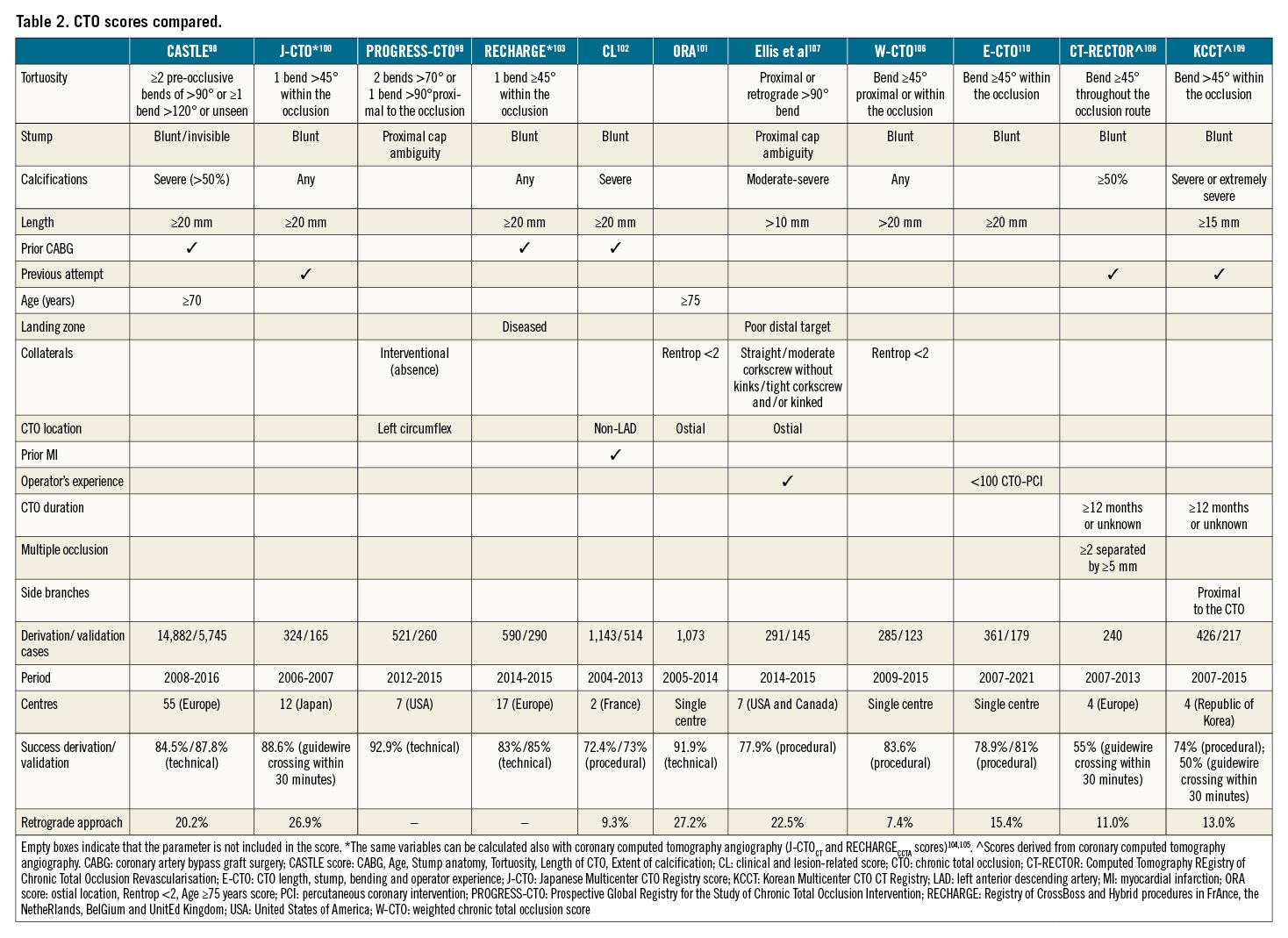
Figure 3. Role of dual injection in antegrade CTO PCI. A) Dual injection is fundamental to highlight the length (>20 mm) of a CTO of the mid LAD (yellow dotted line), in order to visualise not only the proximal, but also the distal coronary bed (distal LAD in this case retrogradely filled from RCA-septal collaterals). B & C) MSCT showing the proximally occluded LAD segment (indicated in a yellow circle). Note the presence of calcium at the proximal and distal end of the occlusion, while the midsegment appears spared. The preintervention MSCT allows the operator to anticipate potential difficulties. In this case, it might be expected that appropriate changes in guidewire stiffness (step-up/step-down) through the microcatheter are required. CTO: chronic total occlusion; LAD: left anterior descending artery; MSCT: multislice computed tomography; PCI: percutaneous coronary intervention; RCA: right coronary artery
The use of a microcatheter is mandatory in order to avoid dissections/subintimal tracking of the coronary vessel proximal to the CTO while manipulating progressively stiffer wires. The microcatheter allows rapid exchange of specialised guidewires during the negotiation of the occluded lesion (i.e., wire escalation) and avoids the need to modify the small distal bend already present in most preshaped dedicated wires. Furthermore, the use of a coil-based structure in the construction of microcatheters facilitates progression within the CTO body, enhancing active support and the penetration power of the guidewire (Supplementary Table 1).
Guidewires used for contemporary antegrade wiring reflect different modalities of tissue tracking and can be divided into 3 categories (Supplementary Table 2):
- Tapered polymer-jacketed wires used for soft tissue tracking. These are low tip-load wires with tip diameters of 0.008-0.009" that allow the wiring of CTO lesions with prevalent cholesterol-rich or fibro-fatty elements.
- Intermediate tip-load guidewires with enhanced torqueability, made to negotiate fibrous and fibrous-calcific intimal plaques. Good examples are the tapered guidewires of the Gaia family (Asahi Intecc) with a composite core that allows enhanced control within the occlusion. In addition, polymer-jacketed untapered guidewires (e.g., Pilot 200 [Abbott Vascular], Gladius EX [Asahi Intecc], Raider [Teleflex], etc.) can be used antegradely, in case the vessel course is uncertain.
- High tip-load guidewires are used to penetrate heavily calcified proximal CTO caps (e.g., Confianza 12g [Asahi Intecc], Hornet 14g [Boston Scientific], Warrior [Teleflex], Infiltrac [Abbott Vascular], etc.). The recommended use involves “puncturing” the first few millimetres of the proximal, often calcified, CTO cap, followed by downgrading the guidewire to a lower tip-load in order to reduce the risk of wire exit.
Parallel wiring
The parallel wire technique can be used in case of failure of direct AW17. Instead of withdrawing the first wire that enters into the subintimal space, a second guidewire is introduced within the CTO segment “parallel” to the first one (which works as a reference for the operator) and is used to penetrate the distal cap. A contemporary modification of this historical technique is performed using a DLMC, which facilitates the interchange between the 2 guidewires (Central illustration, Supplementary Figure 2)135.
Antegrade dissection re-entry
A long-segment occlusion significantly decreases the possibility of successful antegrade intimal tracking (“true-to-true lumen wiring”). This is especially true if there is tortuosity making the vessel course uncertain within the occlusion1748 or if calcifications deflect the wire during advancement. Controlled use of the subintimal space mandates the utilisation of a dedicated re-entry device, namely the Stingray balloon (Central illustration, Figure 4), in a stepwise fashion: (i) after angiographic confirmation of the antegrade subintimal wire tracking, (ii) the Stingray balloon is advanced into the subintimal space distal to the occluded segment, (iii) the inflation of 2 small parallel balloons enhances stability of position and wire direction during puncture, (iv) a high tip-load guidewire (e.g., Stingray guidewire, Hornet 14g; Confianza Pro 12g, etc.) can be used to establish a focal false-to-true luminal communication, (v) the high tip-load guidewire can be advanced towards the distal vessel after the re-entry (“stick-and-drive”) or exchanged for a polymer-jacketed intermediate tip-load guidewire after puncturing (“stick-and-swap”, e.g., with a Pilot 200)136. The ADR can be used as a first- (intentional) or second-line (bailout) crossing technique47. A small randomised trial (CrossBoss First Trial), using a dedicated catheter that can be screwed within the occlusion to create a small, controlled subintimal track for the Stingray, failed to show improved success or reduced time to crossing137. The larger RECHARGE (REgistry of CrossBoss and Hybrid procedures in FrAnce, the NetheRlands, BelGium and UnitEd Kingdom) registry observed a primary success rate of this procedure among ADR experts of 66%, reaching 86% when other routes, including retrograde, were used138. This indicated that ADR can be a useful addition to the CTO technical armamentarium but that it is not a stand-alone technique in most cases.
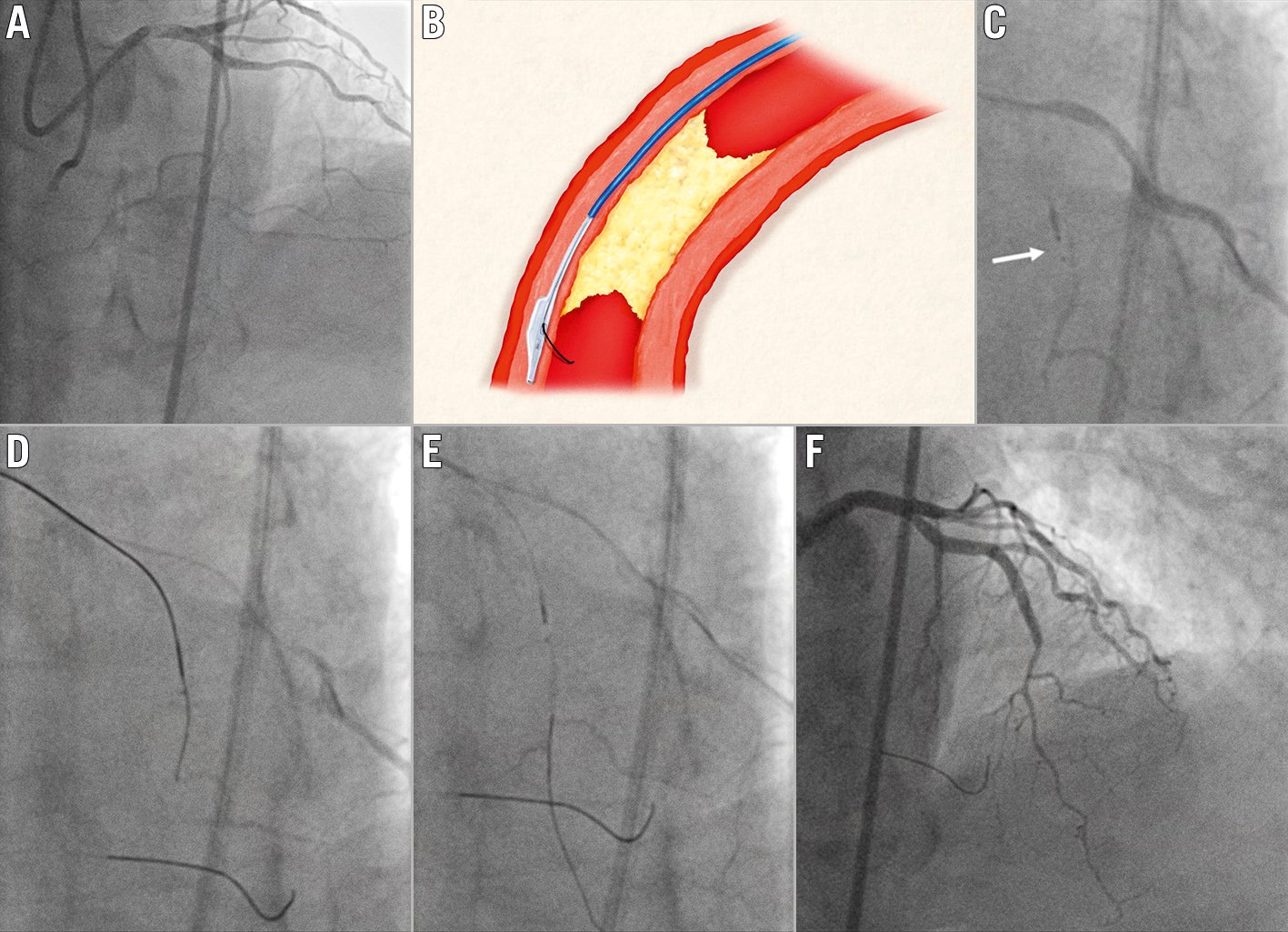
Figure 4. Antegrade dissection re-entry using the Stingray balloon. A) Clinical case of a chronically occluded LAD. B) Schematic representation of use of a Stingray balloon, positioned in the subintimal space distal to the occlusion; a wire puncturing towards the vessel lumen, in the proximity of the distal CTO cap, is used to re-enter. C) Placement of the Stingray balloon subintimally (white arrow). D) Puncture towards the distal lumen using a Confianza 12g guidewire. E) Wiring of the distal vessel using a Pilot 200 guidewire (“stick-and-swap” technique). F) Final angiographic result after stent implantation. CTO: chronic total occlusion; LAD: left anterior descending artery
As an alternative to the Stingray system, a contemporary DLMC can be used to perform ADR. The monorail port is loaded over the subintimal guidewire while a stiff, high tip-load guidewire is used through the more proximal over-the-wire port to achieve distal re-entry (Figure 5). The ReCross (IMDS) is the only available DLMC with 2 over-the-wire lumen and 3 distal exit ports, 2 of the latter being placed in opposite directions, potentially allowing re-entry in the vessel lumen with 1 of the 2 guidewires used139.
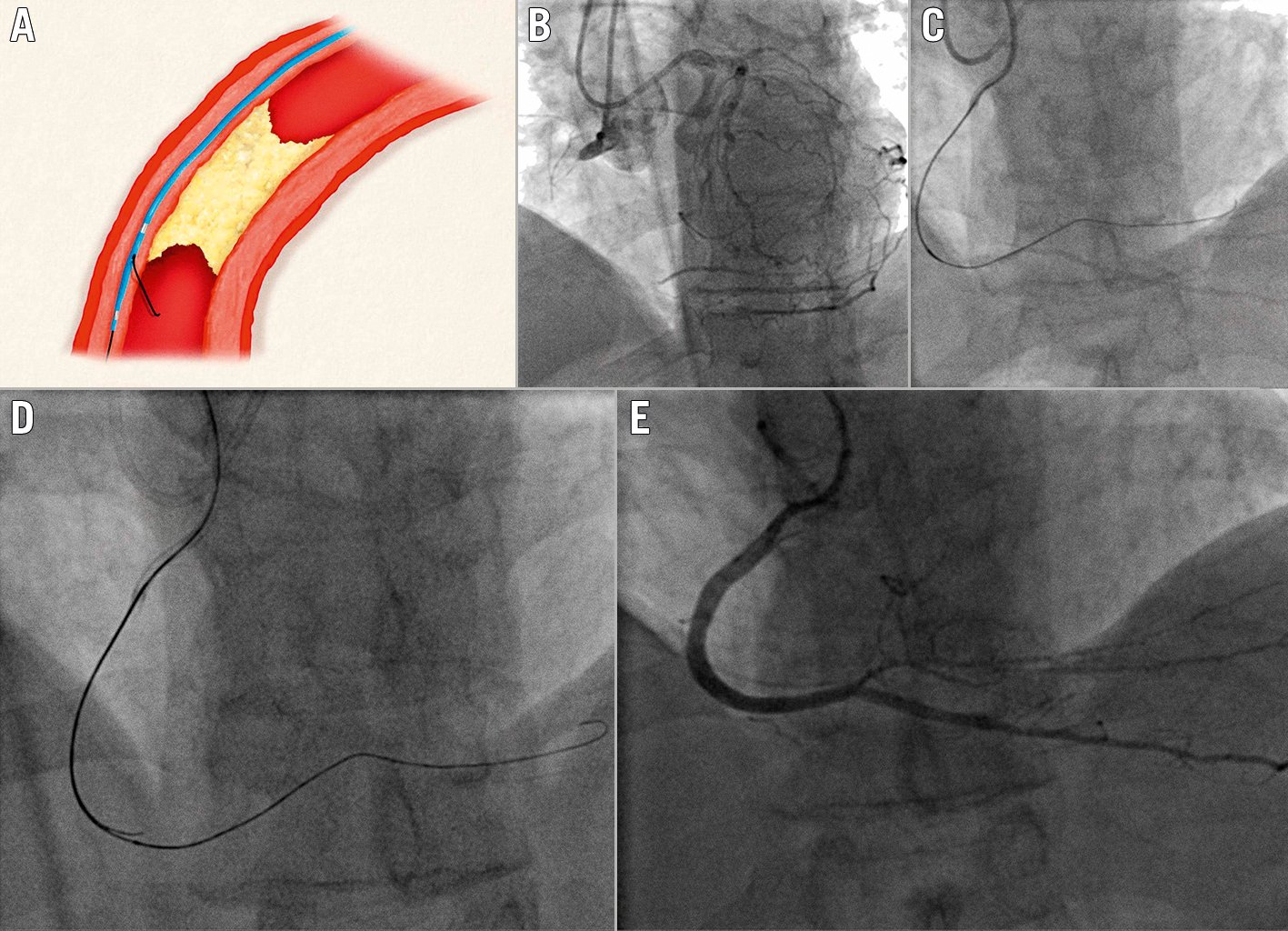
Figure 5. Antegrade dissection re-entry using a dual lumen microcatheter (DLMC). A) Schematic representation of use of the DLMC, advanced over the monorail port, in the subintimal space, distally to the occlusion. B) Chronically occluded RCA. C) Subintimal position of the first antegrade guidewire. D) DLMC on-site and puncture through the proximal OTW port towards the distal vessel lumen using a Confianza 12g. E) Final angiographic result. DLMC: dual lumen microcatheter; OTW: over-the-wire; RCA: right coronary artery
In particular, IVUS guidance can be used to facilitate wire re-entry into the true lumen (Figure 6, Figure 7). The ultrasound probe is positioned subintimally (first guidewire) with direct visualisation of the extra- and intraplaque space, redirecting a second stiff wire with a larger bend from extra- to intraplaque, reaching the distal true lumen. It must be acknowledged that IVUS-guided re-entry is an extremely complex technique, limited to a small number of elite operators and not routinely used for re-entry in most centres.
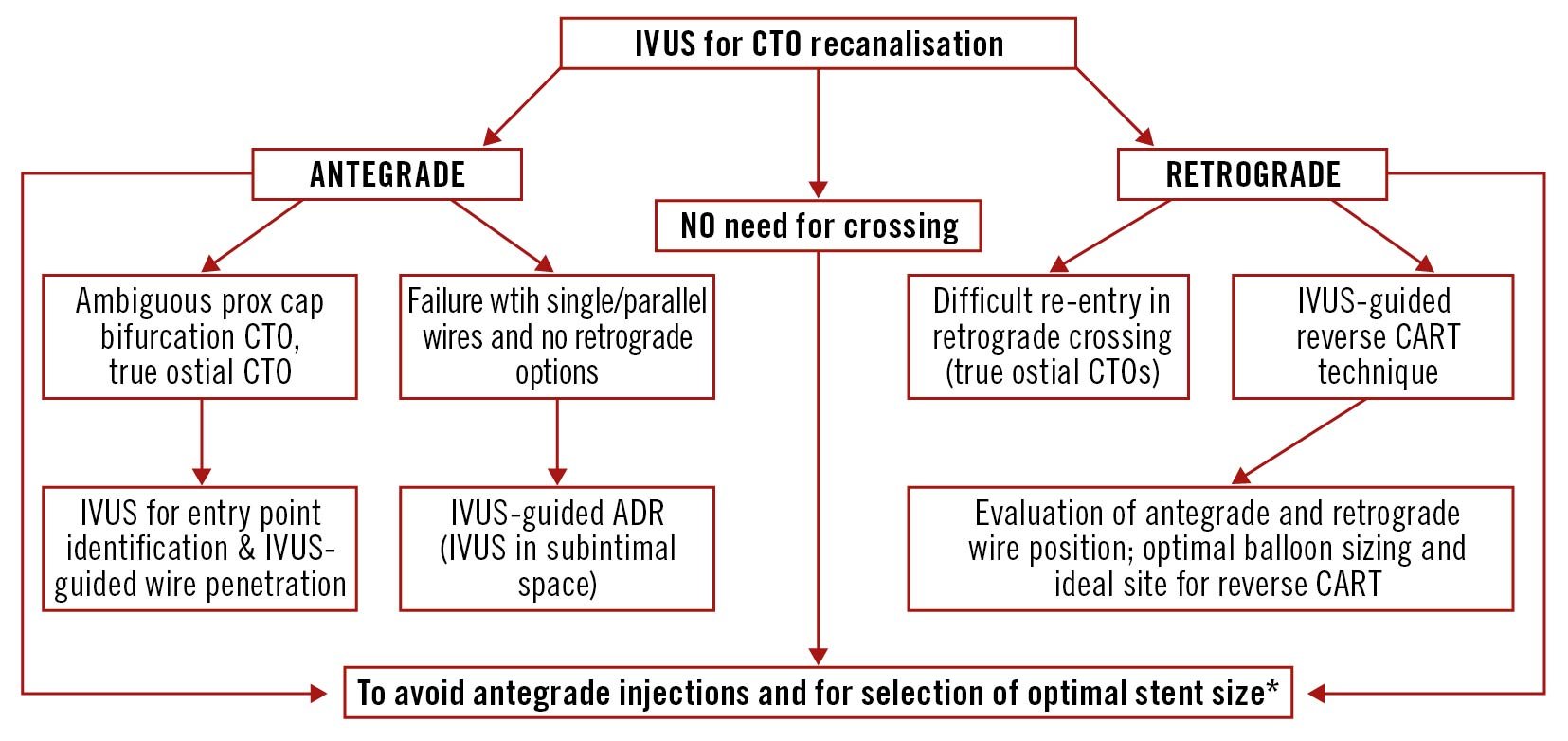
Figure 6. Potential applications of IVUS in CTO PCI. *Consider also predictors of lumen area enlargement after CTO recanalisation (perimedial high echoic band, occlusion duration >3 months, poor collateral flow, statin use, LAD occlusion and absence of moderate/severe calcifications)174178 179180. ADR: antegrade dissection re-entry; CART: controlled antegrade and retrograde tracking; CTO: chronic total occlusion; IVUS: intravascular ultrasound; LAD: left anterior descending artery; PCI: percutaneous coronary intervention; prox: proximal
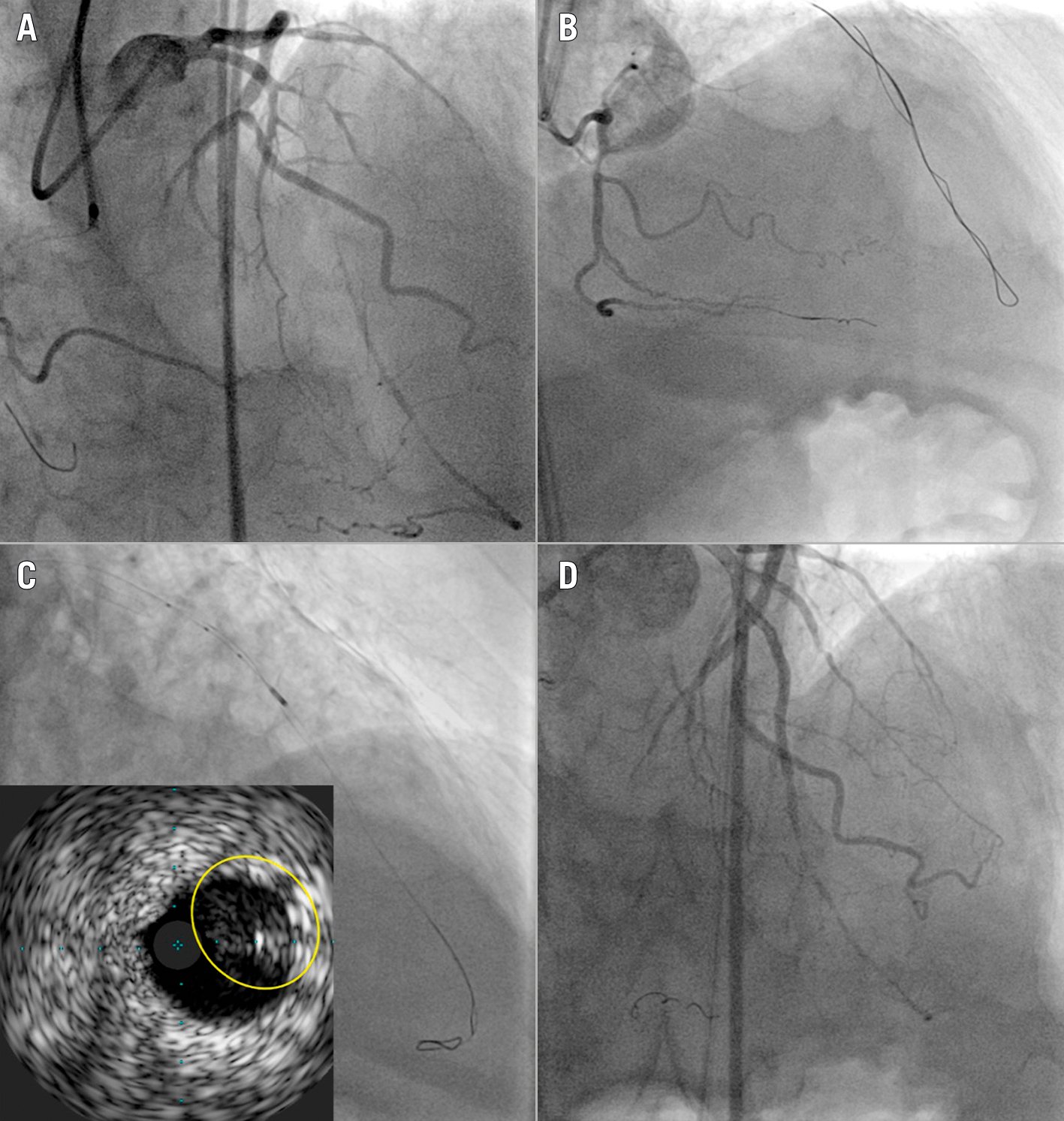
Figure 7. IVUS-guided antegrade re-entry. A) Angiography of a CTO of the proximal LAD. B) A knuckled Fielder XT-wire advanced distal to the CTO. C) IVUS confirms the subintimal distal position of the knuckled wire and facilitates re-entry into the true distal lumen using a Confianza 12g guidewire (yellow circle). D) Final angiographic result. CTO: chronic total occlusion; IVUS: intravascular ultrasound; LAD: left anterior descending artery
Proximal cap ambiguity
Proximal cap ambiguity has been previously defined as the inability to unequivocally determine the proximal entry point into a CTO, due to the presence of side branches, vessel tortuosity, or a flush ostial coronary artery occlusion that prevents the engagement of the guide catheter140.
When there is an ambiguous proximal CTO cap with a side branch (SB) at the site of the occlusion, antegrade IVUS-guided wiring can be performed. The ultrasound probe is advanced into the SB and used to visualise the ostium of the occluded artery. A second wire can be advanced to puncture the cap under real-time IVUS visualisation of the wire’s position (Central illustration, Figure 6, Figure 8).
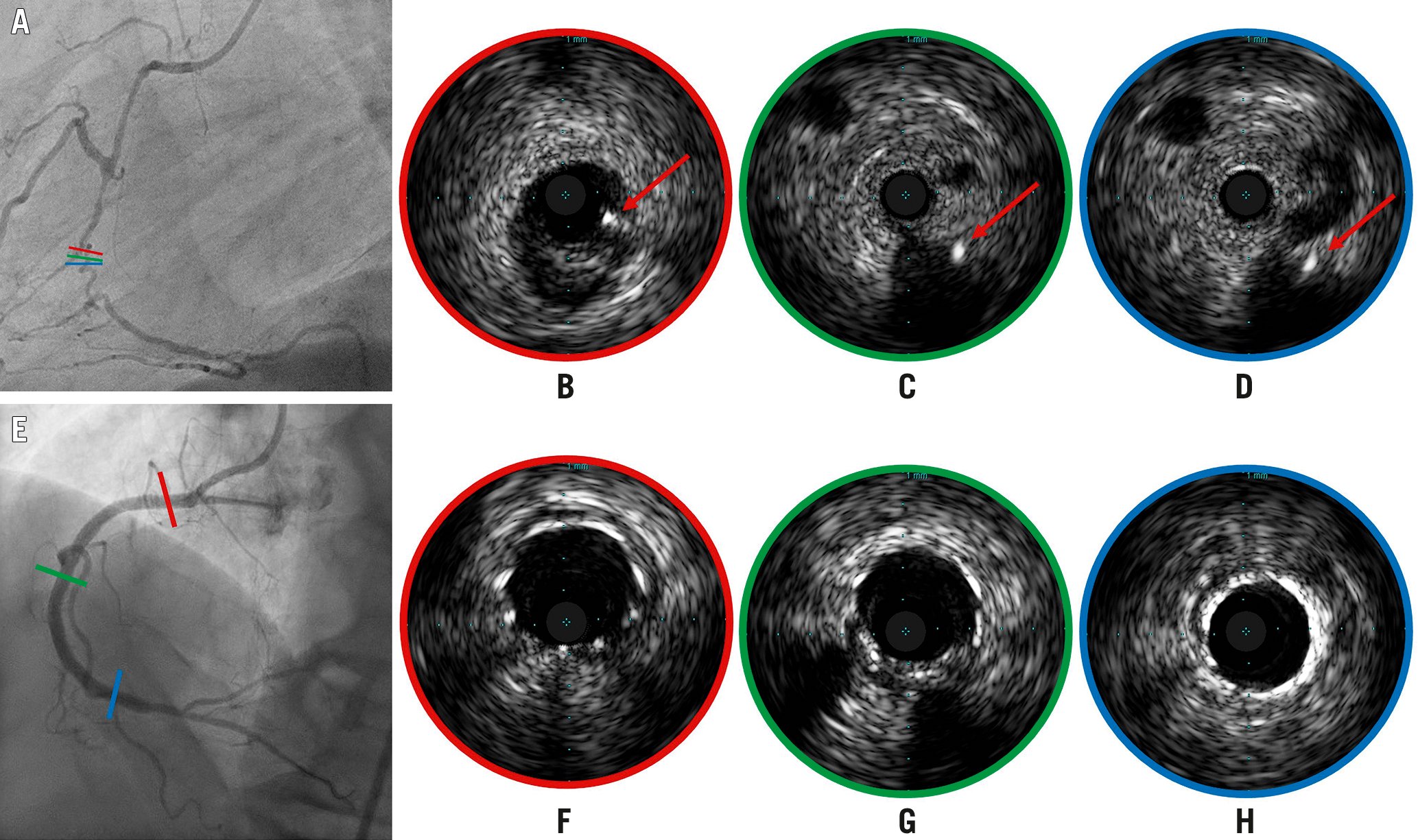
Figure 8. IVUS-guided puncture of an angiographically ambiguous proximal CTO cap. A) Blunt occlusion of the mid-RCA. A right ventricular branch allows insertion of an IVUS probe with its piezoelectric crystal positioned at the CTO ostium; coloured lines indicate the position of IVUS cross-sections. B - D) A Gaia third guidewire (Asahi Intecc) (red arrows) punctures, under IVUS guidance, the proximal (B), the mid (C) and the distal (D) segment of the CTO. E) Final angiographic result after deployment and expansion of multiple stents from the third segment to the ostium guided by repeated IVUS runs; coloured lines indicate the position of IVUS cross-sections confirming optimal expansion (F - H, respectively, from proximal to distal segment). CTO: chronic total occlusion; IVUS: intravascular ultrasound; RCA: right coronary artery
In the absence of a suitable SB to accommodate the IVUS catheter, initiation of a dissection plane proximal to the CTO cap (“move-the-cap” techniques) (Central illustration, Figure 9) may be needed to safely start subintimal tracking within the unknown arterial course141:
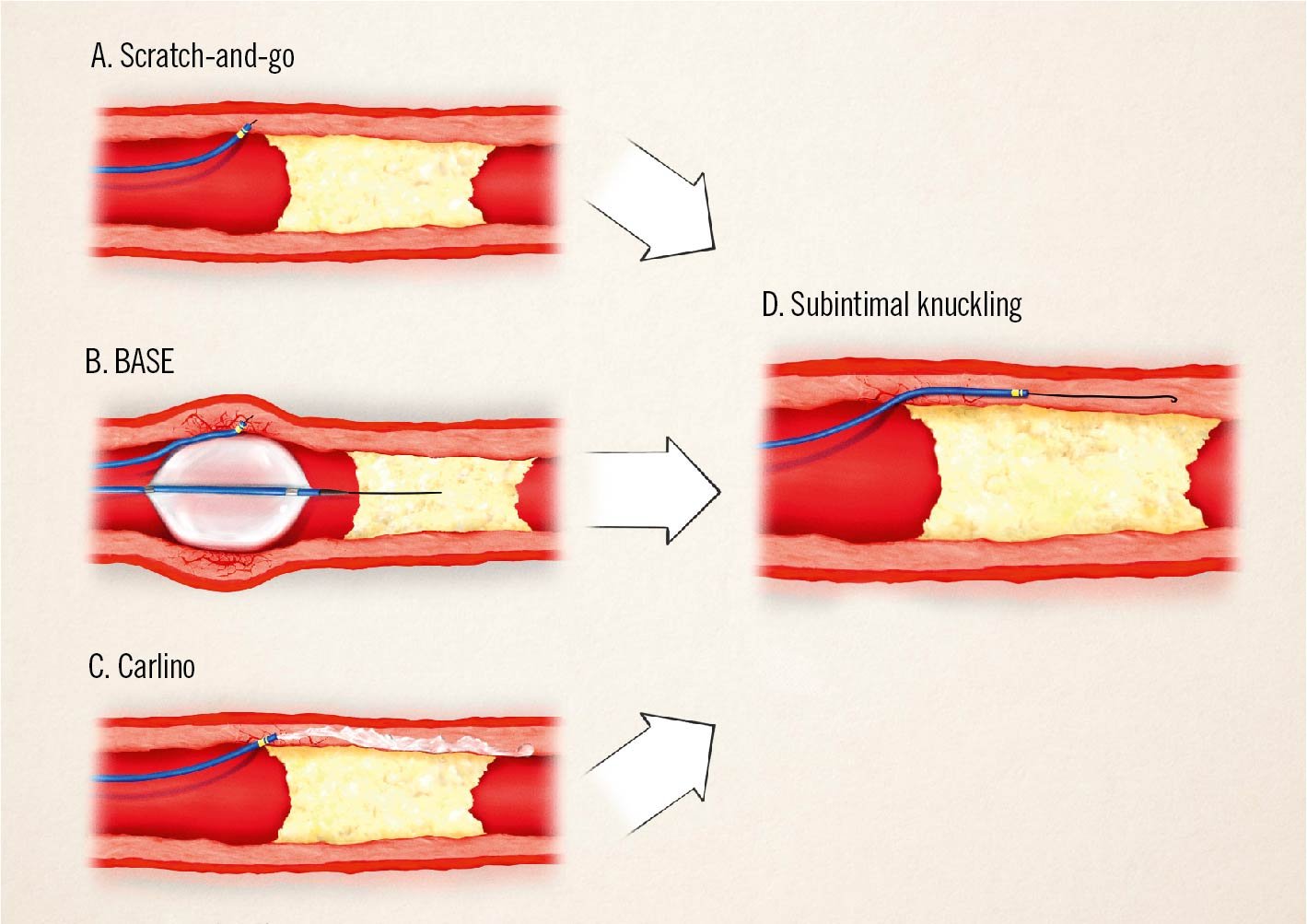
Figure 9. Move-the-cap techniques. In the “scratch-and-go” technique, a high tip-load guidewire is used to directly enter in the extraplaque space (A), and the tip of the microcatheter is positioned inside the subintimal space. The high tip-load guidewire is then exchanged for a polymer-jacketed guidewire, used as “knuckled” wire to advance in the subintimal space (D). In the BASE technique, a slightly oversized balloon is dilated proximally to the occluded segment, resulting in disruptions of the intimal layer (B), which can be used to enter a guidewire in the extraplaque space with the support of a microcatheter and start a knuckle (D). The Carlino technique uses contrast to create intimal disruptions (C), which can be negotiated by a guidewire as above. BASE: balloon-assisted subintimal entry
- Balloon-assisted subintimal entry (BASE) (Figure 9B, Figure 9D): a slightly oversized balloon is dilated immediately proximal to the CTO in order to create focal disruptions of the intimal layer which allow communication between the vessel lumen and the extraplaque space, allowing wire passage in this space. In the case of an SB proximal to the occlusion, a modified version of this technique (side-BASE) can be used to reduce the risk of SB loss and to facilitate the entry of a polymer knuckled wire into the subintimal space142.
- Carlino technique143 (Figure 9C, Figure 9D): microinjections of contrast through the tip of a microcatheter positioned immediately proximal to the CTO, using focal hydraulic dissection as a tool of proximal cap disruption, as well as vessel course definition.
- “Scratch-and-go” technique141(Figure 9A, Figure 9D): direct use of a high tip-load penetrative guidewire to puncture into the extraplaque space, proximally to the occluded segment. The guidewire is exchanged over a microcatheter to a polymer-jacketed one, which is advanced forward as a “knuckled” wire.
Advances in retrograde recanalisation
The concept of reopening a CTO using retrograde access was already proposed by Geoffrey Hartzler using a degenerated or occluded distal bypass connection and became a more universal option with septal wire passage, proposed by Osamu Katoh in 1998. It took another 5 years to establish a systematic approach resulting in the early technique of CART144 and then further dissemination outside of Japan (Table 3)26145146. This approach often required the dilatation of septal collaterals. Further development towards today’s standards included the development of dedicated wires and microcatheters for atraumatic collateral passage. Collateral crossing was no longer restricted to septal connections, with about a third of retrograde recanalisations performed through epicardial collaterals21134147.
Collateral assessment and selection
The first and most important step when considering the retrograde approach is the selection of an “interventional” collateral pathway. There are more than 20 individual pathways described148149. Recently presented scores that predict a successful collateral passage emphasise the relevance of the collateral connection size (CC)150, and the tortuosity of the collaterals149151152, but another important factor is the operator’s experience. If septal and epicardial collaterals coexist, the septal pathway should be preferred because it is less prone to catastrophic perforations requiring complex sealing manoeuvres.
Collateral wiring
A general rule for the retrograde approach is that extreme caution is necessary to avoid any damage to the collateral donor vessel and the fragile collaterals. Septal surfing or selective injection are 2 basic approaches to identify septal collaterals. They are often a subject for discussion but they can coexist in daily practice. Septal surfing leads to perforation in 25% of all attempted collaterals, without clinical consequence in most cases153. If there is a high degree of tortuosity in a septal, it is wise to start with a selective injection through a microcatheter. It is unanimously agreed that surfing is not advised for epicardial collaterals, where we must have a precise idea of the vessel course, best obtained in multiple viewing angles.
Microcatheter passage
After collateral wire passage, a microcatheter needs to be advanced into the distal coronary just beyond the distal cap154; this is normally achieved by holding the distal wire and rotating/advancing the microcatheter. Balloon dilatation of the septal channel with small 1.0-1.25 mm diameter balloons at a low pressure of 2-4 atm is rarely required to facilitate microcatheter passage. Epicardial channels require specific care to avoid overstretching and damage. It should be noted that epicardial channels are better approached with tapered thin microcatheters such as the Caravel (Asahi Intecc) or the Turnpike LP (Teleflex)22. An important rule to avoid perforations related to collateral passage is to desist from forcing the microcatheter across extreme bends, especially in epicardial collaterals. If the microcatheter cannot advance, we still have the opportunity to use the distal wire as a marker wire. This technique, used in the early phases of the retrograde approach to facilitate antegrade wire direction145, reduces the need for contrast injection during the manipulation of the antegrade wire.
Connecting the retrograde and antegrade lumen
The ideal course of a retrograde approach is the crossing of the retrograde wire, supported by the retrograde microcatheter, from the distal true lumen into the proximal true lumen of the occluded vessel (Central illustration). This process is threatened by the translational movement of the heart, which may cause considerable instability of the microcatheter tip. If the retrograde wire does not proceed proximally to the occlusion or enters a false lumen, the reverse CART technique should be used155. This technique requires both an antegrade and a retrograde wire positioned within the occlusion; one wire is advanced to meet the other wire. The wires need to be parallel within the occlusion at some point, moving (“dancing”) together during the heartbeat. Then, a balloon is advanced over the antegrade wire to dilate the occluded segment and allow the retrograde wire to find a route into the proximal vessel156 (Central illustration, Figure 10). This part of the retrograde approach can sometimes be time consuming, depending on the relative position of the antegrade and retrograde wires inside or outside of the plaque. To identify the position of the wires, IVUS is often required to decide the best strategy for connecting the 2 wires (Figure 6).
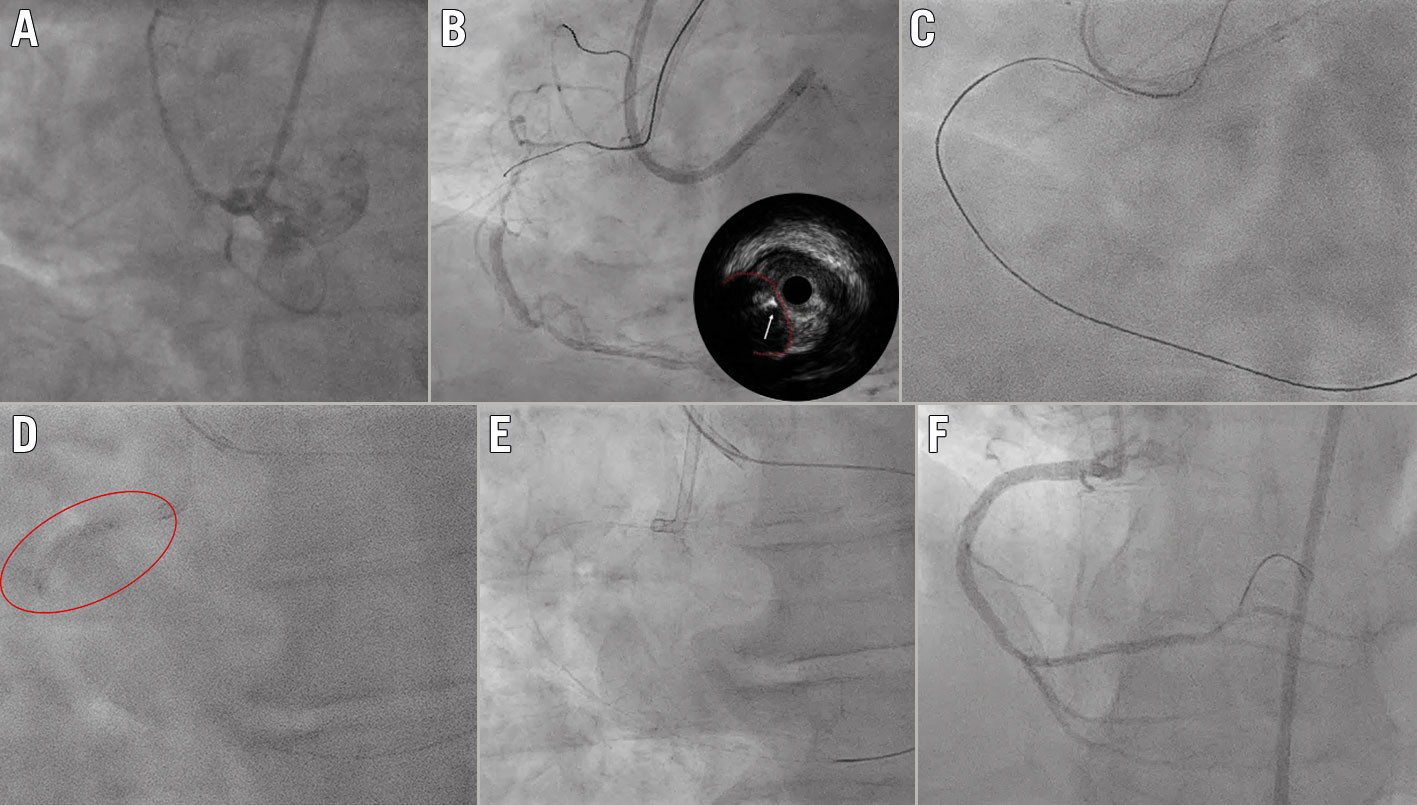
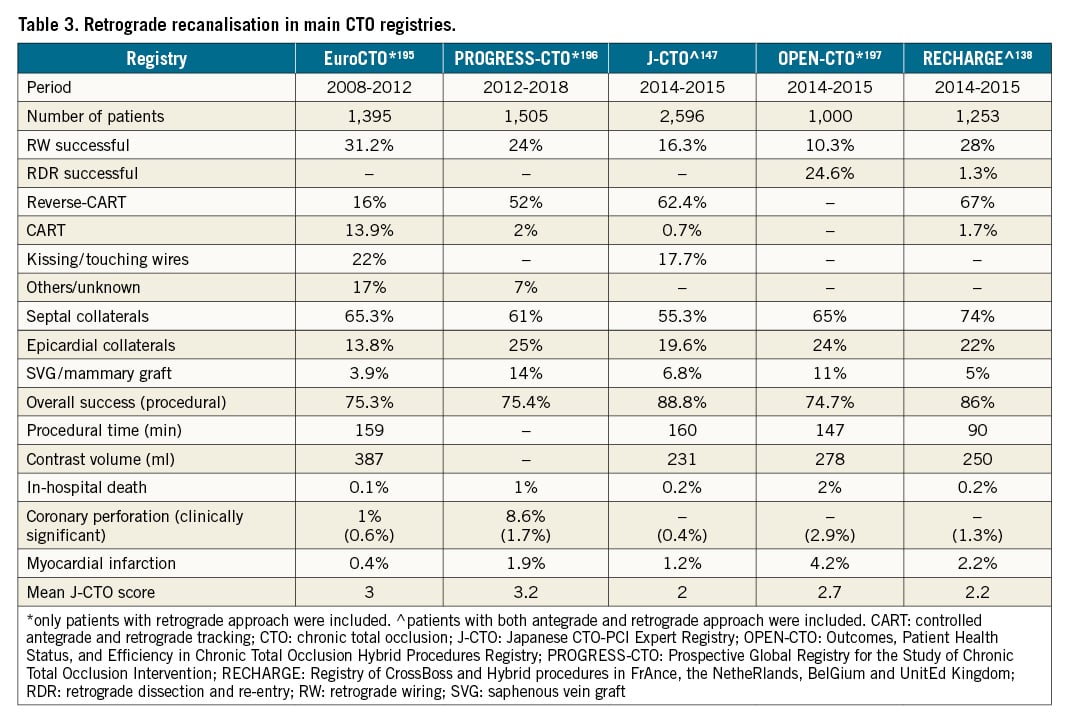
Figure 10. Reverse CART. A) Blunt occlusion of proximal RCA. B) Failed crossing of the occlusion by antegrade IVUS-guided attempt with Asahi Intecc ULTIMATEbros 3, Gaia second and Gaia third guidewires (white arrow; the red dotted line indicates the proximal CTO cap). C) A Confianza Pro 12 guidewire (Asahi Intecc), supported by a Corsair Pro XS microcatheter (Asahi Intecc), slid inside the occlusion retrogradely, parallel to the Gaia third antegrade wire, but couldn’t be steered towards the proximal lumen. D) A 2.0 mm balloon was advanced over the antegrade wire (red circle) and reverse CART was performed. E) Externalisation with RG3 guidewire (Asahi Intecc). F) Final angiographic result. CART: controlled antegrade and retrograde tracking; CTO: chronic total occlusion; IVUS: intravascular ultrasound; RCA: right coronary artery
A recent expert consensus defined the evolving concepts of the reverse CART technique with more precise terms157. We may speak of the conventional reverse CART technique, with a larger balloon being the target of a retrograde wire advanced far into the CTO body. On the other hand, we have the directed reverse CART, where the concept is to advance a wire antegrade as far as possible, followed by a rather small balloon of 2.0-2.5 mm used as a target for the retrograde wire. This concept works best with modern wires, which provide excellent torque control within the occluded segment. Finally, a third type is the extended reverse CART technique – by definition, a form of retrograde dissection re-entry technique – where the operator fails to penetrate the proximal cap but can advance the retrograde wire more proximally and then the connection is made close to, but proximal to, the proximal cap.
Retrograde dissection re-entry
Not synonymous with reverse CART, but often thought to be, is the retrograde dissection re-entry (RDR) technique48 (Figure 11). With this technique, knuckled wires are tracked antegrade and retrograde until they overlap within the occluded segment, which can be quicker than a wire-based probing towards an antegrade or retrograde target. Care must be taken to avoid side branch loss if within the dissection zone158. This technique is often required when the occluded segment is long and the vessel course ambiguous. The advantage of a knuckled wire like the Fielder XT (Asahi Intecc) or the Gladius MG (Asahi Intecc) is that they are unlikely to penetrate the adventitia and, rather, follow the course of the main vessel. Likewise, some very calcified lesions can only be overcome by going around the calcium with this approach.
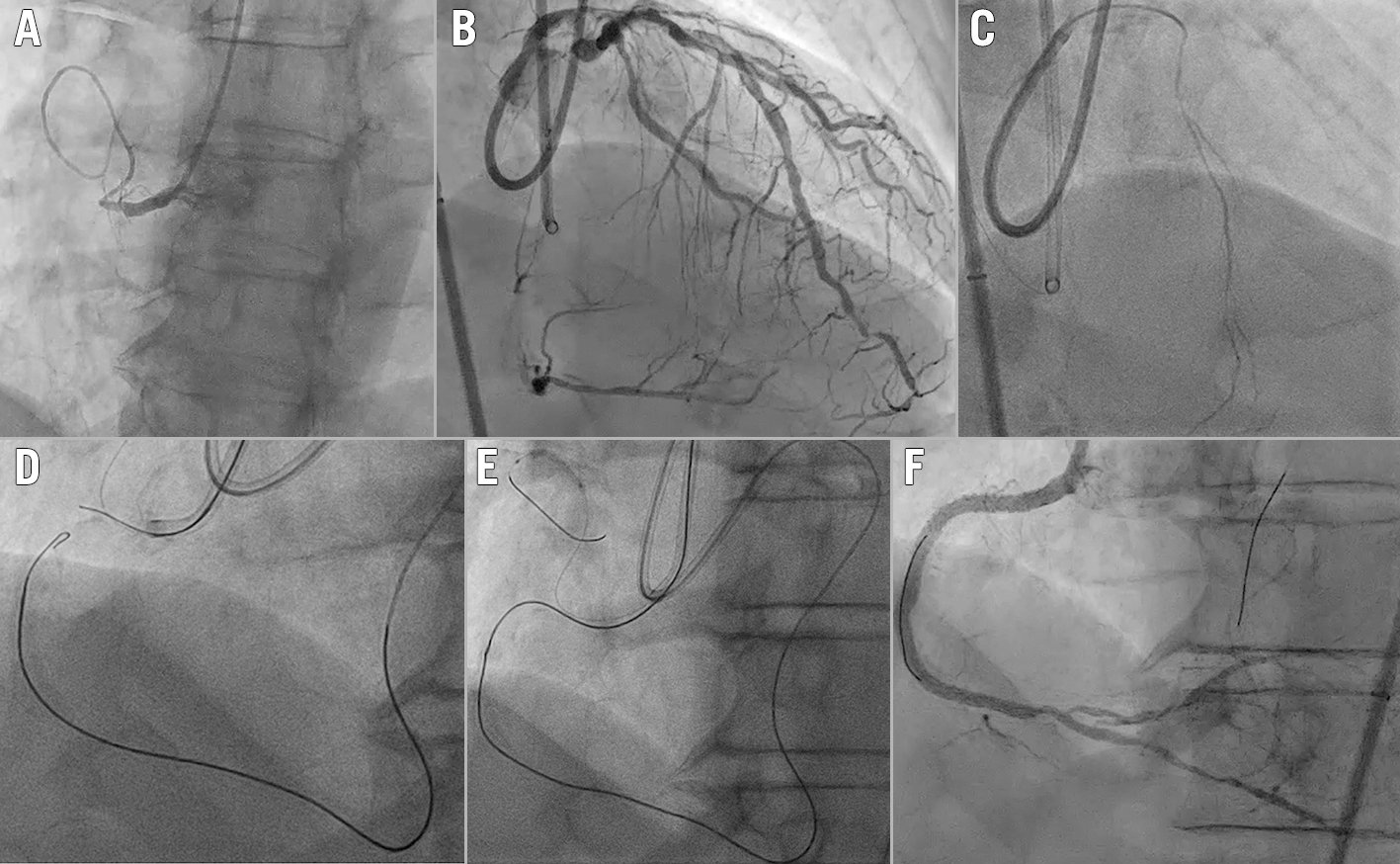
Figure 11. Retrograde dissection re-entry. A) Blunt severely calcified occlusion of the proximal RCA. B) Retrograde septal collaterals to the RCA from the LAD. C) Selective injection from the tip of a Corsair Pro XS microcatheter (Asahi Intecc) to choose the most suitable septal channel. D) After collateral wire and microcatheter passage up to the distal cap of the occlusion, the knuckled retrograde wire was steered into the subintimal space. E) The antegrade wire was advanced, creating the connection between antegrade and retrograde. F) Final angiographic result. LAD: left anterior descending artery; RCA: right coronary artery
Externalisation of the retrograde guidewire
Once the retrograde wire is passed into the true proximal vessel lumen, the next step is usually the externalisation of the wire, i.e., passage of the retrograde wire into the antegrade guiding catheter and out of the catheter through the Y-connector22. When there is significant disease of the segment proximal to the occlusion or poor alignment of the guiding catheter and the proximal vessel, a guide catheter extension can help to facilitate the wire externalisation159 (Central illustration). In some cases when the externalisation cannot be achieved because the retrograde microcatheter is too short or does not cross the occlusion, the retrograde wire can enter the antegrade microcatheter within the guiding catheter with a tip-in manoeuvre160. Alternatively, the antegrade wire can be inserted into the retrograde microcatheter by aligning the 2 microcatheters within the occlusion or in the antegrade guiding catheter (called the bridge or rendez-vous technique).
For true ostial occlusions, precluding antegrade intubation, the wire should be advanced into the aortic root and caught by a snare through the antegrade guiding catheter. The snaring of the wire can be facilitated when the wire and the snare are both advanced into the right brachiocephalic artery.
Specific CTO complications
The specific risk of the retrograde approach is damage to the collateral channel and/or the donor vessel. Flow-limiting dissections and thrombus formation in the donor vessel should be avoided by maintaining an activated clotting time well beyond 300 sec22, avoiding deep intubation of the retrograde guiding catheter, especially when removing the retrograde microcatheter, and using an additional safety wire along the main donor vessel for any rescue angioplasty required in case of vessel damage. Collateral perforation, especially in epicardial collaterals on the cardiac surface close to the pericardium, may cause catastrophic tamponade that is difficult to repair161162163. The perforation of septal collaterals is often self-limiting, but focal compression due to septal haematoma or localised bleeding of epicardial collaterals within a closed pericardial space after previous cardiac surgery are also life-threatening164165. In a recent US registry, the risk of collateral perforations with high mortality was increased in post-bypass patients, which needs to be taken into account when treating this specific patient group166.
IVUS in CTO: more than stent optimisation
IVUS is a fundamental tool in CTO PCI (Figure 6). It can be used in the antegrade approach to facilitate wire penetration in ambiguous proximal caps or to guide a dissection re-entry strategy from the subintimal space. In the retrograde approach, it can be used for difficult re-entry in true ostial CTOs or to guide the reverse CART technique. After CTO wire crossing, IVUS is useful to distinguish intimal or subintimal guidewire tracking and helps in the presence of distal disease.
"STUMPLESS" CTOs
When there is a side branch sufficiently large to accommodate an IVUS probe and which is close to an ambiguous proximal cap (Central illustration, Figure 12), IVUS can reveal the plaque morphology of the proximal cap and guide the choice of guidewire for the correct cap penetration. When a 7 Fr (or larger) guiding catheter has been selected, the IVUS probe can be left in place in “live view” to guide the microcatheter-supported wire puncture. In some cases, the simultaneous presence of both the IVUS probe and the microcatheter is made impossible by severe disease or tortuosity of the proximal vessel, causing excessive interference. Still, the IVUS probe can be advanced after the wire puncture to confirm the correct entry point and guidewire position.
Figure 12. IVUS for entry point identification and penetration. A, A') IVUS probe in side branch for identification of left circumflex CTO proximal cap. B, B’) Gaia second guidewire (Asahi Intecc) too eccentric, wrong puncture. C, C’) Perfect central puncture with Gaia second supported by Finecross microcatheter (Terumo) at ostial LAD. CTO: chronic total occlusion; IVUS: intravascular ultrasound; LAD: left anterior descending artery
IVUS-guided re-entry
If the wire has failed to cross the CTO and reached a subintimal position distal to the occlusion, antegrade injections must be strictly avoided. An IVUS probe can follow the wire and help direct a second wire to a different track starting from the proximal stump, offering real-time confirmation of its position in the distal true lumen167168.
If this fails, the IVUS catheter can be left in the subintimal distal position together with a microcatheter. A stiff guidewire with a 45 degree bend in the last 7-8 mm (longer than usual) is then advanced to penetrate from the extra- to intraplaque space under IVUS guidance. IVUS helps to identify the most appropriate puncture site, to avoid calcific segments and segments with excessive separation between false and true lumen, and can be used to facilitate re-entry also with a conventional ADR technique using sequential insertion of IVUS followed by the Stingray positioned in the optimal site, identified with IVUS.
IVUS for reverse CART: correct position, balloon sizing, wire connection
IVUS can be of help when reverse CART fails155156169. When the antegrade and retrograde wires are both intraplaque or are both in the subintimal space, antegrade balloon dilatation easily creates a connection of the 2 wires in the same space.
IVUS is also helpful to identify the other 2 more difficult situations. When the antegrade guidewire is intraplaque but the retrograde guidewire is in the subintimal space, IVUS can be used to correctly identify a properly sized balloon diameter (ideally 1:1) to facilitate wire connection after inflation. Retrograde crossing is more difficult when the antegrade wire is in the subintimal space (extraplaque) but the retrograde wire is inside the plaque, which is often very calcified. Inflation of the antegrade balloon only serves to enlarge the subintimal space, increasing subintimal haematoma, but without cracking the calcium in the CTO body. Increasing the balloon size is ineffective, and IVUS can be very useful to facilitate the connection between the 2 wires, identifying a different segment with a more favourable scenario. The success in this case is usually achieved by pushing a knuckled retrograde wire in the subintimal space towards the antegrade guidewire, or using a puncture with a retrograde stiff wire from intra- to extraplaque to make both wires subintimal168.
DIFFICULT RETROGRADE RE-ENTRY IN TRUE OSTIAL CTOs
In true ostial occlusions, a retrograde approach is often required but it is of paramount importance to avoid advancing the wire subintimally in the left main or along the aortic wall, creating dangerous dissections.
For true ostial left anterior descending (LAD) artery CTOs, an IVUS probe placed in the ostial circumflex could confirm the retrograde guidewire position into the LAD true lumen before reaching the distal left main, avoiding a potentially catastrophic re-entry from the subintimal space and risking acute circumflex occlusion. In this situation, generally, the best solution is to try to advance the antegrade wire and go for a reverse CART distal to the bifurcation, with the guiding extension-assisted re-entry and IVUS confirmation. If this option fails, at least the operator will be ready to maintain a large balloon across the ostium of the left circumflex before deploying the stent of the LAD ostium, finishing with a controlled crush technique.
For true ostial right coronary artery CTOs, an IVUS probe can be inserted without the need for a guidewire into the aorta and steered using an appropriate guiding catheter towards the ostium. IVUS probes with lower frequency and greater penetration are preferred in these situations. Once the retrograde guidewire penetrates the ostial segment, IVUS detects the wire position and easily distinguishes if it is free in the ascending aorta or still stuck in the aortic wall (Figure 13).
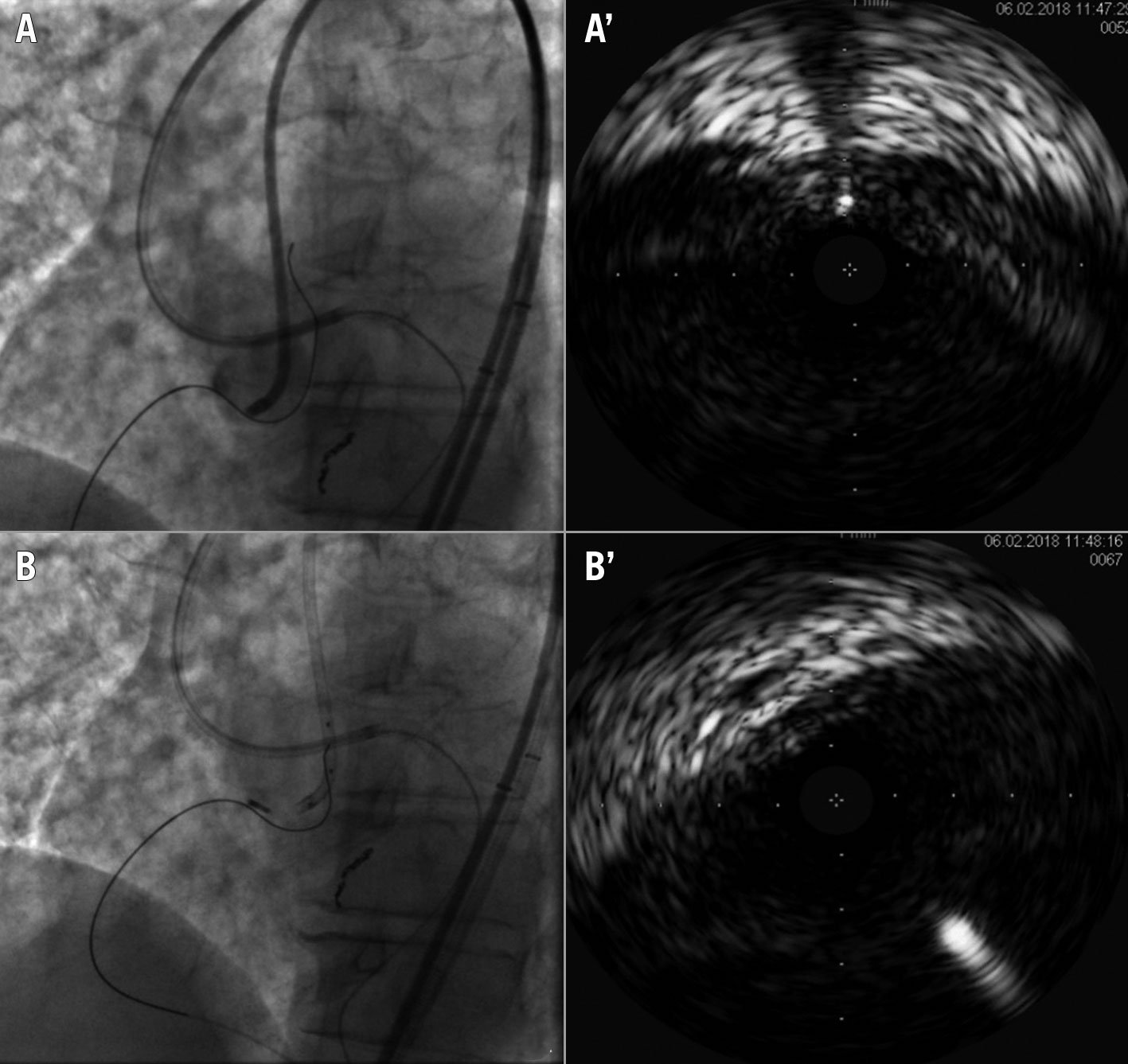
Figure 13. IVUS for ostial RCA re-entry. A, A') ULTIMATEbros 3 guidewire (Asahi Intecc) retrograde crossing in aorta but IVUS shows the wire in the aortic wall . B, B’) New attempt of ULTIMATEbros 3 retrograde crossing and IVUS confirmation of a correct position within the aortic lumen. IVUS: intravascular ultrasound; RCA: right coronary artery
IVUS for stent implantation and optimisation
IVUS-guided stenting after the initial balloon predilatation helps to avoid contrast injections that could create or worsen antegrade or retrograde subintimal dissections. This golden rule should always apply, including techniques expected to achieve a true-to-true lumen approach. Fortunately, subintimal tracking has been shown to have no negative clinical impact at mid-term follow up170171.
IVUS is essential to evaluate the true vessel size (media-to-media) and to understand if there are some points of negative remodelling in which stent overexpansion can lead to vessel rupture. Optimal expansion always improves outcome after stenting172173 but its potential utility is greater after CTO stenting. A systematic underestimation of vessel size, often by as much as 1-2 mm, has been shown by Park et al174 in 58 CTO patients serially restudied with IVUS 3 months after the initial CTO recanalisation. Not surprisingly, stent optimisation appears to be the strongest predictor of long-term success since the introduction of DESs in CTO interventions169175.
In the Korean CTO Registry176, a propensity score-matched analysis between 206 IVUS-guided PCI and 201 patients undergoing angiography guidance alone showed that after 2 years, IVUS guidance led to significantly less stent thrombosis than angiography alone (0% vs 3%; p=0.014) and to a non-significant reduction in the incidence of myocardial infarction (1% vs 4%; p=0.058). The rates of major adverse cardiovascular events (MACE) were similar in both groups.
In the Korean randomised CTO-IVUS study177, 402 patients with CTOs were randomised to angio-guided vs IVUS-guided PCI after successful wire crossing of the CTO. After 12 months, MACE rates were significantly lower in the IVUS-guided group (2.6% vs 7.1%; hazard ratio 0.35; 95% confidence interval: 0.13–0.97; p=0.035). The IVUS-guided group were more likely to receive larger stents and high-pressure dilatation after stenting with greater minimal lumen diameter compared with the angiography group.
General consensus on the criteria to be followed for a safe and effective optimal stent expansion after CTO recanalisation is still lacking. The key points are the selection of the distal landing zone, avoiding excessive distal plaque burden but also resisting the temptation to extend the stented segment too far beyond the occlusion where positive remodelling after flow restoration is expected to increase lumen size and modify the estimated percent plaque burden. This is probably the most difficult, often subjective, decision during CTO recanalisation and IVUS definitely helps, distinguishing a distal small hypoperfused vessel without significant plaque burden from a severely diseased vessel needing treatment. Predictors of lumen area enlargement after CTO recanalisation have been described: occlusion duration >3 months, poor collateral flow, statin use, LAD occlusion and the absence of moderate/severe calcification, and one of these, the presence of a perimedial high echoic band, can be identified on IVUS before stenting174178179180. There is also no agreement on vessel preparation before stenting. While no further expansion before stenting is probably needed when there is extensive subintimal tracking, a true-to-true crossing in a highly calcified segment, as is often present in CTOs, might be a good indication for high-pressure predilatation with appropriately sized balloons. CTOs were one of the few exclusion criteria in the initial registries of intravascular lithotripsy (IVL)181, but the safety shown in these registries and the fact that dilatation during delivery is performed at very low pressure (4 atm), minimising the risk of perforation, suggests a major role of IVL in stent optimisation during CTO PCI, as confirmed in successful initial series182183. A relatively small distal stent should be selected matching the distal lumen, especially when diffuse disease is present. The more proximal stent can be larger, matching the proximal segment unless clear segments of negative remodelling are present within the occlusion. After stenting, serial ultrasound examination will detect segments of residual underexpansion guiding further high-pressure dilation with non-compliant balloons.
Future developments
Progress in CTO recanalisation has been a slow process of refinement of dedicated material, with persistent need of specific training of experienced operators. The Stingray has been the only innovative technology that has stood the test of time, enabling distal wire re-entry via a stepwise teachable procedure, but it is difficult to call it a “breakthrough” technology. Its penetration does not exceed 25% even among the highest volume users and may remain as low as 2% among other CTO operators who apply it sparingly and only as a bailout technique98184. After failure of so many other “revolutionary” devices in the last 30 years, there is deep scepticism that a real breakthrough technology that will allow consistent success with low complications for operators with limited PCI experience will ever come. Still there are several promising programs, from the use of local collagenase to modify the lesion before crossing185186 to the application of forward-looking lithotripsy to disrupt intraluminal calcifications187 or a bipolar radiofrequency wire system (PlasmaWire System; RetroVascular/Asahi Intecc) for plaque ablation and channel creation inside the occlusion188. Time will tell whether effective devices fulfilling these promises will be developed. Aside from massive calcification of the lesion, “ambiguity” of the CTO course is the main enemy now that very effective stiff wires can be steered within the occlusion. In peripheral revascularisation and in many structural procedures, CT angiography can be fused with conventional angiography to guide the procedure, taking advantage of its superior 3-dimensional reconstruction of the vessel course189. Respiratory changes in a beating heart remain a challenge for the application of reliable fusion programs, but the information obtained with CT angiography on calcium distribution, true occlusion length and vessel direction is invaluable and may already prompt changes in the CTO recanalisation strategy190191.
Conclusions
With 15-20% of all patients undergoing coronary angiography having 1 or more CTOs and 1/4-1/5 of all CTOs approached by PCI, the challenge for modern interventional cardiology is to streamline the technique and expand training. A cautious attitude is obligatory before embarking on procedures that remain complex and demanding. Still, it is not acceptable that patients are left on medical therapy or referred to surgery, not because of clinical appropriateness, but because no operators able to approach the procedure complexity are available or the procedural costs exceed the reimbursement rate. All interventional cardiologists should know the basic principles of CTO treatment. The most complex CTO procedures should be referred or performed together with specifically trained operators in high-volume centres. These, together with a fair reimbursement policy, are indispensable steps to offer this treatment option to all CTO patients with valid clinical indications to recanalisation.
Acknowledgments
The authors would like to thank Dr. Claire Raphael (Cleveland Clinic, OH, USA) for her valuable support in the final revision of the manuscript.
Conflict of interest statement
C. Di Mario is the recipient of institutional research grants from AMGEN, Abbott Vascular, Behring, Boston Scientific, Chiesi Pharmaceuticals, Daiichi Sankyo, Edwards Lifesciences, Medtronic, Shockwave Medical, and Philips Volcano. K. Mashayekhi reports consulting/speaker/proctoring honoraria from Abbott Vascular, Abiomed, Ashai Intecc, AstraZeneca, Biotronik, Boston Scientific, Cardinal Health, Daiichi Sankyo, Medtronic, Shockwave Medical, Teleflex, and Terumo. R. Garbo has received consulting/speaker/proctoring honoraria from Abbott Vascular, Asahi Intecc, Boston Scientific, IMDS, Philips Volcano, Teleflex, and Terumo. S. A. Pyxaras reports consulting/speaker/proctorship honoraria from Abiomed, AstraZeneca, Asahi Intecc, and Boston Scientific. G. Werner reports speaker honorarium from Asahi Intecc, Bayer, Biotronik, Daiichi Sankyo, Shockwave Medical, Siemens Healthineers, and Terumo. All authors (except N. Ciardetti) are members of the EuroCTO Club that receives annual grants to support its congress and other activities from Abbott, Asahi Intecc, Biotronik, Boston Scientific, Braun, IMDS, OrbusNeich, Philips Volcano, Shockwave, SIS Medical, Teleflex, and Terumo.
Supplementary data
To read the full content of this article, please download the PDF.

